Abstract
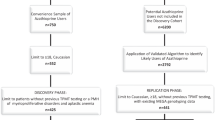
A common pharmacogenomic test is for thiopurine S-methyltransferase (TPMT) status prior to treatment with thiopurine drugs, used to treat auto-immune conditions and pediatric cancer. Guidelines assist practitioners with decisions regarding testing and treatment. The objectives were to conduct a systematic review and critical appraisal of guidance documents with statements regarding TPMT testing and thiopurine dosing. Guidelines, clinical protocols and care pathways from all disciplines were eligible. A quality appraisal was carried out by three appraisers using the Appraisal of Guidelines for Research and Evaluation II. Of the 20 documents found, 5 recommended genotyping while 4 recommended phenotyping. Thirteen documents provided dosing recommendations based on TPMT status. The quality appraisal revealed wide variation across documents. The National Institute for Health and Clinical Excellence and Cincinnati Children’s Hospital guidelines demonstrated the highest overall quality with scores of 79 and 76, respectively. Low-scoring documents failed to use systematic methods to develop recommendations or to provide evidence to support recommendations. Guidance documents that included dosing recommendations demonstrated higher quality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stanek EJ, Sanders CL, Taber KA, Khalid M, Patel A, Verbrugge RR et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther 2012; 91: 450–458.
Shah RR, Shah DR . Personalized medicine: is it a pharmacogenetic mirage? Br J Clin Pharmacol 2012; 74: 698–721.
Amstutz U, Carleton BC . Pharmacogenetic testing: time for clinical practice guidelines. Clin Pharmacol Ther 2011; 89: 924–927.
Zielinski SL . As genetic tests move into the mainstream, challenges await for doctors and patients. J Natl Cancer Inst 2005; 97: 334–336.
MacDermott RP . 6-Mercaptopurine (6-MP) metabolite monitoring and TPMT testing in the treatment of inflammatory bowel disease with 6-MP or azathioprine. UpToDate. Wolters Kluwer Health: Waltham, MA, USA., 2013.
Evans WE . Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Ther Drug Monit 2004; 26: 186–191.
Sahasranaman S, Howard D, Roy S . Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol 2008; 64: 753–767.
Weinshilboum RM, Sladek SL . Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Amer J Hum Gen 1980; 32: 651–662.
Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med 1997; 126: 608–614.
Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. Can Med Assoc J 2004; 170: 1678–1686.
Lathia N, Mittmann N, DeAngelis C, Knowles S, Cheung M, Piliotis E et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer 2010; 116: 3–742–748.
Weycker D, Malin J, Edelsberg J, Glass A, Gokhale M, Oster G . Cost of neutropenic complications of chemotherapy. Ann Oncol 2008; 19: 454–460.
McLeod HL, Isaacs KL . Preemptive pharmacogenetic testing: insufficient data equal unsatisfactory guidance. Ann Intern Med 2011; 154: 842–U108.
Hanauer SB . Advances in IBD: current developments in the treatment of inflammatory bowel diseases. Gastroenterol Hepatol 2009; 5: 407–409.
Stocco G, Cheok MH, Crews KR, Dervieux T, French D, Pei D et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther 2009; 85: 164–172.
Alves S, Amorim A, Ferreira F, Prata MJ . Influence of the variable number of tandem repeats located in the promoter region of the thiopurine methyltransferase gene on enzymatic activity. Clin Pharmacol Ther 2001; 70: 165–174.
Anglicheau D, Sanquer S, Loriot MA, Beaune P, Thervet E . Thiopurine methyltransferase activity: new conditions for reversed-phase high-performance liquid chromatographic assay without extraction and genotypic-phenotypic correlation. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 773: 119–127.
Indjova D, Atanasova S, Shipkova M, Armstrong VW, Oellerich M, Svinarov D . Phenotypic and genotypic analysis of thiopurine s-methyltransferase polymorphism in the bulgarian population. Ther Drug Monit 2003; 25: 631–636.
Winter JW, Gaffney D, Shapiro D, Spooner RJ, Marinaki AM, Sanderson JD et al. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2007; 25: 1069–1077.
Children’s Oncology Group (COG). AALL0232: High Risk B-precursor Acute Lymphoblastic Leukemia (ALL). A Phase III Group-Wide Study. Children’s Oncology Group (COG): Arcadia, CA, USA, 2008.
Stanulla M, Schaeffeler E, Flohr T, Cario G, Schrauder A, Zimmermann M et al. Thiopurine methyltransferase (TPMT) genotype and early treatment response to mercaptopurine in childhood acute lymphoblastic leukemia. JAMA 2005; 293: 1485–1489.
Ujiie S, Sasaki T, Mizugaki M, Ishikawa M, Hiratsuka M . Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2 - *24). Pharmacogenet Genomics 2008; 18: 887–893.
Appraisal of Guidelines for Research and Evaluation (AGREE) Instrument. AGREE Next Steps Consortium (2009). The AGREE II Instrument. Retreived 27 March 2014, from http://www.agreetrust.org.
National Institute for Health and Clinical Excellence (NICE). National Institute for Health and Clinical Excellence (NICE): Crohn's Disease: Management in Adults and Children. National Health Service: London, UK, 2012.
Cincinnati Children's Hospital Medical Center. Evidence-Based Care Guideline for Management of Pediatric Moderate/Severe Inflammatory Bowel Disease. Cincinnati, OH, USA, 2007.
