Abstract
Young adults often experience psychological distress and poor quality of life (QoL). Yet, there are no objective neural markers to accurately guide interventions to help improve these measures. We thus aimed to identify directional relationships between frontoamygdala emotional regulation circuitry activity during emotion processing, personality traits, and symptoms associated with psychological distress, and QoL. One hundred twenty 18–25-year olds, n=51 psychologically distressed and n=69 healthy individuals, completed a face emotion-processing task during functional magnetic resonance imaging, clinical and behavioral measures, and QoL assessment. Penalized regression, accounting for large numbers of independent variables, showed that increased state and trait anxiety, cohort and measures of general and anhedonic depression severity predicted poorer QoL (all exponents>0.87). Only state and trait anxiety predicted emotion processing-related frontoamygdala activity (all exponents=1.00). State and trait anxiety fully mediated the relationship between amygdala activity and QoL (P-value increased from 0.001 to 0.29: left amygdala, and from 0.003 to 0.94: right amygdala). State anxiety fully mediated the relationship between left ventrolateral prefrontal cortical (vlPFC) activity and QoL (P-value increased from 0.01 to 0.18). Testing an alternative mediational pathway showed that the relationship between state and trait anxiety and QoL was not mediated by amygdala or left vlPFC activity. We thereby identify specific, directional relationships linking amygdala and left vlPFC activity, state and trait anxiety, and poor QoL across different diagnoses. Our findings highlight roles of amygdala and left vlPFC activity as neural predictors of anxiety and poor QoL, and as potentially important targets for novel interventions to reduce anxiety and, in turn, improve QoL in young adults.
Similar content being viewed by others
Introduction
Almost one-fifth of all 18–25-year olds seek help from mental health professionals for psychological distress,1 which ranges from anxiety and depressive symptoms to personality traits, functional disabilities and behavioral problems. These symptoms and problem behaviors can have adverse impact on quality of life (QoL) assessment, which focuses on the subjective perception of one’s relationships, physical health, daily functioning, general sense of well-being and life satisfaction.2 Anxiety and depression in particular have been linked to poor QoL.3, 4 A large meta-analysis demonstrated significant QoL impairments in anxious individuals, across all anxiety disorders.3 In addition, severe impairments in QoL were observed in depressed individuals across 11 treatment trials.4
There are unfortunately no neural markers that provide objective targets to facilitate treatment to improve QoL in young adults. An important step toward these goals is to identify patterns of neural activity associated with symptoms and problem behaviors that are, in turn, associated with psychological distress and poor QoL. The neural correlates of anxiety have been extensively studied using tasks that present affective stimuli such as faces with emotional expressions,5, 6 aversive images7 and sounds,8 and emotion regulation9 and fear learning paradigms.10 Across studies, several brain regions are frequently implicated in both pathological and non-pathological anxiety, including the amygdala, ventromedial and lateral prefrontal cortices, and anterior cingulate cortex.11 Enhanced amygdala activity is one of the more consistent findings in individuals with elevated state12 and trait anxiety,5 and in those with anxiety disorders,13, 14 whereas specific patterns of frontal cortical activation are task dependent. These findings are thought to reflect dysfunction in frontoamygdala emotion regulation circuitry, and include amygdala hyper-reactivity to fear and threat cues, deficits in prefrontal regulation and attention processes, and impaired fear extinction. Abnormal activation patterns in amygdala, prefrontal cortices and anterior cingulate cortex have also been observed in depressed individuals15 suggesting similar dysfunction in frontoamygdala circuitry during emotion processing.
While the above studies show associations between anxiety and depression and poor QoL, and aberrant patterns of activity in amygdala–frontal cortical circuitry in anxiety and depression, there are no studies that examined specific, directional relationships among all three components: neural function, symptoms, and QoL. A critical next step is thus to identify such relationships among these three components to aid identification of modifiable neural targets for interventions to not only ameliorate personality traits and symptoms but also to improve QoL.
