Abstract
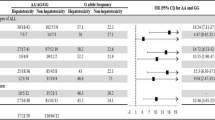
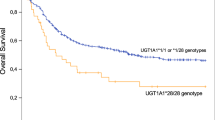
Drug-related toxicities represent an important clinical concern in chemotherapy, genetic variants could help tailoring treatment to patient. A pharmacogenetic multicentric study was performed on 508 pediatric acute lymphoblastic leukemia patients treated with AIEOP-BFM 2000 protocol: 28 variants were genotyped by VeraCode and Taqman technologies, deletions of GST-M1 and GST-T1 by multiplex PCR. Toxicities were derived from a central database: 251 patients (49.4%) experienced at least one gastrointestinal (GI) or hepatic (HEP) or neurological (NEU) grade III/IV episode during the remission induction phase: GI occurred in 63 patients (12.4%); HEP in 204 (40.2%) and NEU in 44 (8.7%). Logistic regression model adjusted for sex, risk and treatment phase revealed that ITPA rs1127354 homozygous mutated patients showed an increased risk of severe GI and NEU. ABCC1 rs246240 and ADORA2A rs2236624 homozygous mutated genotypes were associated to NEU and HEP, respectively. These three variants could be putative predictive markers for chemotherapy-related toxicities in AIEOP-BFM protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010; 115: 3206–3214.
Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 2011; 118: 2077–2084.
Vecchi V, Arico M, Basso G, Ceci A, Madon E, Mandelli F et al. Risk-directed therapy for childhood acute lymphoblastic leukemia. Results of the Associazione Italiana Ematologia Oncologia Pediatrica '82 studies. Cancer 1993; 72: 2517–2524.
AIRTUM Working Group CCM AIEOP Working Group. Italian cancer figures, report 2012: Cancer in children and adolescents. Epidemiol Prev 37 (1 Suppl 1): 1–225.
Gervasini G, Vagace JM . Impact of genetic polymorphisms on chemotherapy toxicity in childhood acute lymphoblastic leukemia. Front Genet 2012; 3: 249.
Stocco G, Franca R, Verzegnassi F, Londero M, Rabusin M, Decorti G . Pharmacogenomic approaches for tailored anti-leukemic therapy in children. Curr Med Chem 2012; 20: 2237–2253.
Franca R, Rebora P, Basso G, Biondi A, Cazzaniga G, Crovella S et al. Glutathione S-transferase homozygous deletions and relapse in childhood acute lymphoblastic leukemia: a novel study design in a large Italian AIEOP cohort. Pharmacogenomics 2012; 13: 1905–1916.
Farrow AC, Buchanan GR, Zwiener RJ, Bowman WP, Winick NJ . Serum aminotransferase elevation during and following treatment of childhood acute lymphoblastic leukemia. J Clin Oncol 1997; 15: 1560–1566.
Rizzari C, Putti MC, Colombini A, Casagranda S, Ferrari GM, Papayannidis C et al. Rationale for a pediatric-inspired approach in the adolescent and young adult population with acute lymphoblastic leukemia, with a focus on asparaginase treatment. Hematol Rep 2014; 6: 5554.
Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G et al. PharmGKB summary: methotrexate pathway. Pharmacogenet Genomics 2011; 21: 679–686.
Hider SL, Thomson W, Mack LF, Armstrong DJ, Shadforth M, Bruce IN . Polymorphisms within the adenosine receptor 2a gene are associated with adverse events in RA patients treated with MTX. Rheumatology 2008; 47: 1156–1159.
Li L, Agarwal S, Elmquist WF . Brain efflux index to investigate the influence of active efflux on brain distribution of pemetrexed and methotrexate. Drug Metab Dispos. 2013; 41: 659–667.
Bleyer WA, Dedrick RL . Clinical pharmacology of intrathecal methotrexate. I. Pharmacokinetics in nontoxic patients after lumbar injection. Cancer Treat Rep 1977; 61: 703–708.
Ohta A, Sitkovsky M . Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414: 916–920.
Kishi S, Cheng C, French D, Pei D, Das S, Cook EH et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood 2007; 109: 4151–4157.
Heller T, Oellerich M, Armstrong VW, von Ahsen N . Rapid detection of ITPA 94C>A and IVS2+21A>C gene mutations by real-time fluorescence PCR and in vitro demonstration of effect of ITPA IVS2+21A>C polymorphism on splicing efficiency. Clin Chem 2004; 50: 2182–2184.
Stocco G, Cheok MH, Crews KR, Dervieux T, French D, Pei D et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009; 85: 164–172.
Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 2011; 71: 575–584.
Kim H, Kang HJ, Kim HJ, Jang MK, Kim NH, Oh Y et al. Pharmacogenetic analysis of pediatric patients with acute lymphoblastic leukemia: a possible association between survival rate and ITPA polymorphism. PLoS ONE 2012; 7: e45558.
Wan Rosalina WR, Teh LK, Mohamad N, Nasir A, Yusoff R, Baba AA et al. Polymorphism of ITPA 94C>A and risk of adverse effects among patients with acute lymphoblastic leukaemia treated with 6-mercaptopurine. J Clin Pharm Ther 2012; 37: 237–241.
Stocco G, Yang W, Crews KR, Thierfelder WE, Decorti G, Londero M et al. PACSIN2 polymorphism influences TPMT activity and mercaptopurine-related gastrointestinal toxicity. Hum Mol Genet 2012; 21: 4793–4804.
