Abstract
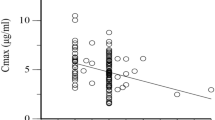
Previous studies have shown controversial results on whether acetylator status causes isoniazid-induced hepatotoxicity (IIH). Moreover, the contribution of CYP2E1, a hepatic enzyme implicated in the formation of hepatotoxins, to the risk of developing IIH remains unclear. The objectives of this study were (i) to assess the quantitative relationship between the level of isoniazid serum concentration and the incidence of IIH and (ii) to evaluate the extent of implication of the N-acetyltransferase-2 (NAT2) and CYP2E1 polymorphisms genes to induce this disorder. Seventy-one patients with tuberculosis receiving a conventional antituberculosis regimen were included. NAT2 and CYP2E1 genotypes were determined using polymerase chain reaction. Three restriction enzymes, RsaI, PstI and DraI were used to detect CYP2E1 RFLP and four different restriction enzymes, KpnI, TaqI, BamHI and Ddel were used to determine NAT2 acetylator status. Therapeutic drug monitoring (TDM) of isoniazid (serum concentration performed 3 h after the morning dose: C3) was performed. Cases of isoniazid-induced hepatotoxicity were diagnosed according to Benichou et al. Receiver Operating Characteristics curve analysis was used to evaluate the relationship between risk factors and the incidence of IIH. Eleven (15.4%) patients have developed IIH. Demographic factors, including age, weight and gender were not associated with the incidence of hepatotoxicity. High serum concentration of isoniazid (C3) was found to be a risk factor of IIH (area under the curve: 0.74, P=0.007, 95% confidence interval (95% CI): 0.56–0.93), with a cutoff value at 3.69 mg l−1 (odds ratio (OR): 13.2, P=0.0007, 95% CI: 2.9–59). Multivariate analysis showed that only a C3 over 3.69 mg l−1 remains a risk factor of IIH. NAT2 and CYP2E1 variants were not found to increase the risk of IIH when analyzed separately. However, combined analysis of the NAT2/CYP2E1 gene polymorphisms showed that patients with both DraI C/D and slow acetylator have an increased risk of IIH compared with other combined NAT2/CYP2E1 genotype profiles (OR: 8.41, P=0.01, 95% CI: 1.54–45.76). Our results suggest that a serum concentration of isoniazid over 3.69 mg l−1 and a combined genotype CYP2E1 DraI(C/D)/slow acetylator are major risk factors for IIH. Therefore, TDM of isoniazid and the determination of both NAT2 and CYP2E1 genotypes could be useful for the prediction and prevention of IIH in Tunisian tuberculosis patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174: 935–952.
Higuchi N, Tahara N, Yanagihara K, Fukushima K, Suyama N, Inoue Y et al. NAT2 6A, a haplotype of the N-acetyltransferase 2 gene, is an important biomarker for risk of anti-tuberculosis drug-induced hepatotoxicity in Japanese patients with tuberculosis. World J Gastroenterol 2007; 13: 6003–6008.
Cho HJ, Koh WJ, Ryu YJ, Ki CS, Nam MH, Kim JW et al. Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis 2007; 87: 551–556.
Possuelo LG, Castelan JA, de Brito TC, Ribeiro AW, Cafrune PI, Picon PD et al. Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from Southern Brazil. Eur J Clin Pharmacol 2008; 64: 673–681.
Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY et al. Polymorphism of the N- acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology 2002; 35: 883–889.
Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B et al. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol 2006; 62: 423–429.
Mitchell JR, Thorgeirsson UP, Black M, Timbrell JA, Snodgrass WR, Potter WZ et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther 1975; 18: 70–79.
Fretland AJ, Leff MA, Doll MA, Hein DW . Functional characterization of human N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms. Phamacogenetics 2001; 11: 207–215.
Zang Y, Doll MA, Zhao S, States JC, Hein DW . Functional characterization of single-nucleotide polymorphisms and haplotypes of human N-acetyltransferase 2. Carcinogenesis 2007; 28: 1665–1671.