In the current study, we recruited young adults (18–25 years) across the range of personality traits and problem behaviors common in this age group, irrespective of the presence or absence of a psychiatric diagnosis. This approach thereby allowed us to include young adults across a wide range of anxiety and depressive symptoms to personality traits, functional disabilities and behavioral problems. Participants were individuals who sought help from mental health professionals for psychological distress, and healthy individuals (see Materials and Methods for definition of these groups). Our aim was to elucidate specific, directional relationships between frontoamygdala circuitry activity during emotion processing, personality traits and symptoms, and QoL measures in young adults. In order to determine these relationships, we first conducted a series of penalized regressions to identify those personality traits and symptoms associated with both QoL and neural circuitry activity during a face emotion processing task known to activate frontoamygdala circuitry.16 We then determined the extent to which these identified personality traits and symptoms mediated the relationship between frontoamygdala circuitry and QoL. On the basis of the present literature, we hypothesized:
Hypothesis 1: Anxiety and/or depressive measures would be most strongly associated with poor QoL.
Hypothesis 2: Emotion processing-related amygdala and prefrontal cortical activity would be positively associated with severity of anxiety/depressive symptoms.
Hypothesis 3: Anxiety and depressive measures would mediate relationships between amygdala and prefrontal cortical activity and poor QoL.
Materials and methods
Participants
Participants were 133 individuals, 18–25 years old: n=58 seeking help from mental health professionals at counseling or psychiatric services for psychological distress (including depressive and anxiety symptoms, and other behavioral and emotional problems such as failing to cope with everyday stressors and interpersonal relationships), irrespective of presence or absence of psychiatric diagnosis, and n=75 healthy individuals not presently seeking help from such services, and with no previous personal or family history of psychiatric illness in first-degree relatives. All individuals were assessed with the Structured Clinical Interview for DSM-5, Research Version (SCID-5-RV17) before participation in the study. We recruited individuals from both groups to ensure inclusion of a range of personality traits and problem behaviors (Table 1). Participants were recruited via community advertisement, student counseling services, and a participant registry. Exclusion criteria are in Supplementary Information.
Thirteen participants were excluded for excessive motion (>4 mm), signal loss, and/or severe artifacts in their imaging data. The final sample comprised 120 individuals (51 distressed and 69 healthy individuals). The University of Pittsburgh Human Research Protection Office approved the study, and all participants provided written informed consent.
Behavioral and clinical measures
Participants were assessed on the following clinical and behavioral measures: Spielberger State-Trait Anxiety Inventory;18 clinician-administered Hamilton Anxiety Rating Scale HAM-A;19 Hamilton Rating Scale for Depression HAM-D;20 Mood and Anxiety Symptom Questionnaire,21 including the general distress anxiety subscale, general distress depression subscale, general distress mixed subscale, anxious arousal subscale and anhedonic depression subscale; Zuckerman sensation seeking scale;22 Behavioral Inhibition and Activation System Scales BIS/BAS;23 Barratt Impulsiveness Scale;24 UPPS-P Impulsive Behavior Scale;25 and Young Mania Rating Scale.26 Participants also completed the short form of the Quality of life Enjoyment and Satisfaction Questionnaire2 to assess their perception of life enjoyment and satisfaction. Only one participant was taking psychotropic medication.
Neuroimaging paradigm
Participants completed the dynamic faces task (12 min 36 s), which has been previously described in detail.27 Briefly, stimuli were grayscale emotional faces (happy, angry, fearful, and sad) taken from the NimStim face database,28 and grayscale ovals (matched in luminance with the face stimuli) that served as control stimuli. The task included three, 12-trial blocks for each emotional face type and six 6-trial blocks of shapes presented pseudorandomly. During the face trials, a face changed in emotional expression from neutral to emotional over 1 s in 5% increments. During shape trials, an oval shape changed in size to parallel the changes in the face trials. In the middle of each trial (200 to 6500 ms), a semitransparent foreground color flash (blue, orange or yellow) overlaid the image. Participants identified the color of the foreground color flash using a response pad.
