Abstract
The striatum and thalamus are subcortical structures intimately involved in addiction. The morphology and microstructure of these have been studied in murine models of cocaine addiction (CA), showing an effect of drug use, but also chronological age in morphology. Human studies using non-invasive magnetic resonance imaging (MRI) have shown inconsistencies in volume changes, and have also shown an age effect. In this exploratory study, we used MRI-based volumetric and novel shape analysis, as well as a novel fast diffusion kurtosis imaging sequence to study the morphology and microstructure of striatum and thalamus in crack CA compared to matched healthy controls (HCs), while investigating the effect of age and years of cocaine consumption. We did not find significant differences in volume and mean kurtosis (MKT) between groups. However, we found significant contraction of nucleus accumbens in CA compared to HCs. We also found significant age-related changes in volume and MKT of CA in striatum and thalamus that are different to those seen in normal aging. Interestingly, we found different effects and contributions of age and years of consumption in volume, displacement and MKT changes, suggesting that each measure provides different but complementing information about morphological brain changes, and that not all changes are related to the toxicity or the addiction to the drug. Our findings suggest that the use of finer methods and sequences provides complementing information about morphological and microstructural changes in CA, and that brain alterations in CA are related cocaine use and age differently.
Similar content being viewed by others
Introduction
The striatum and thalamus are subcortical structures greatly affected in the physiopathology of cocaine addiction in animal models and possibly in humans.1 Studies in animal models show microstructural changes in striatal medium spiny neurons, as well as striatal indirect, direct and thalamo-striatal pathways.2 Using neuroimaging in mice, Wheeler et al.3 showed volumetric reduction in ventral and posterior striatum, and expansion in the dorsal striatum in a cocaine addiction model. However, non-invasive neuroimaging studies in human cocaine addicts (CAs) have shown inconsistent and conflicting neuroanatomical abnormalities in striatum and thalamus.4, 5
A number of studies have observed lower volume in striatum,6, 7 larger striatal and thalamic volumes,8, 9, 10 reduced volume in anterior and increase in posterior striatum,11 or no volumetric differences whatsoever.12, 13 These discrepancies may be explained by methodological factors (that is, the use of volume as a metric, the segmentation method and small sample sizes), but, also, polysubstance use and cocaine delivery (inhaled vs smoked) could play an important a role. Compared to inhaled cocaine, crack cocaine (smoked) is clinically related to stronger craving, addiction and deterioration in cognition.14, 15 Crack cocaine use and addiction is also most prevalent in the lower socioeconomic stratum and poses a great health risk to addicts both directly and indirectly, as well as being a great social problem and expense.16, 17
To better understand the neuroanatomical effects of cocaine as the main drug of use and the mechanism of addiction, there is a need for large-scale studies using more sophisticated computational neuroanatomical methods and novel neuroimaging sequences in human addicts, while focusing on one type of cocaine delivery separately. Also, it would be important to study brain morphology in a wider range of ages (young and older addicts), as it has been shown that brain changes related to cocaine abuse tend to worsen with age in young mice3 and in humans.18 Because substance addiction is known to affect both the brain gross anatomy (volume and shape) and tissue composition (microstructure), our study employs an imaging protocol allowing both types of analysis. Volumetric and shape analyses are performed based on high-resolution T1-weighted magnetic resonance imaging data. Shape analysis refers to the study of the three-dimensional shape of the subcortical nuclei using the information of the automatic segmentation and shape-based models.19 The average nonlinear deformations are used to estimate displacement of the nuclei with respect to the model (inward or contraction and outward or expansion displacement), as well as surface area calculated from a tessellated surface of the segmentation. These metrics, surface area and displacement, are related to biological processes and pathology (for example, schizophrenia), and provide different and complementary information than volume.20, 21
