Abstract
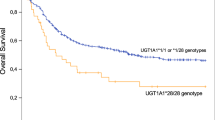
To date, studies of irinotecan pharmacogenetics have mostly focused on the effect of the UGT1A1*28 allele on irinotecan-related toxicity. However, the clinical utility of routine UGT1A1*28 genotyping to pre-emptively adjust irinotecan dosage is dependent upon whether UGT1A1*28 also affects patient survival following irinotecan therapy. Previous observational studies evaluating the influence of UGT1A1*28 on survival have shown contradictory results. A systematic review and meta-analysis of both published and unpublished data were performed to summarize the available evidence of the relationship between the UGT1A1*28 allele and patient survival related to irinotecan therapy. Overall and progression-free survival meta-analysis data were available for 1524 patients and 1494 patients, respectively. The difference in the survival between patients of different UGT1A1*28 genotypes (homozygous, heterozygous or wild-type) who had received irinotecan was not found to be statistically significant. There was also no evidence of irinotecan dose, regimen or line of therapy having an impact on this association.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL . UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 2007; 99: 1290–1295.
Palomaki GE, Bradley LA, Douglas MP, Kolor K, Dotson WD . Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? An evidence-based review. Genet Med 2009; 11: 21–34.
Hu ZY, Yu Q, Zhao YS . Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysis. Eur J Cancer 2010; 46: 1856–1865.
Hu ZY, Yu Q, Pei Q, Guo C . Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase risk. Clin Cancer Res 2010; 16: 3832–3842.
Innocenti F, Ratain MJ . Pharmacogenetics of irinotecan: clinical perspectives on the utility of genotyping. Pharmacogenomics 2006; 7: 1211–1221.
Berg AO, Armstrong K, Botkin J, Calonge N, Haddow J, Hayes M et al. Recommendations from the EGAPP working group: Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11: 15–20.
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–1388.
Marcuello E, Altes A, Menoyo A, del Rio E, Gomez-Pardo M, Baiget M . UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer 2004; 91: 678–682.
Hoskins JM, McLeod HL . UGT1A and irinotecan toxicity: Keeping it in the family. J Clin Oncol 2009; 27: 2419–2421.
Deeken JF, Slack R, Marshall JL . Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: To test or not to test, that is the question. Cancer 2008; 113: 1502–1510.
Winder T, Lenz H-J . Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010; 36: 550–556.
Liu X, Cheng D, Kuang Q, Liu G, Xu W . Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in Caucasians. Pharmacogenomics J, advance online publication, 26 March 2013 doi:10.1038/tpj.2013.10(e-pub ahead of print).
Gold HT, Hall MJ, Blinder V, Schackman BR . Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer 2009; 115: 3858–3867.
Obradovic M, Mrhar A, Kos M . Cost-effectiveness of UGT1A1 genotyping in second-line, high-dose, once every 3 weeks irinotecan monotherapy treatment of colorectal cancer. Pharmacogenomics 2008; 9: 539–549.
Glimelius B, Garmo H, Berglund A, Fredriksson LA, Berglund M, Kohnke H et al. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J 2011; 11: 61–71.
Dias MM, Sorich MJ, McKinnon RA . Impact of the UGT1A1*28 allele on response to irinotecan: A systematic review and meta-analysis. Pharmacogenomics 2012; 13: 889–899.
Moher D, Liberati A, Tetzlaff J, Altman D . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009; 6: 1–6.
Liberati A, Altman D, Tetzlaff J, Murlow C, Gotzsche P, Ioannidis J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: 1–28.
Stroup D, Berlin J, Morton S, Olkin I, Williamson G, Rennie D et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008–2012.
West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF et al. Systems to rate the strength of scientific evidence, Evidence Report, Technology Assessment No. 47, Publication No. 02-E016 edn, vol. April. Agency for Healthcare Research and Quality, Research Triangle Institute, University of North Carolina, Evidence-based Practice Center: Rockville, MD, USA, 2002, pp 1–200.
The Cochrane Collaboration Higgins JPT, Green S 2009 Cochrane Handbook for Systematic Reviews of Interventions.
Parmar MKB, Torri V, Stewart L . Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834.
Jorgensen AL, Williamson PR . Methodological quality of pharmacogenetic studies: issues of concern. Stat Med 2008; 27: 6547–6569.
Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E et al. STrengthening the REporting of Genetic Association studies (STREGA)—an extension of the STROBE statement. PLoS Med 2009; 6: 0151–0163.
Janssens ACJW, Ioannidis JPA, van Duijn CM, Little J, Khoury MJ . Strengthening the reporting of genetic risk prediction studies: the GRIPS statement. PLoS Med 2011; 8: 1–4.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR . Introduction to Meta-Analysis. John Wiley & Sons Ltd: West Sussex, 2009.
Garg AX, Hackam D, Tonelli M . Systematic review and meta-analysis: when one study is just not enough. Clin J Am Soc Nephrol 2008; 3: 253–260.