Neuroimaging data acquisition
Functional neuroimaging data were collected using a 3.0 Tesla Siemens Trio 2 MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil in the Magnetic Resonance Research Center (MRRC) at the University of Pittsburgh Medical Center. A total of 504 blood-oxygen-level-dependent (BOLD) images were acquired with a simultaneous multi-slice (SMS) gradient echo EPI sequence and an oblique axial angle (18 slices acquired with SMS factor=3 for a total of 54 slices, TR=1500 ms, TE=30 ms, Field of View (FOV)=220 × 220 mm, matrix=96 × 96, slice thickness=2.3 mm, Flip Angle=55°, Bandwidth=1860 Hz/Px). We also acquired structural 3D axial MPRAGE images (TR=1500 ms, TE=3.19 ms, Flip Angle 8°, FOV=256 × 256 mm, 1 mm isotropic voxels, 176 continuous slices), and fieldmaps (TR=500 ms, TE1=4.92 ms, TE2=7.38 ms, FOV=220 × 220 mm, matrix=96 × 96, Flip Angle=45°, Bandwidth=1302 Hz/Px).
Neuroimaging data analysis
Data were preprocessed using a combination of software packages (SPM, FSL, AFNI) implemented in Nipype.29 Preprocessing included realignment, coregistration, distortion correction, normalization, despiking, and smoothing (Supplementary Information). A first-level fixed-effect general linear model (GLM) was constructed for each participant using Statistical Parametric Mapping software, Version-8 (SPM8), with the four emotion (anger, fear, sad and happy) and shape conditions. Motion parameters were included as covariates of no interest to control for participant movement. A regressor to correct for physiological fluctuations was also included, derived from the mean signal within white matter, cerebrospinal fluid and high temporal standard deviation voxels.30, 31 A high-pass filter (256 s), and autoregressive (AR(1)) modeling were also implemented at the first level.
Extraction of neural measures
A first-level single subject statistical parametric map was generated for the emotion>shape contrast and used in a second-level (group) analysis to identify neural activity across participants during face emotion processing. We extracted the first eigenvariate from individual contrast images for all significant clusters, using a family-wise error correction threshold of α=0.05, in amygdala and all frontal regions (that is, entire frontal cortex), given our focus on frontoamygdala emotion regulation circuitry.
Data analytic plan
Variable selection methods were used to identify the salient variables related to our outcome variables in steps 1–3 below. These salient variables were then tested and used in mediation analyses, as described below.
-
1
Identify the behavioral trait, clinical, behavioral, and demographic variables (Table 2) predictive of QoL using penalized regression. We conducted elastic-net penalized least squares regression analysis for variable selection, using the GLMNET package in R.32 Elastic-net is a modified form of least squares regression that penalizes complex models with a regularization parameter(λ),33 is sensitive to correlated variables,33 and is robust to assumption violations.34, 35, 36 The regularization parameter shrinks coefficients toward zero, and eliminates unimportant terms entirely.32 Cross validation identifies the optimal penalty term (λ) that minimizes mean cross validated error, reduces the chances of overfitting, and enforces recommended sparsity in the solution. Lambda.1se was selected as a more conservative model in which more coefficients are set to zero.
Table 2 Demographic, clinical and behavioral data for all participants (n=120) -
2
Identify frontoamygdala regions showing significant activity during emotion processing (emotion>shape contrast) across participants.
-
3
Identify the behavioral trait, clinical, behavioral and demographic measures predictive of extracted measures of neural activity identified in Step 2 using penalized regression. As in Step 1, we used elastic-net with cross validation and implemented the mgaussian family within the package to account for the multivariate approach.
-
4
Test regression assumptions for the mediation model implemented in Step 5. Testing included normality of residuals and outlier tests of Mahalanobis, Cook’s, and Leverage values. Extreme values on two of the three outlier tests warranted exclusion.