Warren RB, Smith RL, Campalani E, Eyre S, Smith CH, Barker JN et al. Genetic variation in efflux transporters influences outcome to methotrexate therapy in patients with psoriasis. J Invest Dermatol 2008; 128: 1925–1929.
Soontornmalai A, Vlaming ML, Fritschy JM . Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 2006; 138: 159–169.
Kilic E, Spudich A, Kilic U, Rentsch KM, Vig R, Matter CM et al. ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain 2008; 131: 2679–2689.
Lamba J, Hebert JM, Schuetz EG, Klein TE, Altman RB . PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics 2012; 22: 555–558.
Aplenc R, Glatfelter W, Han P, Rappaport E, La M, Cnaan A et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. Br J Haematol 2003; 122: 240–244.
Moore AS, Norris R, Price G, Nguyen T, Ni M, George R et al. Vincristine pharmacodynamics and pharmacogenetics in children with cancer: a limited-sampling, population modelling approach. J Paediatr Child Health 2011; 47: 875–882.
Davies SM, Borowitz MJ, Rosner GL, Ritz K, Devidas M, Winick N et al. Pharmacogenetics of minimal residual disease response in children with B-precursor acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 2008; 111: 2984–2990.
Guha N, Chang JS, Chokkalingam AP, Wiemels JL, Smith MT, Buffler PA . NQO1 polymorphisms and de novo childhood leukemia: a HuGE review and meta-analysis. Am J Epidemiol 2008; 168: 1221–1232.
van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol 2003; 59: 585–592.
Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab 1998; 83: 144–151.
Cuzzoni E, De Iudicibus S, Bartoli F, Ventura A, Decorti G . Association between BclI polymorphism in the NR3C1 gene and in vitro individual variations in lymphocyte responses to methylprednisolone. Br J Clin Pharmacol 2012; 73: 651–655.
Marino S, Verzegnassi F, Tamaro P, Stocco G, Bartoli F, Decorti G et al. Response to glucocorticoids and toxicity in childhood acute lymphoblastic leukemia: role of polymorphisms of genes involved in glucocorticoid response. Pediatr Blood Cancer 2009; 53: 984–991.
Lauten M, Matthias T, Stanulla M, Beger C, Welte K, Schrappe M . Association of initial response to prednisone treatment in childhood acute lymphoblastic leukaemia and polymorphisms within the tumour necrosis factor and the interleukin-10 genes. Leukemia 2002; 16: 1437–1442.
French D, Hamilton LH, Mattano LA Jr, Sather HN, Devidas M, Nachman JB et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 2008; 111: 4496–4499.
Stocco G, Crews KR, Evans WE . Genetic polymorphism of inosine-triphosphate-pyrophosphatase influences mercaptopurine metabolism and toxicity during treatment of acute lymphoblastic leukemia individualized for thiopurine-S-methyl-transferase status. Expert Opin Drug Saf 2012; 9: 23–37.
Dervieux T, Wessels JA, van der Straaten T, Penrod N, Moore JH, Guchelaar HJ et al. Gene-gene interactions in folate and adenosine biosynthesis pathways affect methotrexate efficacy and tolerability in rheumatoid arthritis. Pharmacogenet Genomics 2009; 19: 935–944.
Kelly JL, Novak AJ, Fredericksen ZS, Liebow M, Ansell SM, Dogan A et al. Germline variation in apoptosis pathway genes and risk of non-Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev 2010; 19: 2847–2858.
Huang RS, Duan S, Kistner EO, Bleibel WK, Delaney SM, Fackenthal DL et al. Genetic variants contributing to daunorubicin-induced cytotoxicity. Cancer Res 2008; 68: 3161–3168.
Enjuanes A, Benavente Y, Bosch F, Martin-Guerrero I, Colomer D, Perez-Alvarez S et al. Genetic variants in apoptosis and immunoregulation-related genes are associated with risk of chronic lymphocytic leukemia. Cancer Res 2008; 68: 10178–10186.
Acknowledgements
PR was partially supported by the grant MIUR-SIR 2014 (RBSI14LOVD). We thank AGMEN (Associazione Genitori Malati Emopatici Neoplastici)-Friuli Venezia Giulia (Italy) and IRCCS Burlo Garofolo in Trieste (Italy) for supporting the pharmacogenetic project.
Author contributions
RF was the principal investigator and contributed to study design, genetic analysis, data interpretation and paper writing; PR is responsible for the study design and statistical analysis; NB recruited patients and collected the clinical data; FF and VC discussed results and revised the manuscript; AB, AC, CM, MZ, RP, FP, GB, MCP and FL recruited patients and collected the clinical data; PD contributed for genetic analysis; MGV and GD discussed results and revised the manuscript; MR contributed to study design, co-ordinated the clinical part, discussed results and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Franca, R., Rebora, P., Bertorello, N. et al. Pharmacogenetics and induction/consolidation therapy toxicities in acute lymphoblastic leukemia patients treated with AIEOP-BFM ALL 2000 protocol. Pharmacogenomics J 17, 4–10 (2017). https://doi.org/10.1038/tpj.2015.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.83
This article is cited by
-
ABC transporter superfamily. An updated overview, relevance in cancer multidrug resistance and perspectives with personalized medicine
Molecular Biology Reports (2021)
-
PACSIN2 rs2413739 influence on thiopurine pharmacokinetics: validation studies in pediatric patients
The Pharmacogenomics Journal (2020)
-
Pharmacogenetic Predictors of Treatment-Related Toxicity Among Children With Acute Lymphoblastic Leukemia
Current Hematologic Malignancy Reports (2017)