Diffusion kurtosis imaging (DKI) is a novel imaging technique known to be very sensitive to tissue microstructure, even in regions of crossing fibers. However, it has not been used for the study of substance abuse and addiction. The basis of DKI is that in tissue, microstructural components hinder free (that is, Gaussian) diffusion of water.22 The measured diffusion magnetic resonance imaging (MRI) signal yields this deviation from normal diffusion as a non-zero kurtosis,23 providing an indirect measure more sensitive to the microstructural physical composition than fractional anisotropy or other diffusion tensor-based methods. DKI, however, may or may not be related to macrostructural volume or shape changes measured with T1-weighted images, as DKI measures microstructural changes. One typically reported DKI metric is the mean kurtosis (MKT), which has been associated with microstructural changes in a host of diseases such as stroke,24 but has also been shown to be sensitive to more subtle brain alterations as in mild traumatic brain injury,25, 26 mild chronic stress27 and even the brain remodeling that is part of normal aging.28 For this study, we employed a fast DKI (fDKI) sequence allowing rapid acquisition of the data needed for estimation of the tensor-based MK (MKT).29, 30, 31 The fDKI method uses a tensor-based MKT definition that differs slightly from the traditional MKT; nevertheless, the agreement between MKT and the rapidly obtainable MKT is well established32 and clinical applications are favorable.33 In our exploratory study, we compare the striatum and thalamus morphology and microstructure of active crack CAs with healthy controls (HCs), using novel analytic computational neuroanatomical methods and a recently introduced fDKI sequence.30 We also included a post hoc striatum and thalamus subdivision analysis due to the importance of the different subnuclei in the pathology such as the ventral striatum.1, 34
Materials and methods
Participants
We recruited 54 CAs and 48 HCs from March to December of 2015 as part of a principal addiction project. HCs were matched by age (±2 years), sex and handedness. Education was matched as closely as possible. The recruitment criteria are shown in Supplementary Table 1. We decided to study a subset of crack CAs for the three following reasons: (1) the known difference in terms of effects and dependency between inhaled and smoked cocaine;15 (2) the small sample size of our inhaled CAs before exclusion and elimination (n=13); and (3) crack cocaine is a more important socioeconomical issue in Mexico and the world due to the toxicity of it, the stronger craving and the low social stratum where it is mostly used. We then excluded 9 participants (HCs=6 and CAs=3), eliminated 1 participant due to claustrophobia (HC) and 3 due to excessive movement during image acquisition (HCs=1 and CAs=2). Our final sample size for the morphological analysis was 36 CAs (3F)/40 HCs (2F) with a median age of 30 (18–48) years old. For the fDKI analysis, we had a final sample size of 17 CAs/18 HCs, as the DKI sequence was acquired in a smaller subset of participants and due to the outlier rejection using Thompson’s Tau.35
The study was approved by the local ethics committee and performed at the Instituto Nacional de Psiquiatría ‘Ramón de la Fuente Muñiz’ in Mexico City, Mexico. The study was carried out according to the Declaration of Helsinki. All participants were invited through posters placed in several centers for addiction treatment. HCs were recruited from the Institute (that is, administrative workers) and using fliers around the city. Participants had the study fully explained to them and provided verbal and written informed consent. The participants underwent clinical and cognitive tests besides the MRI as part of the main ongoing addiction project (see Supplementary Methods and Results and Supplementary Table 2 for details), which are not part of this study. Tobacco use in this population is unavoidable; therefore, we determined years of tobacco use and tobacco dependency in CAs and HCs (see Supplementary Methods and Results). Psychiatric comorbidities, lifetime medication and polysubstance use are reported in Supplementary Table 3, respectably. Participants were asked to abstain from crack cocaine as well as other drugs and alcohol, for at least 24 h prior to the study and were urine-tested for the presence of the drugs before the MRI scan. The clinical and MRI sessions were done the same day as minimum and 4 days apart as maximum.
MRI acquisition
Brain images were acquired using a Phillips Ingenia 3 T MR system (Phillips Healthcare, Best, The Netherlands, and Boston, MA, USA), with a 32-channel dS Head coil. We acquired structural T1-weighted data and DKI data using the fast kurtosis acquisition scheme. Specifically, the fast kurtosis protocol from Hansen et al.30 was employed requiring a total of 19 diffusion weighted volumes: one b=0 scan for normalization and nine distinct directions at each of b=1000 s mm−2 and b=2500 s mm−2. The data were acquired using a spin-echo single-shot echo-plannar imaging, time repetition (TR)/time echo (TE)=11820/115 ms, flip-angle=90° and inversion recovery for cerebrospinal fluid suppression. A total of 50 consecutive axial slices were acquired with isotropic resolution of 2 mm, matrix=112 × 112, scan time=4:46 min. T1-weighted images were acquired using a three-dimensional FFE SENSE sequence, TR/TE=7/3.5 ms, field of view=240, matrix=240 × 240 mm, 180 slices, gap=0, plane=sagittal, voxel=1 × 1 × 1 mm (five participants were acquired with a voxel size=0.75 × 0.75 × 1 mm), scan time=3.19 min. As part of a major addiction project, resting-state functional MRI and HARDI sequences were also acquired and are not part of this particular study. The order of the sequences was resting-state functional MRI, T1-weighted, HARDI and fDKI, and it was maintained across participants. Total scan time was ~50 min.