-
5
Examine the extent to which behavioral trait, clinical and behavioral measures identified in Steps 1 and 3 that were associated with both QoL and neural activity during emotion processing mediated relationships between neural activity and QoL. A multiple mediation model was used to test each mediator while simultaneously accounting for the shared association of correlated mediators.37
-
6
We also tested an alternative pathway, in which emotion processing-related neural activity mediated the relationship in Step 1 (that is, between behavioral trait, clinical and behavioral measures and QoL; Supplementary Figure 4).
Post hoc analyses
We examined relationships between diagnostic categories, neural and clinical/behavioral variables identified in Steps 1–3, and QoL. In addition, we compared results from simple and multiple mediator models (Supplementary Table 2). Finally, we conducted mediation analyses using neural activity to each specific emotion versus shape contrast (Supplementary Information).
Results
Clinical and behavioral measures related to QoL
Here, an exponentiated coefficient (exp) represented the rate ratio change in QoL corresponding to one unit change in predictor variable. Seven variables were identified as predictors of poor QoL: increased state (exp=0.906) and trait (exp=0.911) anxiety, cohort (exp=1.00), increased Hamilton depression ratings (exp=0.897), and, from the Mood and Anxiety Symptom Questionnaire, increased general distress depression (exp=0.876), general distress mixed (exp=0.9004), and anhedonic depression (exp=0.952). The distributions of State Anxiety and Trait Anxiety scores in relation to QoL are in Supplementary Figures 1 and 2, respectively.
Neural regions showing significant activity during emotion processing
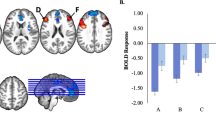
Across participants there was significant activity for the emotion>shape contrast in five regions: right and left amygdala, medial prefrontal cortex, and right and left vlPFC (Brodmann area 47); family-wise error whole-brain corrected threshold α=0.05, Figure 1. Neural activity for each separate emotion expression>shape is in Supplementary Figure 3.
Clinical and behavioral measures predictive of neural activity during emotion processing
Two clinical variables only were identified as non-zero predictors of neural activity during emotion processing: state and trait anxiety. Each of the five brain regions that showed significant activity for the emotion>shape contrast had exp=1.00, that is, a unit increase in anxiety measures predicted a unit increase in neural activity.
Testing regression assumptions
To test model assumptions, we conducted a regression analysis with the two clinical variables that predicted neural activity during emotion processing (state and trait anxiety), and extracted measures of activity in the five brain regions that showed significant activity for the emotion>shape contrast (left and right amygdala, medial prefrontal cortex, left and right vlPFC). One participant was excluded from the following mediation analyses due to extreme outlier values. Regression assumptions were met (Supplementary Information).
Mediation analyses
Given that only state and trait anxiety were significantly associated with QoL and neural activity during emotion processing, these two clinical measures were tested as potential multiple mediators of relationships between activity in the five neural regions above and QoL. This resulted in five mediation models (one model for each neural region identified in Step 3, and simultaneously testing both state and trait anxiety).
-
State and trait anxiety fully mediated the relationship between left amygdala and QoL (t(117)=−3.35, P=0.001 going to t(115)=−1.07, P=0.29; Figure 2a). The indirect effects of state anxiety on the relationship between left amygdala and QoL were significant (Boot strapped confidence interval (BootCI)=−5.58 to −0.69), as were the indirect effects of trait anxiety on the relationship between left amygdala and QoL (BootCI=−8.54 to −1.83).