Chakravarty K, McDonald H, Pullar T, Taggart A, Chalmers R, Oliver S et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology 2008; 47: 924–925.
Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S . British Association of Dermatologists' guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol 2011; 165: 711–734.
Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571–607.
Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51: 2193–2213.
Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther 2011; 89: 387–391.
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009; 61: 451–485.
National Comprehensive Cancer Network. NCNN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia.Version 1.2012. National Comprehensive Cancer Network: Fort Washington, PA, USA, 2012.
Valdes R Jr., Payne DA, Linder MW (eds) Clinical Practice Considerations. In: Laboratory Medicine Practice Guidelines: Laboratory Analysis and Application of Pharmacogenetics to Clinical Practice. The National Academy of Clinical Biochemistry: Rockville, MD, USA, 2010.
Sanderson J, Ansari A, Marinaki T, Duley J . Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem 2004; 41: 294–302.
Fabre MA, Jones DC, Bunce M, Morris PJ, Friend PJ, Welsh KI et al. The impact of thiopurine S-methyltransferase polymorphisms on azathioprine dose 1 year after renal transplantation. Transpl Int 2004; 17: 531–539.
Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S et al. World Gastroenterology Organization Practice Guidelines for the Diagnosis and Management of IBD in 2010. Inflamm Bowel Dis 2010; 16: 112–124.
Czaja AJ, Freese DK . Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002; 36: 479–497.
Gleeson D, Heneghan MA British Society of Gastroenterology. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 2011; 60: 1611–1629.
Lichtenstein GR, Abreu MT, Cohen R, Tremaine W . American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006; 130: 935–939.
Sandhu BK, Fell JME, Beattie RM, Mitton SG . British Society of Paediatric Gastroenterology Hepatology and Nutrition (BSPGHAN). Guidelines for the Management of Inflammatory Bowel Disease (IBD) in Children in the United Kingdom. UK IBD Working Group, 2008.
Anstey AV, Wakelin S, Reynolds NJ . Guidelines for prescribing azathioprine in dermatology. Br J Dermatol 2004; 151: 1123–1132.
PURINETHOL® (mercaptopurine) Product Monograph. (2013). US Food and Drug Agency. Accessed 27 March 2014 http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009053s032lbl.pdf.
Ma Q, Lu AYH . Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacological Rev 2011; 63: 437–459.
Booth RA, Ansari MT, Loit E, Tricco AC, Weeks L, Doucette S et al. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med 2011; 154: 814–823, W-295-818.
Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr 2012; 55: 340–361.
The British Society for Paediatric and Adolescent Rheumatology. The British Society for Paediatric and Adolescent Rheumatology. Azathioprine use in paediatric rheumatology. https://www.bspar.org.uk/DocStore/FileLibrary/PDFs/BSPAR%20guidance%20for%20Azathioprine%202011.pdf. (accessed 13 August 2014).
Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for Thiopurine Methyltransferase Genotype and Thiopurine Dosing: 2013 update. Clin Pharmacol Ther 2013; 93: 324–325.
Bunnik EM, Schermer MHN, Janssens ACJW . Personal genome testing: test characteristics to clarify the discourse on ethical, legal and societal issues. BMC Med Ethics 2011; 12: 11.
McDonnell J, Meijler A, Kahan JP, Bernstein SJ, Rigter H . Panellist consistency in the assessment of medical appropriateness. Health Pol 1996; 37: 139–152.
Kahan JP, Park RE, Leape LL, Bernstein SJ, Hilborne LH, Parker L et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care 1996; 34: 512–523.
Donnan JR, Ungar WJ, Mathews M, Hancock-Howard RL, Rahman P . A cost effectiveness analysis of thiopurine methyltransferase testing for guiding 6- mercaptopurine dosing in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2011; 57: 231–239.
Relling MV, Klein TE . CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther 2011; 89: 464–467.
Relling MV, Guchelaar HJ, Roden DM, Klein TE . Pharmacogenetics: call to action. Clin Pharmacol Ther 2011; 90: 507; author reply 507–508.
Ooi CJ, Fock KM, Makharia GK, Goh KL, Ling KL, Hilmi I et al. The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol 2010; 25: 453–468.
Acknowledgements
Funding for this research was provided by a program grant from the Ontario Ministry of Health and Long-Term Care Drug Innovation Fund. The views expressed are those of the authors and not of the Ontario Ministry of Health and Long-Term Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Burnett, H., Tanoshima, R., Chandranipapongse, W. et al. Testing for thiopurine methyltransferase status for safe and effective thiopurine administration: a systematic review of clinical guidance documents. Pharmacogenomics J 14, 493–502 (2014). https://doi.org/10.1038/tpj.2014.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2014.47
This article is cited by
-
Pharmacotherapy of Generalized Myasthenia Gravis with Special Emphasis on Newer Biologicals
Drugs (2022)
-
Guideline appraisal with AGREE II: online survey of the potential influence of AGREE II items on overall assessment of guideline quality and recommendation for use
BMC Health Services Research (2018)
-
Relationship between Azathioprine metabolites and therapeutic efficacy in Chinese patients with neuromyelitis optica spectrum disorders
BMC Neurology (2017)
-
Ethical Perspectives on Translational Pharmacogenetic Research Involving Children
Pediatric Drugs (2015)