T1-weighted preprocessing and image processing
T1-weighted images were converted from DICOM format to MINC for preprocessing. T1 images were preprocessed using an in-house preprocessing pipeline with the software Bpipe (http://cobralab.ca/software/mincbeast_bpipe.html),36 which makes use of the MINC Tool-Kit (http://www.bic.mni.mcgill.ca/ServicesSoftware/ServicesSoftwareMincToolKit) and Advanced Normalization Tools.37 Briefly, we performed N4 bias field correction,38 linear registration to Montreal Neurology Institute space using Advanced Normalization Tools, and we cropped the region around the neck in order to improve registration quality, followed by transformation back to native space.
The native space preprocessed files were input into the MAGeT-Brain morphological analysis pipeline (http://cobralab.ca/software/MAGeTbrain.html).39 MAGeT-Brain is modified multi-atlas segmentation technique designed to take advantage of hard-to-define atlases and uses a minimal number of atlases for input into the segmentation process (atlases’ labels in Supplementary Methods and Results). We obtained segmentations and volumetric measures for whole striatum and thalamus, as well as their subdivisions. For shape analysis, indices of surface displacement20, 40 and surface area41 for the striatum and thalamus were derived. Briefly, surface displacements were derived based on the average of the nonlinear portions of the 21 transformations estimated using MAGeT-Brain as the dot product between the surface normal and the local nonlinear registration vector at each point. Native surface area was estimated using a median surface representation based on the 21 surfaces from the MAGeT-Brain pipeline in native space. Surface area was estimated by assigning one-third of the surface area of each triangle to each vertex within the triangle. Finally, surface area and displacement values were blurred with a surface based diffusion-smoothing kernel of 5 mm.42 These measures were provided for the striatum (~6300 vertices per hemisphere) and the thalamus (~3000 vertices per hemisphere).
DKI preprocessing
DKI data were corrected for motion and eddy currents using FSL version 5.0.9 (Oxford Centre for Functional MRI of the Brain, Oxford, UK). Tensor-based MKT was calculated as previously described in Hansen et al.30 using Matlab (MathWorks, Natick, MA, USA). MKT was calculated on unsmoothed data, as we aimed for region of interest-based statistics (it should be noted that motion and eddy current corrections introduce slight blurring due to resampling). T1-weighted images were skull-stripped43 and linearly co-registered to the MKT images.44 To extract region of interest-based MKT values, we used the labels derived individually from the MAGeT-Brain pipeline, focusing on whole left and right striatum, thalamus and nucleus accumbens (NAcc). The labels were resampled in DKI space using the T1-to-DKI linear transformation, and mean MKT values from each label and each participant were calculated and stored in a CSV file for statistical analysis.
Statistical analysis
All data were analyzed using R Statistics (R Core Team, Vienna, Austria) and R Studio (Boston, MA, USA). All variables were tested for normality using the Shapiro–Wilks test. Participant data were analyzed using t-tests and χ2-tests when appropriate. All tests were corrected for multiple comparisons using false discovery rate (FDR)45 at 10% threshold and adjusted P-values are shown. Effect sizes were determined using sums of squares (SS) and partial-eta-squared (partial-eta-squared=SS(effect)/(SS(effect)+SS(error)). For striatum and thalamus whole volumes and subdivisions, we tested for group differences and group × age interactions using analysis of covariance at α level of 0.05 and using total brain volume, age (only group differences) and sex as covariates.
Also, we tested for linear relationships between years of consumption of crack cocaine in CA participants and striatum and thalamus volumes using multiple regression using total brain volume and sex as covariates with α of 0.05. Post hoc we then performed two-tailed correlations between ‘years of consumption’, and striatal and thalamic subnuclei volume. Finally, we performed a linear regression of the right pulvinar volume and years consuming cocaine, with total brain volume, age and sex as covariates. A liberal FDR threshold was chosen due to the small sample size, the high variability of results in previous studies and consistency with previous reports on shape analysis.40, 46 For visualization purposes only, we created corrected volume variables using the residuals of the linear model.
Vertex-wise general linear model analyses of surface morphometric measures were performed using RMINC (https://github.com/mcvaneede/RMINC), a statistical image analysis software package built to work in the R environment. All vertex-wise results were also corrected for multiple comparisons using FDR at 10% threshold. For surface area and displacement, we tested for group differences and group × age interactions using general linear model with total surface area, age and sex as covariates in surface area and only age and sex for displacement. We superimposed the striatum and thalamus subdivision atlases to label the subnuclei location of the significant clusters. Finally, we created plots using the values of surface area and displacement observed in the peak vertex of significant clusters.
For the DKI data, we tested for group differences and group × age interactions (analysis of covariance, α=0.05) using age (only for the former) and sex as covariates. Again, FDR at 10% was used. We performed parametric correlation between years of consumption of crack cocaine (CA only) and striatum/thalamus MKT.