Figure 2 Mediation models examining the effect of state and trait anxiety on the relationship between neural activity and quality of life (QoL). (a) State and Trait anxiety mediating the relationship between left amygdala and QoL. (b) State and Trait anxiety mediating the relationship between right amygdala and QoL. (c) State and Trait anxiety mediating the relationship between left ventrolateral prefrontal cortex (vlPFC) and QoL. (d) State and Trait anxiety mediating the relationship between right vlPFC and QoL. (e) State and Trait anxiety mediating the relationship between medial prefrontal cortex (mPFC) and QoL. Abbreviations: a1: causal effect of X on M1; a2: causal effect of X on M2; c: direct effect of X on Y; c’: mediated effect of X on Y; b1: effect of M1 on Y; b2: effect of M2 on Y; M1=State anxiety; M2=Trait anxiety; X=Neural region of interest; Y=Quality of life; a1 b1: specific indirect effect of M1 on the relationship between X and Y; a2 b2: specific indirect effect of M2 on the relationship between X and Y; *P<0.005; **P<0.001; † trend significance; ζ 95% confidence interval does not contain 0; Solid line: significant relationship; Heavy solid line: full mediation; Dashed line: non-significant relationship.
-
State and trait anxiety fully mediated the relationship between right amygdala and QoL (t(117)=−3.09, P=0.003 going to t(115)=−0.08, P=0.94; Figure 2b). The indirect effects of state anxiety on the relationship between right amygdala and QoL were significant (BootCI=−5.42 to −0.83), as were the indirect effects of trait anxiety and the relationship between right amygdala and QoL (BootCI=−9.11 to −2.32).
-
State anxiety fully mediated the relationship between left vlPFC and QoL (t(117)=−1.97, P=0.01 going to t(115)= −1.33, P=0.18; Figure 2c). State anxiety had an indirect effect on the relationship between left vlPFC and QoL (BootCI=−2.93 to −0.03). Trait anxiety did not have an indirect effect on the relationship between left vlPFC and QoL (BootCI=−4.18 to 0.68).
-
The relationship between right vlPFC and QoL was not significant (t(117)=−1.33, P=0.19) and was not mediated by state and trait anxiety (t(115)=−0.53, P=0.60; Figure 2d). Neither state anxiety (BootCI=−3.63 to 0.36) nor trait anxiety (BootCI=−4.55 to 1.37) showed indirect effects on the relationship between right vlPFC and QoL.
-
The relationship between medial prefrontal cortex and QoL was not significant (t(117)=−0.18, P=0.86) and was not mediated by state and trait anxiety (t(115)=−0.53, P=0.60; Figure 2e). Neither state anxiety (BootCI=−0.79 to 1.15) nor trait anxiety (BootCI=−1.19 to 1.96) showed indirect effects on the relationship between medial prefrontal cortex and QoL.
Alternative pathway
Neither right or left amygdala, nor left vlPFC, activity mediated the relationship between anxiety and QoL (Supplementary Information).
Post hoc results: effect of diagnostic categories on left and right amygdala and left vlPFC activity, state and trait anxiety and QoL
Young adults with and without anxiety disorders differed significantly on both state (t(49)=−2.48, P=0.017) and trait (t(49)=−2.49, P=0.016) anxiety, with higher anxiety shown in those with an anxiety disorder. The groups did not differ on left or right amygdala activity, left vlPFC activity, or on QoL, however. Young adults with and without major depressive disorder (MDD) significantly differed on QoL (t(49)=−2.63, P=0.011), with higher QoL reported by those without MDD. The groups did not differ on left or right amygdala activity, left vlPFC activity, or on state anxiety, however. There was a trend for trait anxiety to be higher in depressed than non-depressed young adults (t(49)=−1.89, P=0.066). Diagnostic categories of bipolar disorder, eating disorder, externalizing disorder, trauma-related disorder, sleep disorder, somatoform disorder and adjustment disorder were too infrequent to compare (Table 1).
Discussion
We examined directional relationships between neural function during face emotion processing, clinical and behavioral measures, and QoL in young adults, irrespective of the presence or absence of a psychiatric disorder. State and trait anxiety fully mediated the relationship between amygdala activity during emotion processing and poor QoL. State anxiety also fully mediated the relationship between left vlPFC activity during emotion processing and poor QoL. By contrast, amygdala and left vlPFC activity did not mediate the relationship between state and trait anxiety and QoL. This is the first study, to our knowledge, to identify specific, directional relationships between neural activity, symptoms, and poor QoL.
