Abstract
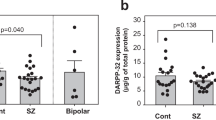
Synapsins are neuronal phosphoproteins crucial to regulating the processes required for normal neurotransmitter release. Synapsin II, in particular, has been implied as a candidate gene for schizophrenia. This study investigated synapsin II mRNA expression, using real-time reverse transcriptase–PCR, in coded dorsolateral prefrontal cortical samples provided by the Stanley Foundation Neuropathology Consortium. Synapsin IIa was decreased in patients with schizophrenia when compared with both healthy subjects and patients with bipolar disorder, whereas synapsin IIb was only significantly reduced in patients with schizophrenia when compared with healthy subjects but not in patients with bipolar disorder. Furthermore, lifetime antipsychotic drug use was positively associated with synapsin IIa expression in patients with schizophrenia. Results suggest that impairment of synaptic transmission by synapsin II reduction may contribute to dysregulated convergent molecular mechanisms, which result in aberrant neural circuits that characterize schizophrenia, while implicating involvement of synapsin II in therapeutic mechanisms of currently prescribed antipsychotic drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dyck BA, Skoblenick KJ, Castellano JM, Ki K, Thomas N, Mishra RK . Behavioral abnormalities in synapsin II knockout mice implicate a causal factor in schizophrenia. Synapse 2009; 63: 662–672.
Dyck BA, Mishra RK . Regulation of Synapsin II by Dopaminergic Mechanisms. In: Kudo E, Fujii Y (eds). Dopamine: Functions, Regulation and Health Effects. Nova Science Publishers: New York, 2012 pp 215–234.
Dyck BA, Beyaert MG, Ferro MA, Mishra RK . Medial prefrontal cortical synapsin II knock-down induces behavioral abnormalities in the rat: examining synapsin II in the pathophysiology of schizophrenia. Schizophr Res 2011; 130: 250–259.
Kasai K, Iwanami A, Yamasue H, Kuroki N, Nakagome K, Fukuda M . Neuroanatomy and neurophysiology in schizophrenia. Neurosci Res 2002; 43: 93–110.
Goldman-Rakic PS . Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6: 348–357.
Weinberger DR, Berman KF, Zec RF . Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43: 114–124.
Winterer G, Weinberger DR . Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 2004; 27: 683–690.
Cesca F, Baldelli P, Valtorta F, Benfenati F . The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 2010; 91: 313–348.
Ferreira A, Rapoport M . The synapsins: beyond the regulation of neurotransmitter release. Cell Mol Life Sci 2002; 59: 589–595.
Guest KA, Dyck BA, Shethwala S, Mishra RK . Atypical antipsychotic drugs upregulate synapsin II in the prefrontal cortex of post-mortem samples obtained from patients with schizophrenia. Schizophr Res 2010; 120: 229–231.
Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P . Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 2000; 28: 53–67.
Gitler D, Xu Y, Kao HT, Lin D, Lim S, Feng J et al. Molecular determinants of synapsin targeting to presynaptic terminals. J Neurosci 2004; 24: 3711–3720.
Kao HT, Song HJ, Porton B, Ming GL, Hoh J, Abraham M et al. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci 2002; 5: 431–437.
Chen Q, He G, Qin W, Chen QY, Zhao XZ, Duan SW et al. Family-based association study of synapsin II and schizophrenia. Am J Hum Genet 2004; 75: 873–877.
Chen Q, He G, Wang XY, Chen QY, Liu XM, Gu ZZ et al. Positive association between synapsin II and schizophrenia. Biol Psychiatry 2004; 56: 177–181.
Saviouk V, Moreau MP, Tereshchenko IV, Brzustowicz LM . Association of synapsin 2 with schizophrenia in families of Northern European ancestry. Schizophr Res 2007; 96: 100–111.
Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL et al. Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet 1995; 60: 252–260.
Chong VZ, Skoblenick K, Morin F, Xu Y, Mishra RK . Dopamine-D1 and -D2 receptors differentially regulate synapsin II expression in the rat brain. Neuroscience 2006; 138: 587–599.
Chong VZ, Young LT, Mishra RK . cDNA array reveals differential gene expression following chronic neuroleptic administration: implications of synapsin II in haloperidol treatment. J Neurochem 2002; 82: 1533–1539.
Seeman P, Kapur S . Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci USA 2000; 97: 7673–7675.
Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 2005; 102: 3513–3518.
Seeman P . Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets 2006; 10: 515–531.
Seeman P . Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses 2010; 4: 56–73.
Seeman P . All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2 receptors. CNS Neurosci Ther 2011; 17: 118–132.