Finally, to better understand the results of the ‘age’ interaction, we first performed two-tailed correlation between chronological age and years of consumption. Then, we performed the analysis of covariance analyses for striatum and thalamus volume, MKT, and shape again using ‘years of consumption’ as an additional covariate. We also performed multiple regression analysis of the thalamus MKT using ‘age’ as a covariate to understand the influence of chronological age in the correlation.
Results
Participant data are shown in Supplementary Table 1. There were no significant relationships between the morphological data and years of tobacco use in our data (see Supplementary Methods and Results for details on the analysis). Chronological age and years of consumption were positively correlated (r=0.53, P=0.001).
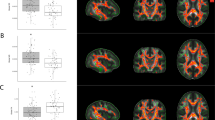
In whole volume, we did not find group effects (HCs>CAs), though CA average volume was lower than HC in striatum and thalamus (Table 1). We found significant group × age interactions in striatum, in postcommisural caudate nucleus, but not in the thalamus (Table 1). The interaction shows that striatum and caudate volume increased with age in the CA group and decreased in the HC group (Figure 1). When ‘years of consumption’ was included as a covariate, the group × age interactions in bilateral whole striatum were maintained (Table 2).
Striatum volume group × age interaction. Scatter plot of left and right striatum corrected volume with regression lines and shadowed confidence intervals at 0.95 per group. Group × age interaction statistics, left striatum: F (1,70)=4.31, uncorrp=0.04, PFDR=0.08, es=0.06; right striatum: F (1,70)=5.18, uncorrp=0.02, PFDR=0.08, es=0.05. CA, cocaine addict; FDR, false discovery rate; HC, healthy control.
There were no significant relationships between years consuming crack cocaine and whole striatum/thalamus volume. The post hoc correlations with the subnuclei found no correlation with years consuming cocaine in the striatum. In the thalamic subdivisions, we found a significant correlation between years consuming with right pulvinar volume (r=−0.52, uncorrp=0.001, PFDR=0.05). The multiple regression analysis confirmed this relationship (Supplementary Table 7). Thus, the longer years of crack cocaine consumption the smaller right pulvinar volume. Introducing ‘age’ as a covariate to the multiple regression model did not affect the R2, but it did reduce the significance and β value of years of consumption (Table 2).
In displacement, we found a significant group difference (HCs>CAs) in the left striatum (t=3.14, PFDR<0.10) localized in the ventral striatum/NAcc area (Figure 2). We also found a non-significant group × age interaction (t=3.28, PFDR<0.20) in the same area. In general, the CA group had more contraction (or inward displacement) than the HC group in the cluster peak. Introducing the ‘years of consumption’ covariate for the left striatum displacement, we found a consistent significant group difference with slightly increased t-value, although the group × age interaction became weaker (Table 2). The left striatum displacement cluster peak vertex was not significantly correlated with years consuming (r=0.23, P=0.19); however, it was positively correlated with age (r=0.48, P=0.003). The multiple regression analysis of the cluster peak showed an influence solely by age (Table 2; Supplementary Table 8). There were no significant clusters in the thalamus. The surface area did not show significant clusters in striatum and thalamus.
Striatum displacement. Left: caudal anterior view of the left striatum showing the significant group cluster on ventral striatum/NAcc. Right: (a) boxplot of the significant (*) displacement difference between groups in the peak vertex location (PFDR 10%) t=3.14, PFDR<0.10; (b) scatter plot of the group × age interaction in displacement showing regression lines and shadowed confidence intervals at 0.95 per group. The color bar shows the t-values at PFDR threshold of 20% (t=3.28, PFDR<0.20). Negative values represent contraction; positive values represent expansion. CA, cocaine addict; FDR, false discovery rate; HC, healthy control.
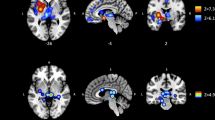
The demographics of the fDKI smaller sample size were similar to the volume analysis. The fDKI analysis did not show a group effect. However, it showed significant group × age interactions in bilateral striatum (left: F (1,30)=4.49, uncorrp=0.04, PFDR=0.05, es=0.13; right: (F (1,30)=3.81, uncorrp=0.06, PFDR=0.06, es=0.11) and in bilateral thalamus (left: F (1,30)=6.12, uncorrp=0.02, PFDR=0.05, es=0.17; right: F (1,30)=5.57, uncorrp=0.03, PFDR=0.05, es=0.16), with a higher effect size on left thalamus (Figure 3). Mostly in the thalamus, MKT decreased with age in CAs, whereas it increased in HCs, although it did show a different pattern in striatum. When ‘years of consumption’ was included as a covariate, the group × age interactions in bilateral striatum and thalamus were not maintained (Table 2).