Walker K, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B . Genetic polymorphism in N-acetyltransferase (NAT): population distribution of NAT1 and NAT2 activity. J Toxicol Environ Health B Crit Rev 2009; 12: 440–472.
Sheng YJ, Wu G, He HY, Chen W, Zou YS, Li Q et al. The association between CYP2E1 polymorphisms and hepatotoxicity due to anti-tuberculosis drugs: a meta-analysis. Infect Genet Evol 2014; 24: 34–40.
Ohno M, Yamaguchi I, Yamamoto I, Fukuda T, Yokota S, Maekura R et al. Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int J Tuberc Lung Dis 2000; 4: 256–261.
Kita T, Tanigawara Y, Chikazawa S, Hatanaka H, Sakaeda T, Komada F et al. N-acetyltransferase 2 genotype correlated with isoniazid acetylation in Japanese tuberculous patients. Biol Pharm Bull 2001; 24: 544–549.
Hiratsuka M, Kishikawa Y, Takekuma Y, Matsuura M, Narahara K, Inoue T et al. Genotyping the N-acetyltransferase 2 polymorphism in the prediction of adverse drug reactions to isoniazid in Japanese patients. Drug Metab Pharmacokinet 2002; 17: 357–362.
Singh J, Garg PK, Thakur VS, Tandon RK . Antitubercular treatment induced hepatotoxicity: does acetylator status matter? Indian J Physiol Pharmacol 1995; 39: 43–46.
An HR, Wu XQ, Wang ZY, Zhang J, Liang Y . NAT2 and CYP2E1 polymorphisms associated with antituberculosis drug-induced hepatotoxicity in Chinese patients. Clin Exp Pharmacol Physiol 2012; 39: 535–543.
Bose PD, Sarm MP, Medhi S, Das BC, Husain S, Kar P et al. Role of polymorphic N-acetyl transferase2 and cytochrome P4502E1 gene in antituberculosis treatment-induced hepatitis. J Gastroenterol Hepatol 2011; 26: 312–318.
Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003; 37: 924–930.
Lee SW, Chung LS, Huang HH, Chuang TY, Liou YH, Wu LS . NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis druginduced hepatitis. Int J Tuberc Lung Dis 2010; 14: 622–626.
Wang T, Yu HT, Wang W, Pan YY, He LX, Wang ZY . Genetic polymorphisms of cytochrome P450 and glutathione S-transferase associated with antituberculosis drug-induced hepatotoxicity in Chinese tuberculosis patients. J Int Med Res 2010; 38: 977–986.
Tang SW, Lv XZ, Zhang Y, Wu SS, Yang ZR, Xia YY et al. CYP2E1, GSTM1 and GSTT1 genetic polymorphisms and susceptibility to antituberculosis drug-induced hepatotoxicity: a nested case-control study. J Clin Pharm Ther 2012; 37: 588–593.
Chamorro JG, Castagnino JP, Musella RM, Nogueras M, Aranda FM, Frías A . Sex, ethnicity, and slow acetylator profile are the major causes of hepatotoxicity induced by antituberculosis drugs. J Gastroenterol Hepatol 2013; 28: 323–328.
Santos NP, Callegari-Jacques SM, Ribeiro Dos Santos AK, Silva CA, Vallinoto AC, Fernandes DC et al. N-acetyl transferase 2 and cytochrome P450 2E1 genes and isoniazid-induced hepatotoxicity in Brazilian patients. Int J Tuberc Lung Dis 2013; 17: 499–504.
Benichou C . Criteria for drug-induced liver disorder: report of an international consensus meeting. J Hepatol 1990; 11: 272–276.
Sa Miller, Dd Dykes, Hf Polesky . A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Stephens EA, Taylor JA, Kaplan N, Yang CH, Hsieh LL, Lucier GW et al. Ethnic variation in the CYP2E1 gene: polymorphism analysis of 695 African-Americans, European-Americans and Taiwanese. Pharmacogenetics 1994; 4: 185–192.