There is growing evidence for a negative impact of anxiety on QoL.3,4,38 Previous studies typically compared clinical and healthy populations, demonstrating substantial QoL impairments in individuals with Generalized Anxiety Disorder, Social Anxiety Disorder, Panic Disorder, Posttraumatic Stress Disorder and Obsessive-Compulsive Disorder.3,38 The degree of QoL impairment was similar across these disorders3,38 suggesting that anxiety severity in general, rather than diagnostic categories or other psychiatric symptoms, predict QoL. This is supported by our current findings that show that state and trait anxiety are associated with poor QoL in a large sample of young individuals, irrespective of diagnosis. QoL may be related to symptom severity, but the two are separate measures.2 Thus, measures of QoL are gaining interest as important targets for intervention, and as complementary measures of treatment outcome.4
Numerous studies demonstrated enhanced amygdala activity during emotion processing in individuals with anxiety disorders,11, 13, 14 as well as those with high trait anxiety.5 Furthermore, individual differences in trait anxiety positively correlated with (basolateral) amygdala activity to unconsciously presented fearful stimuli.39 Enhanced amygdala activity was also observed in individuals with high state anxiety to unattended and attended fearful faces.12 In the present study, using multiple clinical and behavioral measures in a penalized regression analysis, we show that only state and trait anxiety showed a non-zero association with amygdala activity to emotional stimuli. This finding provides strong support for a link between amygdala activity during emotion processing and dimensional measures of anxiety, which, alongside the above link between state and trait anxiety and QoL, underscores the role of amygdala activity–anxiety relationships in predicting psychosocial functional outcomes in young adults.
The amygdala has widespread connections with subcortical and cortical regions,40 and is important for acquisition, expression, and recognition of fear/threat-related cues,41 and more generally in emotion learning and processing of socially relevant emotional stimuli, for example, facial expressions.42 Thus, elevated amygdala activity to emotional stimuli, including socially salient facial emotional cues, in young adults may underlie enhanced threat perception, especially in emotionally salient social contexts, that in turn may be linked with vulnerability to emotional dysregulation, anxiety, compromised psychosocial function and poor QoL. Importantly, these associations were evident across a range of different psychiatric disorders, and in individuals without a present psychiatric disorder, in our sample of young adults, and were not present for other symptoms or personality traits. These findings thus further highlight links between greater anxiety severity, irrespective of the presence of absence of a psychiatric disorder and poorer QoL.
Several prefrontal cortical regions are implicated in anxiety, including ventromedial and lateral prefrontal cortices, and dorsal and ventral anterior cingulate cortex.11, 13 These regions subserve different processes relevant to emotion processing and regulation, including effortful, attentional control of emotion in lateral prefrontal cortices;43 decision-making regarding probability of stimulus outcome associations in vlPFC;1 directed attention and conflict monitoring in dorsal anterior cingulate cortex;44 and fear extinction in ventromedial prefrontal cortex and ventral anterior cingulate cortex.45 Anxious individuals show relative increases and decreases in activity in these regions, including reduced ventromedial prefrontal cortical activity associated with impaired fear extinction recall;10 increased dorsal anterior cingulate cortical activity,46, 47 potentially underlying increased attention to potential threat; and increased48 and decreased49 lateral prefrontal cortical activity, which may underlie inefficient, effortful attentional control of emotion, attention bias to threat-cues, and aberrant, inferential decision-making regarding emotional stimulus outcome associations.50, 51 Our present finding linking greater left vlPFC activity to emotional stimuli with greater state anxiety in young adults may thus reflect either inefficient attentional control of emotion or a greater tendency to make inferences about facial expressions in individuals with greater state anxiety. Interestingly, in a longitudinal neuroimaging study, we previously showed a positive relationship between increasing state anxiety and increasing activity in a similar region in the left vlPFC during an attentional control of emotional distractor task in emotionally dysregulated youth.52 The left laterality of these findings is intriguing, but may reflect undue approach toward, and attention to, emotional stimuli in anxious individuals, given the role of the left hemisphere in approach-related emotion processing.53
Interestingly, depression severity was not associated with emotion processing-related neural activity, and thus did not mediate neural activity-QoL relationships. This may suggest that anxiety rather than depressive symptoms are the most proximal behavioral manifestations of emotion processing-related neural activity, especially in the amygdala.