Liu X, Cheng D, Kuang Q, Liu G, Xu W . Association between UGT1A1*28 polymorphisms and clinical outcomes of irinotecan-based chemotherapies in colorectal cancer: a meta-analysis in Caucasians. PLoS ONE 2013; 8: 1–10.
Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, D'Andrea M et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 2006; 24: 3061–3068.
Kweekel DM, Gelderblom H, Van der Straaten T, Antonini NF, Punt CJA, Guchelaar HJ . UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group study. Br J Cancer 2008; 99: 275–282.
Ruzzo A, Graziano F, Loupakis F, Santini D, Catalano V, Bisonni R et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFIRI chemotherapy. Pharmacogenomics J 2008; 8: 278–288.
Lara PN Jr., Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009; 27: 2530–2535.
Boige V, Mendiboure J, Pignon J-P, Loriot M-A, Castaing M, Barrois M et al. Pharmacogenetic assessment of toxicity and outcome in patients with metastatic colorectal cancer treated with LV5FU2, FOLFOX, and FOLFIRI: FFCD 2000-05. J Clin Oncol 2010; 28: 2556–2564.
Martinez-Balibrea E, Abad A, Martínez-Cards A, Ginés A, Valladares M, Navarro M et al. UGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first-line irinotecan and fluorouracil combination therapy. Br J Cancer 2010; 103: 581–589.
McLeod HL, Sargent DJ, Marsh S, Green EM, King CR, Fuchs CS et al. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J Clin Oncol 2010; 28: 3227–3233.
Shulman K, Cohen I, Barnett-Griness O, Kuten A, Gruber SB, Lejbkowicz F et al. Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer 2011; 117: 3156–3162.
Font A, Sanchez JM, Taron M, Martinez-Balibrea E, Sanchez JJ, Manzano JL et al. Weekly regimen of irinotecan/docetaxel in previously treated non-small cell lung cancer patients and correlation with uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) polymorphism. Invest New Drugs 2003; 21: 435–443.
Han JY, Lim HS, Eun SS, Yoo YK, Yong HP, Lee JE et al. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 2006; 24: 2237–2244.
Liu CY, Chen PM, Chiou TJ, Liu JH, Lin JK, Lin TC et al. UGT1A1*28 polymorphism predicts irinotecan-induced severe toxicities without affecting treatment outcome and survival in patients with metastatic colorectal carcinoma. Cancer 2008; 112: 1932–1940.
Schulz C, Heinemann V, Schalhorn A, Moosmann N, Zwingers T, Boeck S et al. UGT1A1 gene polymorphism: impact on toxicity and efficacy of irinotecan-based regimens in metastatic colorectal cancer. World J Gastroenterol 2009; 15: 5058–5066.
Sugiyama T, Hirose T, Kusumoto S, Shirai T, Yamaoka T, Okuda K et al. The UGT1A1*28 genotype and the toxicity of low-dose irinotecan in patients with advanced lung cancer. Oncol Res 2009; 18: 337–342.
Seo BG, Kwon HC, Oh SY, Lee S, Kim SG, Kim SH et al. Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep 2009; 22: 127–136.
Nakamura Y, Soda H, Oka M, Kinoshita A, Fukuda M, Fukuda M et al. Randomized phase II trial of irinotecan with paclitaxel or gemcitabine for non-small cell lung cancer: Association of UGT1A1*6 and UGT1A1*27 with severe neutropenia. J Thorac Oncol 2011; 6: 121–127.
Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 2008; 26: 2690–2698.
Toffoli G, Cecchin E, Gasparini G, D'Andrea M, Azzarello G, Basso U et al. Genotype-driven Phase I Study of irinotecan administered in combination with Fluorouracil/Leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28: 866–871.
Marcuello E, Paez D, Pare L, Salazar J, Sebio A, del Rio E et al. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 2011; 105: 53–57.
Ducreux M, Malka D, Mendiboure J, Etienne P-L, Texereau P, Auby D et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000–05): an open-label, randomised, phase 3 trial. The Lancet Oncology 2011; 12: 1032–1044.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Dias, M., Pignon, JP., Karapetis, C. et al. The effect of the UGT1A1*28 allele on survival after irinotecan-based chemotherapy: a collaborative meta-analysis. Pharmacogenomics J 14, 424–431 (2014). https://doi.org/10.1038/tpj.2014.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2014.16
This article is cited by
-
Pharmacogenomics and functional imaging to predict irinotecan pharmacokinetics and pharmacodynamics: the predict IR study
Cancer Chemotherapy and Pharmacology (2021)
-
UGT1A1 polymorphism has a prognostic effect in patients with stage IB or II uterine cervical cancer and one or no metastatic pelvic nodes receiving irinotecan chemotherapy: a retrospective study
BMC Cancer (2020)
-
Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics
Clinical Pharmacokinetics (2018)
-
UGT1A polymorphisms associated with worse outcome in colorectal cancer patients treated with irinotecan-based chemotherapy
Cancer Chemotherapy and Pharmacology (2018)
-
Irinotecan-induced toxicity pharmacogenetics: an umbrella review of systematic reviews and meta-analyses
The Pharmacogenomics Journal (2017)