Salama SA, Sierra-Torres CH, Oh HY, Hamada FA, Au WW . A multiplex- PCR/RFLP procedure for simultaneous CYP2E1, mEH and GSTM1 genotyping. Cancer Lett 1999; 143: 51–56.
Purabi DB, Manash PS, Subhash M, Bhudev CD, Syed AH, Premashis K . Role of polymorphic N-acetyl transferase2 and cytochrome P4502E1 gene in antituberculosis treatment induced hepatitis. Gastroenterol Hepatol 2011; 26: 312–318.
Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R . Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 2008; 23: 192–202.
Jeong I, Park JS, Cho YJ, Yoon HI, Song J, Lee CT et al. Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels. J Korean Med Sci 2015; 30: 167–172.
Gurumurthy P, Krishnamurthy MS, Nazareth O, Parthasarathy R, Sarma GR . Lack of relationship between hepatic toxicity and acetylator phenotype in three thousand South Indian patients during treatment with isoniazid for tuberculosis. Am Rev Respir Dis 1984; 129: 58–61.
Vivien JN, Thibier R, Lepeuple A . La pharmacocinétique de l’isoniazide dans la race blanche. Rev Fr Mal Resp 1973; 1: 753–772.
Evans DA . N-Acetyltransferase. Pharmacol Ther 1989; 42: 157–234.
Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 2001; 10: 1239–1248.
Tanira MO, Simsek M, Al Balushi K, Al Lawatia K, Al Barawani H, Bayoumi RA . Distribution of arylamine N-acetyltransferase 2 (nat2) genotypes among Omanis. J Sci Res Med Sci 2003; 5: 9–14.
Lin HJ, Han CY, Lin BK, Hardy S . Ethnic distribution of slow acetylator mutations in the polymorphic N-acetyltransferase (NAT2) gene. Pharmacogenetics 1994; 4: 125–134.
Hamdy SI, Hiratsuka M, Narahara K, Endo N, El-Enany M, Moursi N et al. Genotype and allele frequencies of TPMT, NAT2, GST, SULT1A1 and MDR-1 in the Egyptian population. Br J Clin Pharmacol 2003; 55: 560–569.
Sabbagh A, Langaney A, Darlu P, Gerard N, Krishnamoorthy R, Poloni ES . Worldwide distribution of NAT2 diversity: implications for NAT2 evolutionary history. BMC Genet 2008; 9: 21.
Guaoua S, Ratbi I, Laarabi FZ, Elalaoui SC, Jaouad IC, Barkat A et al. Distribution of allelic and genotypic frequencies of NAT2 and CYP2E1 variants in Moroccan population. BMC Genet 2014; 15: 156.
Kunak SC, Ada AO, Karacaoglan V, Soydas E, Bilgen S, Iscan M . Drug/xenobiotic metabolizing enzyme polymorphisms in a Turkish population. African J Pharm Pharmacol 2012; 6: 2068–2074.
Acknowledgements
Ethical approval was obtained from the local committee. An informed consent was obtained from all the subjects included in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ben Fredj, N., Gam, R., Kerkni, E. et al. Risk factors of isoniazid-induced hepatotoxicity in Tunisian tuberculosis patients. Pharmacogenomics J 17, 372–377 (2017). https://doi.org/10.1038/tpj.2016.26
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.26
This article is cited by
-
Development of a population pharmacokinetic model and Bayesian estimators for isoniazid in Tunisian tuberculosis patients
The Pharmacogenomics Journal (2021)
-
The clinical impact of drug-induced hepatotoxicity on anti-tuberculosis therapy: a case control study
Respiratory Research (2019)
-
Association between ATT and Hepatotoxicity: Food for Thought
The Indian Journal of Pediatrics (2019)
-
CYP genetic variants and toxicity related to anti-tubercular agents: a systematic review and meta-analysis
Systematic Reviews (2018)
-
Isoniazid
Reactions Weekly (2017)