Striatum and thalamus MKT group × age interaction. Scatter plot of (top) striatum MKT and (bottom) thalamus MKT with regression lines and shadowed confidence intervals at 0.95 per group. Group × age interactions, left striatum: F (1,30)=4.49, uncorrp=0.04, PFDR=0.05, es=0.13; right striatum: F (1,30)=3.81, uncorrp=0.06, PFDR=0.06, es=0.11; left thalamus: F (1,30)=6.12, uncorrp=0.02, PFDR=0.05, es=0.17; right thalamus: F (1,30)=5.57, uncorrp=0.03, PFDR=0.05, es=0.16. CA, cocaine addict; FDR, false discovery rate; HC, healthy control; MKT, mean kurtosis.
Striatum MKT showed no significant correlations. We found significant negative correlations between years consuming with left thalamus MKT (r=−0.62, uncorrp=0.008, PFDR=0.03) and not significant with right thalamus MKT (r=−0.47, uncorrp=0.06, PFDR=0.1), meaning the longer years they consumed the lower MKT values. The multiple regression analysis with age as covariate maintained the significant correlation in left thalamus MKT (Table 2; Supplementary Table 9). We did not find significant correlations between striatum and thalamus MKT and volume.
Discussion
Our findings suggest that morphometric changes in active crack cocaine addiction are subtle, and that volumetric analysis is less sensitive than shape analysis and DKI to measure these changes. However, we also found that volume and shape in the striatum were more related to abnormal aging in CAs, whereas morphometric differences in thalamus and DKI differences in both striatum and thalamus were more related to years consuming the drug. In our sample, striatum and thalamus volumes were not different between HCs and crack CAs. This agrees with the study by Martinez et al.,11 where they also showed no significant volumetric differences in striatum using manual segmentation. In their study, they also admitted patients with active crack cocaine addiction and had a smaller sample size than ours. Narayana et al.12 did not find differences as well using whole-brain voxel-based morphometry with a similar sample size, without specifying the type of cocaine delivery. In studies that include the thalamus, polysubstance users had greater volume in the right thalamus compared to cocaine users, and another study found that cocaine-dependent subjects have decreased volume in the left thalamus compared to HCs.10 Inability to detect group differences could be the result of several factors: variability of cocaine consumption, quality of the cocaine used, polysubstance use or type of HCs used for comparison. In our case, the HCs were closely paired for age, sex, handedness and education. We found that striatum volume increased with age in our CA group, and the effect was more prominent in the left postcommissural caudate. In healthy human population, striatum and thalamus volumes decrease with normal aging, suggesting age-related atrophy in our sample.47, 48 Bartzokis et al.49 showed accelerated age-related decline in cortical volume in CAs, although they did not study striatum nor thalamus specifically. Gray matter volume decrease shown by MRI is related to a decrease in synaptic spine density in murine models.50 The volume increase in our CA group could suggest an increase in dendritic spines, supporting changes in synaptic plasticity due to years of cocaine consumption.34 However, volume could be affected by non-specific microstructural changes; hence, an increase in volume by itself does not necessarily suggest an increase in dendritic spines. Instead, it could also suggest reorganization in circuitry, scarring or inflammation.34, 51, 52 Our analysis also showed that years of consumption did not affect volume in the striatum, which seems to imply that the differences found are related to abnormal aging. However, we found a positive medium–high correlation between years of consumption and age, which suggests that these variables are difficult to differentiate and this complicates interpretation.
Thalamus volume was not affected by age in the CA group as in striatum. A deeper investigation showed that longer years consuming crack cocaine were associated with decreased right pulvinar volume. Age did not affect the statistical model; therefore, we suspect the effect may be more related to years of consumption. The pulvinar is a thalamic nucleus highly connected to the visual cortex and the superior colliculus, and recent studies suggest one of its main functions is visual attention and modulation.53 It is highly connected to the striatum as well54 and related to the addictive pathology.34 In fact, striatal availability of dopamine D2 receptor predicts thalamic functional responses to reward in CAs.55 This suggests that cocaine abuse may be related to atrophy of the thalamus and could be part of the habit reinforcement stage of the addiction cycle theory.56 If the volume decrease is related to a decrease in dendritic spines, it may also explain inhibition and attention issues seen in cocaine addiction due to the visual–motor connectivity of the pulvinar.51 However, at the moment, this is only possible to investigate in murine models.