Moghaddam B . Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 2002; 51: 775–787.
Stone JM, Morrison PD, Pilowsky LS . Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol 2007; 21: 440–452.
Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE . Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull 2013; 39: 120–129.
Gitler D, Cheng Q, Greengard P, Augustine GJ . Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 2008; 28: 10835–10843.
Bogen IL, Boulland JL, Mariussen E, Wright MS, Fonnum F, Kao HT et al. Absence of synapsin I and II is accompanied by decreases in vesicular transport of specific neurotransmitters. J Neurochem 2006; 96: 1458–1466.
Kleinbaum DG, Kupper LL, Muller KE, Nizam A . Applied Regression Analysis and Other Multivariable Methods. Duxbury Press: Pacific Grove, CA, USA, 1998.
van Os J, Verdoux H, Maurice-Tison S, Gay B, Liraud F, Salamon R et al. Self-reported psychosis-like symptoms and the continuum of psychosis. Soc Psychiatry Psychiatr Epidemiol 1999; 34: 459–463.
Andreasen NC, Rezai K, Alliger R, Swayze VW, Flaum M, Kirchner P et al. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49: 943–958.
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50: 825–844.
Liddle PF . Inner connections within domain of dementia praecox: role of supervisory mental processes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 1995; 245: 210–215.
Pillai A . Decreased expression of Sprouty2 in the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder: a correlation with BDNF expression. PLoS One 2008; 3: e1784.
Muller HK, Wegener G, Popoli M, Elfving B . Differential expression of synaptic proteins after chronic restraint stress in rat prefrontal cortex and hippocampus. Brain Res 2011; 1385: 26–37.
Ferreira AF, Real CC, Rodrigues AC, Alves AS, Britto LR . Moderate exercise changes synaptic and cytoskeletal proteins in motor regions of the rat brain. Brain Res 2010; 1361: 31–42.
Fdez E, Hilfiker S . Vesicle pools and synapsins: new insights into old enigmas. Brain Cell Biol 2006; 35: 107–115.
Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F . The role of synapsins in neuronal development. Cell Mol Life Sci 2010; 67: 1383–1396.
Matus-Leibovitch N, Nevo I, Vogel Z . Differential distribution of synapsin IIa and IIb mRNAs in various brain structures and the effect of chronic morphine administration on the regional expression of these isoforms. Mol Brain Res 1997; 45: 301–316.
Van Putten T, Marder SR, Mintz J, Poland RE . Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry 1992; 149: 500–505.
Imai C, Sugai T, Iritani S, Niizato K, Nakamura R, Makifuchi T et al. A quantitative study on the expression of synapsin II and N-ethylmaleimide-sensitive fusion protein in schizophrenic patients. Neurosci Lett 2001; 305: 185–188.
Gabriele JP, Chong VZ, Pontoriero GF, Mishra RK . Decreased expression of a 40-kDa catecholamine-regulated protein in the ventral striatum of schizophrenic brain specimens from the Stanley Foundation Neuropathology Consortium. Schizophr Res 2005; 74: 111–119.
De L V, Likhodi O, Van Tol HH, Kennedy JL, Wong AH . Tryptophan hydroxylase 2 gene expression and promoter polymorphisms in bipolar disorder and schizophrenia. Psychopharmacology (Berl) 2005; 183: 378–382.
Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM . Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med 2005; 2: e263.
Frank O, Giehl M, Zheng C, Hehlmann R, Leib-Mosch C, Seifarth W . Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J Virol 2005; 79: 10890–10901.
Konradi C, Heckers S . Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry 2001; 50: 729–742.
Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006; 20: 389–409.
Skoblenick KJ, Argintaru N, Xu Y, Dyck BA, Basu D, Tan ML et al. Role of AP-2alpha transcription factor in the regulation of synapsin II gene expression by dopamine D1 and D2 receptors. J Mol Neurosci 2010; 41: 267–277.
Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF . Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res 2006; 81: 47–63.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research. We are grateful to Dr Mary Webster (Stanley Foundation Neuropathology Consortium) for donating the RNA samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tan, M., Dyck, B., Gabriele, J. et al. Synapsin II gene expression in the dorsolateral prefrontal cortex of brain specimens from patients with schizophrenia and bipolar disorder: effect of lifetime intake of antipsychotic drugs. Pharmacogenomics J 14, 63–69 (2014). https://doi.org/10.1038/tpj.2013.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2013.6
Keywords
This article is cited by
-
The Role of Synapsins in Neurological Disorders
Neuroscience Bulletin (2018)
-
Dysregulations of Synaptic Vesicle Trafficking in Schizophrenia
Current Psychiatry Reports (2016)