There were study limitations. Individuals were included with a range of diagnostic categories. There were no differences in neural activity in any of the five brain regions activated during emotion processing across diagnostic categories, however, although individuals with MDD did have poorer QoL than those without such a diagnosis, similar to previous studies.4 We showed no significant difference in QoL in individuals with versus those without anxiety disorders indicating that severity of state and trait anxiety rather than presence of anxiety disorder impacts daily functioning. The study sample included a majority of female participants, which likely reflects increased help-seeking behavior54 and general research participation by women.55 There was no gender effect on any of the measures of interest, however.
In summary, our findings identify specific directional relationships linking neural activity during emotion processing, state and trait anxiety, and poor QoL in a large sample of young adults regardless of diagnosis. This is the first study, to our knowledge, to jointly examine neural activity, symptoms, and QoL, using dimensional measures and diagnostic categories in young adults. We show a full mediation by state and trait anxiety on the relationship between amygdala activity and QoL, and by state anxiety on the relationship between left vlPFC activity and QoL. Our findings highlight amygdala and left vlPFC activity as neural predictors of poor QoL in young adults, and as potentially important neural targets for novel interventions, such as neuromodulation, to improve QoL by reducing anxiety in this population.
References
Boorman Erie D, Rajendran Vani G, O’Reilly Jill X, Behrens Tim E . Two anatomically and computationally distinct learning signals predict changes to stimulus-outcome associations in hippocampus. Neuron 89: 1343–1354.
Endicott J, Nee J, Harrison W, Blumenthal R . Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29: 321–326.
Olatunji BO, Cisler JM, Tolin DF . Quality of life in the anxiety disorders: a meta-analytic review. Clin Psychol Rev 2007; 27: 572–581.
Rapaport MH, Clary C, Fayyad R, Endicott J . Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry 2005; 162: 1171–1178.
Stein MB, Simmons AN, Feinstein JS, Paulus MP . Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007; 164: 318–327.
Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry 2008; 165: 1193–1202.
Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry 2009; 166: 302–310.
Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR . Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc Cogn Affect Neurosci 2011; 6: 74–81.
Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ . Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry 2009; 66: 170–180.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082.
Taylor JM, Whalen PJ . Neuroimaging and anxiety: the neural substrates of pathological and non-pathological anxiety. Curr Psychiatry Rep 2015; 17: 49.
Bishop SJ, Duncan J, Lawrence AD . State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci 2004; 24: 10364–10368.
Shin LM, Liberzon I . The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191.
Etkin A, Wager TD . Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476–1488.
Gotlib IH, Hamilton JP . Neuroimaging and depression. Curr Direct Psychol Sci 2008; 17: 159–163.
Perlman SB, Almeida JR, Kronhaus DM, Versace A, Labarbara EJ, Klein CR et al. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disord 2012; 14: 162–174.
First MB, Williams JBW, Karg RS, Spitzer RL . Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association: Arlington, VA, 2015.
Spielberger CD, Gorsuch RL, Lushene R . State-Trait Anxiety Invenstory Test Manual Form Y. Consulting Psychological Press: Palo Alto, CA, 1983.
Hamilton M . The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–55.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Clark LA, Watson D . Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991; 100: 316–336.
Zuckerman M . The sensation seeking scale V (SSS-V): Still reliable and valid. Pers Indiv Differ 2007; 43: 1303–1305.
Carver CS, White TL . Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Personal Soc Psychol 1994; 67: 319–333.