The shape analysis showed significant changes in striatum anatomy between the groups, as opposed to volume. We found a greater contraction (or inward displacement) in the CA group than in the HC group in the ventral striatum/NAcc, an important part of the reward system and addiction. The NAcc seems to be involved in the intoxication and withdrawal stages of cocaine addiction, with the consequent drug-seeking behavior.34, 56 A study in adults with prenatal exposure to cocaine also found displacement differences compared to HCs in striatum using shape analysis.57 However, they only studied the caudate and putamen, and their results were not corrected for multiple comparisons, although they support our own findings. A murine model of adolescent cocaine exposure determined displacement differences in striatum, with significant expansion of the lateral surface and contraction of the medial surface after 30 days of abstinence.3 We also found a non-significant age-related shape interaction with group, showing expansion of the ventral striatum/NAcc in the CA group. This is similar to the findings in volume. When we included years of consumption in the statistical model as another covariate, the group difference was maintained but the group × age interaction disappeared, suggesting shape may represent changes related to age and not years of consumption. A study on schizophrenia suggests that the ventral striatum/NAcc contracts with age in HCs,20, 41 and this seems to be true for thalamus as well.58 Our HC group showed the same age-related contraction, whereas the CA group showed expansion, although this was not significant. This evidence may suggest that, although the NAcc is contracted in crack cocaine addiction, there is a tendency to expand with age. Similarly to volume, this could be related to either an increase of dendritic spines or gliosis. To our knowledge, this is the first shape analysis study in cocaine-addicted humans; therefore, bigger sample sizes and longitudinal studies are needed to corroborate these results. It is relevant to mention that the MAGeT algorithm we applied in this study has been shown to give similar results to the gold standard (manual segmentation; achieving Dice Kappa against manual segmentation of ~0.88–0.89), as opposed to other tools that over or under estimate the volumes.39
The DKI analysis, as with the volume, did not show group differences in striatum and thalamus MKT. It did show an age-related group interaction in striatum and thalamus with low effect sizes. MKT decreased with age in the CA group, especially evident in thalamus, and increased with age in the HC group. We compare our results with the recently introduced MKT directly to studies employing conventional MKT. Falangola et al.28 found whole-brain MKT to increase rapidly with age until age 18, followed by a less steep increase until age 47 after which a decline in MKT was observed. For gray matter, the MKT was seen to continue to increase through life, whereas for white matter MKT was found to increase rapidly up to age 18 and then steadily decline. In agreement with that study, MKT increased with age in the HC group, but decreased with aging in the CA group, especially evident in thalamus. Meaning that in our study, crack CAs show an abnormal MKT decline with age, as well as decrease in thalamic MKT with years of cocaine use. Interestingly, introducing years of consumption negatively affected the model, and we found significant correlation between thalamic MKT and years of consumption. This may suggest that MKT may reflect both substance-related and age-related alterations, with a higher specificity for the former. A decreased MKT compared to normal brain tissue would suggest a less complex tissue microstructure with fewer diffusion barriers causing the diffusion process to be less non-Gaussian. Specific causes for this could be loss of neurites, leading to fewer cell connections, decreased tissue integrity and increased extracellular space. This may be corroborated by the significant negative correlations with years of cocaine use that, when compared with the pulvinar volume correlation, could suggest a reflection of thalamic atrophy. To our knowledge, this is the first study to use DKI in substance addiction, hence more studies are needed in this and other types of substance abuse.
There were several limitations to our study. With the low effect sizes in all the morphological contrasts, it seems that higher sample sizes may be needed to find more significant differences in these areas. Nevertheless, we were able to obtain significant results with our limited sample sizes. The DKI analysis had the lowest sample size, and still it showed a similar effect size than the other analyses. And, unlike the rest of the analyses, the DKI showed thalamic effects as well as striatal. Our significant threshold for the multiple comparisons FDR was 10% (q=0.1), which may be considered more liberal than usual and caution should be taken when interpreting our findings. However, this approach was preferred to allow for a more exploratory study and we have successfully used it in our previous studies. With our sample size (n=76) and with the largest effect size in volume (group × age interaction of postcommisural caudate nucleus, es=0.13), we achieved a power of 90%. However, with the lowest significant effect size in volume (group × age interaction of striatum, es=0.05), we achieved a power of 50%. To achieve an 80% power at α=0.05, with those effect sizes, we would need a sample size of at least n=152. This was calculated using the software G*Power (http://www.gpower.hhu.de/en.html) for an analysis of covariance with fixed effects, main effects and interactions, two groups, and three covariates. Tobacco use and dependency was prevalent in half of our CA group, and although we did not find any effects in striatum and thalamus, one study has shown striatal volume and shape relation to craving tobacco.40 Finally, age and years of consumption were positively correlated, and this colinearity should be considered when interpreting the results. One thing to take into consideration is that the variable ‘years of consumption’ may be somewhat subjective, as it is investigated by the clinician but reported by the patient and sometimes by family members. Studies in murine models may help to model the nature of the effects of polysubstance abuse, age and years of consumption.3
Our findings show morphological and microstructural changes in crack CAs, related to age and years of cocaine consumption. Most importantly, we found that striatal morphological changes may be more related to age, whereas thalamic changes may be more related to years consuming. We also found abnormal MKT development with age and years of consumption, highlighting the potential for MKT, and DKI in general, as a method for in vivo investigation of the effects of addiction on brain microstructure. The shape analysis showed contraction of the NAcc when compared to HCs. We showed that volumetric analysis by itself provides incomplete information about morphology and suggest that shape analysis and/or DKI should be used in addition to better characterize brain pathology in substance abuse.
References
Smith RJ, Lobo MK, Spencer S, Kalivas PW . Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol 2013; 23: 546–552.
Robinson TE, Kolb B . Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004; 47 (Suppl 1): 33–46.
Wheeler AL, Lerch JP, Chakravarty MM, Friedel M, Sled JG, Fletcher PJ et al. Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci 2013; 33: 1797–1803.
Mackey S, Paulus M . Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev 2013; 37: 300–316.
Ersche KD, Williams GB, Robbins TW, Bullmore ET . Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol 2013; 23: 615–624.
Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend 2012; 125: 208–214.
Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage 2011; 56: 1021–1026.
Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET . Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 2011; 134: 2013–2024.
Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH . Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry 2001; 158: 486–489.
Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology 2007; 32: 2229–2237.
Martinez D, Broft A, Foltin RW, Slifstein M, Hwang D-R, Huang Y et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 2004; 29: 1190–1202.
Narayana PA, Datta S, Tao G, Steinberg JL, Moeller FG . Effect of cocaine on structural changes in brain: MRI volumetry using tensor-based morphometry. Drug Alcohol Depend 2010; 111: 191–199.
Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn 2009; 71: 223–228.
Hatsukami DK, Fischman MW . Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA 1996; 276: 1580–1588.
Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 2000; 67: 1507–1515.
Fryer RG, Heaton PS, Levitt SD, Murphy KM . Measuring crack cocaine and its impact. Econ Inq 2013; 51: 1651–1681.
Brouwer KC, Case P, Ramos R, Magis-Rodríguez C, Bucardo J, Patterson TL et al. Trends in production, trafficking, and consumption of methamphetamine and cocaine in Mexico. Subst Use Misuse 2006; 41: 707–727.
Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET . Cocaine dependence: a fast-track for brain ageing? Mol Psychiatry 2013; 18: 134–135.
Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 2014; 101: 494–512.
Chakravarty MM, Rapoport JL, Giedd JN, Raznahan A, Shaw P, Collins DL et al. Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: a longitudinal study. Hum Brain Mapp 2015; 36: 1458–1469.
Payer D, Park MT, Kish S, Lerch J, Boileau I, Chakravarty MM . Exploring brain morphology in poly-stimulant abuse: shape, volume, and surface area abnormalities in Ecstasy-cocaine- and methamphetamine-preferring individuals. Drug Alcohol Depend 2015; 146: e65.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K . Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53: 1432–1440.
Kiselev VG . The cumulant expansion: an overarching mathematical framework for understanding diffusion NMR. In: Jones DK (ed.). Diffusion MRI: Theory, Methods, and Applications. Oxford University Press: Oxford, 2011, pp 152–168.
Hui ES, Fieremans E, Jensen JH, Tabesh A, Feng W, Bonilha L et al. Stroke assessment with diffusional kurtosis imaging. Stroke 2012; 43: 2968–2973.
Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012; 29: 2318–2327.
Naess-Schmidt E, Blicher JU, Eskildsen SF, Tietze A, Ostergaard L, Stubbs P et al. Microstructural changes in the thalamus after mild traumatic brain injury – a longitudinal diffusion and mean kurtosis tensor MRI study. Brain Inj 2017; 31: 230–236.
Delgado y Palacios R, Verhoye M, Henningsen K, Wiborg O, Van der Linden A . Diffusion kurtosis imaging and high-resolution MRI demonstrate structural aberrations of caudate putamen and amygdala after chronic mild stress. PLoS ONE 2014; 9: e95077.
Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A et al. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging 2008; 28: 1345–1350.
Hansen B, Lund TE, Sangill R, Jespersen SN . Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med 2013; 69: 1754–1760.
Hansen B, Lund TE, Sangill R, Stubbe E, Finsterbusch J, Jespersen SN . Erratum: Experimental considerations for fast kurtosis imaging. Magn Reson Med 2016; 76: 1455–1468.
Hansen B, Lund TE, Sangill R, Jespersen SN . Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med 2013; 69: 1754–1760.