Patton JH, Stanford MS, Barratt ES . Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995; 51: 768–774.
Whiteside SP, Lynam DR . The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Indiv Differ 2001; 30: 669–689.
Young RC, Biggs JT, Ziegler VE, Meyer DA . A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435.
Fournier JC, Keener MT, Almeida J, Kronhaus DM, Phillips ML . Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disord 2013; 15: 741–752.
Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168: 242–249.
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 2011; 5: 13.
Behzadi Y, Restom K, Liau J, Liu TT . A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37: 90–101.
Fournier JC, Chase HW, Almeida J, Phillips ML . Model specification and the reliability of fMRI results: implications for longitudinal neuroimaging studies in psychiatry. PLoS One 2014; 9: e105169.
Friedman J, Hastie T, Simon N, Tibshirani R GLMNET. 2.0-2 edn2014.
Zou H, Hastie T . Regularization and variable selection via the elastic net. J R Stat Soc B 2005; 67: 301–320.
Friedman J, Hastie T, Tibshirani R . Sparse inverse covariance estimation with the graphical lasso. Biostatistics (Oxford, England) 2008; 9: 432–441.
Friedman J, Hastie T, Tibshirani R . Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010; 33: 1–22.
Tateishi S, Matsui H, Konishi S . Nonlinear regression modeling via the lasso-type regularization. Journal of Statistical Planning and Inference 2010; 140: 1125–1134.
Hayes AF . Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. Guilford Press, 2013.
Barrera TL, Norton PJ . Quality of life impairment in generalized anxiety disorder, social phobia, and panic disorder. J Anxiety Disord 2009; 23: 1086–1090.
Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 2004; 44: 1043–1055.
Jennifer L Freese, David G Amoral . Neuroanatomy of the primate amygdala Whalen PJ & Phelps, EA The Human Amygdala. Guilford Press: New York, NY, USA, 2009; 3–42.
LeDoux JE . Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184.
Hooker CI, Germine LT, Knight RT, D’Esposito M . Amygdala response to facial expressions reflects emotional learning. J Neurosci 2006; 26: 8915–8922.
Ochsner KN, Silvers JA, Buhle JT . Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251: E1–24.
Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG . Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex 2005; 15: 229–237.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE . Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905.
Straube T, Mentzel H-J, Miltner WHR . Neural Mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 2006; 59: 162–170.
McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007; 64: 97–106.
Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 2012; 90: 134–142.
Bishop S, Duncan J, Brett M, Lawrence AD . Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 2004; 7: 184–188.
Phillips ML, Ladouceur CD, Drevets WC . A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13: 833–857.
Cisler JM, Koster EHW . Mechanisms of attentional biases towards threat in the anxiety disorders: an integrative review. Clin Psychol Rev 2010; 30: 203.
Bertocci MA, Bebko G, Dwojak A, Iyengar S, Ladouceur CD, Fournier JC et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry 2017; 2: 336–345.
Davidson RJ . Anterior cerebral asymmetry and the nature of emotion. Brain Cogn 1992; 20: 125–151.
Vessey JT, Howard KI . Who seeks psychotherapy? Psychotherapy: Theory, Research, Practice, Training 1993; Vol. 30: 546–553.
Singer E, van Hoewyk J, Maher MP . Experiments with incentives in telephone surveys. Pub Opin Quart 2000; 64: 171–188.
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH100041 (MLP) and the Emmerling-Pittsburgh Foundation (MLP).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Greenberg, T., Bertocci, M., Chase, H. et al. Mediation by anxiety of the relationship between amygdala activity during emotion processing and poor quality of life in young adults. Transl Psychiatry 7, e1178 (2017). https://doi.org/10.1038/tp.2017.127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.127
This article is cited by
-
A specific neural substrate predicting current and future impulsivity in young adults
Molecular Psychiatry (2021)
-
Understanding the relationships between mental disorders, self-reported health outcomes and positive mental health: findings from a national survey
Health and Quality of Life Outcomes (2020)