Sun PZ, Wang Y, Mandeville E, Chan S-T, Lo EH, Ji X . Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR Biomed 2014; 27: 1413–1418.
Tietze A, Hansen MB, Ostergaard L, Jespersen SN, Sangill R, Lund TE et al. Mean diffusional kurtosis in patients with glioma: initial results with a fast imaging method in a clinical setting. Am J Neuroradiol 2015; 36: 1472–1478.
Volkow ND, Morales M . The Brain on Drugs: From Reward to Addiction. Cell 2015; 162: 712–725.
Thompson R . A note on restricted maximum likelihood estimation with an alternative outlier model. J R Stat Soc Ser B … 1985; 47: 53–55.
Sadedin SP, Pope B, Oshlack A . Bpipe: a tool for running and managing bioinformatics pipelines. Bioinformatics 2012; 28: 1525–1526.
Avants BB, Tustison N, Song G . Advanced Normalization Tools (ANTS) Release 1.5. University of Pennsylvania: PA, USA, 2011.
Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010; 29: 1310–1320.
Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp 2013; 34: 2635–2654.
Janes AC, Park MTM, Farmer S, Chakravarty MM . Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology 2015; 40: 406–411.
Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci USA 2014; 111: 1592–1597.
Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 2005; 27: 210–221.
Eskildsen SF, Coupé P, Fonov V, Manjón JV, Leung KK, Guizard N et al. BEaST: brain extraction based on nonlocal segmentation technique. NeuroImage 2012; 59: 2362–2373.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM . FSL. NeuroImage 2012; 62: 782–790.
Genovese CR, Lazar NA, Nichols T . Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002; 15: 870–878.
Leh SE, Kälin AM, Schroeder C, Park MTM, Chakravarty MM, Freund P et al. Volumetric and shape analysis of the thalamus and striatum in amnestic mild cognitive impairment. J Alzheimers Dis 2015; 49: 237–249.
Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15: 1676–1689.
Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV . Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage 2013; 65: 176–193.
Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E et al. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res 2000; 98: 93–102.
Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol 2013; 47: 645–661.
Everitt BJ, Robbins TW . Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8: 1481–1489.
Zatorre RJ, Fields RD, Johansen-Berg H . Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 2012; 15: 528–536.
Lakatos P, O’Connell MN, Barczak A . Pondering the pulvinar. Neuron 2016; 89: 5–7.
Lenglet C, Lenglet C, Abosch A, Abosch A, Yacoub E, Yacoub E et al. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PLoS ONE 2012; 7: e29153.
Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse 2010; 64: 397–402.
Koob GF, Volkow ND . Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35: 217–238.
Roussotte F, Soderberg L, Warner T, Narr K, Lebel C, Behnke M et al. Adolescents with prenatal cocaine exposure show subtle alterations in striatal surface morphology and frontal cortical volumes. J Neurodev Disord 2012; 4: 22.
Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ et al. Regional changes in thalamic shape and volume with increasing age. Neuroimage 2012; 63: 1134–1142.
Acknowledgements
We thank the people who helped this project in one way or another: Francisco J. Pellicer Graham, Margarita López-Titla, Aline Leduc, Erik Morelos-Santana, Diego Angeles, Alely Valencia, Laura Arreola, Lya Paas, Daniela Casillas, Sarael Alcauter, Luis Concha and Bernd Foerster. We also thank Rocio Estrada Ordoñez and Isabel Lizarindari Espinosa Luna at the Unidad de Atención Toxicologica Xochimilco for all their help and effort. Finally, we thank the study participants for their cooperation and patience. This project was funded by CONACYT-FOSISS project No. 0201493 and CONACYT-Cátedras project No. 2358948.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Garza-Villarreal, E., Chakravarty, M., Hansen, B. et al. The effect of crack cocaine addiction and age on the microstructure and morphology of the human striatum and thalamus using shape analysis and fast diffusion kurtosis imaging. Transl Psychiatry 7, e1122 (2017). https://doi.org/10.1038/tp.2017.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.92
This article is cited by
-
Adolescent substance use initiation and long-term neurobiological outcomes: insights, challenges and opportunities
Molecular Psychiatry (2024)
-
Neuroimaging and immunological features of neurocognitive function related to substance use in people with HIV
Journal of NeuroVirology (2023)
-
Additive cortical gray matter deficits in people living with HIV who use cocaine
Journal of NeuroVirology (2023)
-
The Mexican magnetic resonance imaging dataset of patients with cocaine use disorder: SUDMEX CONN
Scientific Data (2022)
-
Aberrant cortico-striatal white matter connectivity and associated subregional microstructure of the striatum in obsessive-compulsive disorder
Molecular Psychiatry (2022)