Abstract
The environmental contributions to autism spectrum disorder (ASD) and their informative content for diagnosing the condition are still largely unknown. The objective of this study was to investigate associations between early medical events and ASD, as well as autistic traits, in twins, to test the hypothesis of a cumulative environmental effect on ASD risk. A total of 80 monozygotic (MZ) twin pairs (including a rare sample of 13 twin pairs discordant for clinical ASD) and 46 dizygotic (DZ) twin pairs with varying autistic traits, were examined for intra-pair differences in early medical events (for example, obstetric and neonatal factors, first year infections). First, differences in early medical events were investigated using multisource medical records in pairs qualitatively discordant for ASD. The significant intra-pair differences identified were then tested in relation to autistic traits in the remaining sample of 100 pairs, applying generalized estimating equations analyses. Significant association of the intra-pair differences in the MZ pairs were found for the cumulative load of early medical events and clinical ASD (Z=−2.85, P=0.004) and autistic traits (β=78.18, P=0.002), as well as infant dysregulation (feeding, sleeping abnormalities, excessive crying and worriedness), when controlling for intelligence quotient and attention deficit hyperactivity disorder comorbidity. The cumulative load of early medical events in general, and infant dysregulation in particular, may index children at risk of ASD owing to non-shared environmental contributions. In clinical practice, these findings may facilitate screening and early detection of ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is an increasingly diagnosed neurodevelopmental condition with a heterogeneous and life-long presentation. It is exclusively behaviorally defined by an early onset of persistent difficulties in social communication and interaction, alongside repetitive, restricted interests and activities causing impairment in daily life.1 Traits of autism are continuously distributed in the general population and overlap with clinical phenotypes of ASD.2, 3, 4 Comorbidity with other neurodevelopmental disorders is common in ASD.5 Symptoms of ASD are typically measurable at the age of 12 months, still most cases are diagnosed much later.6 Early behavioral signs of ASD are limited in sensitivity and specificity, and current screening tools show low positive predictive values.7 Establishing reliable early detection of the condition remains a clinical research priority, as early identification typically ensures access to intervention and facilitates improved outcomes.8
Twin and family studies have shown both genetic and environmental factors to be influential in ASD etiology with heritability estimates ranging from 38 to 55%9,10 up to 95%,3 and non-shared environmental (NSE) factors to be the predominant environmental influence.9, 11 For autistic traits, a British population-based twin study reported evidence that twins with more neonatal problems had more autistic traits. For example, lower birth weight was associated with more autistic traits in the social domain in middle childhood.12 Multiple single environmental risks have been implicated in ASD, both shared environment (SE) factors, such as maternal valproate intake, exposure to toxic chemicals, enhanced steroidogenic activity, gestational diabetes and maternal obesity, and NSE factors, such as birth injury and trauma, and neonatal anemia.13, 14, 15, 16 Bolton et al.17 in their study on the relevance of obstetric complications on ASD etiology, found that rather than playing any primary causal role, the birth complications associated with autism either represent an epiphenomenon of the condition or derive from shared risk factor(s). A number of environmental risk factors have been suggested to lead to epigenetic dysregulation, and increase ASD risk, such as prenatal exposure to valproate.18 Overall, the current evidence favors a multiple risk factor threshold model hypothesizing that an increased exposure to a composition of environmental stressors increases the risk for ASD in individuals with a genetic vulnerability.19 Further, it is suggested that the cumulative load of risk factors correlates with the severity of ASD.19
As genetic factors influence how individuals respond to different environmental exposures, limited control over the individual genetic factors has been a major weakness of most studies investigating environmental risks for ASD. As monozygotic (MZ) twins are identical on a DNA sequence level, with the exception of putative post-twinning de novo mutations, all differences in phenotypes seen in MZ twin pairs are attributable to effects of NSE.20 Particularly studies investigating MZ pairs discordant for the phenotype in question are powerful to elucidate environmental risk factors.21 Only a few studies have applied a discordant MZ twin pair design in ASD as reviewed by Ronald and Hoekstra.11
The objective of this study was to explore associations between potential environmental risk factors, ASD and autistic traits. Specifically, we sought to identify early medical events likely to be caused by environmental factors that are associated with ASD and autistic traits, and test the hypothesis of their cumulative effect on risk status. For this purpose, we scrutinized the early medical histories of a rare and informative sample of 13 MZ twin pairs discordant for clinical ASD. The findings were then cross-validated in a separate larger cohort of MZ and dizygotic (DZ) twins discordant for autistic traits (N=100 pairs), operationalizing them in a cumulative multifactorial model. The results of this study provide information about early medical events and adverse behavioral manifestations that may index children at risk for ASD, owing to NSE influences. Such evidence could be used to facilitate early detection of ASD, as it might either further support or discourage an existing diagnostic suspicion.
Materials and methods
Diagnostic and behavioral assessments
The participants were diagnosed by three experienced clinicians according to DSM-5 criteria using clinical consensus supported by results from the Autism Diagnostic Interview – Revised,22 the Autism Diagnostic Observation Schedule Second Edition,23 the Kiddie Schedule for Affective Disorders and Schizophrenia24 or the Diagnostic Interview for attention deficit hyperactivity disorder (ADHD) in Adults.25 The Wechsler Intelligence Scales for Children or Adults, Fourth Editions,26, 27 or the Leiter-revised scales28 in combination with the Peabody Picture Vocabulary Test Third Edition29 (in cases of low verbal abilities) and the parent-rated Adaptive Behavior Assessment Scale, 2nd Edition,30 were applied to assess general cognitive and verbal abilities and adaptive functional level. Autistic traits were measured by the parent report version of the Social Responsiveness Scale-2 (SRS-2) using total raw scores as recommended for research settings.31 The SRS-2 includes 65 Likert scaled items, generating a total maximum score of 195. The questions in SRS-2 focus on the individual’s behavior during the past 6 months and inquiries about autistic traits, such as alterations in social cognition, social awareness, social motivation, social communication and inflexible, repetitive behavior. Increasing scores on the SRS-2 indicate more autistic traits. The SRS-2 has demonstrated satisfactory-to-excellent psychometric properties for test–retest reliability (0.80–0.97), interrater reliability (0.75–0.95) and convergent validity (0.35–0.58).32, 33
The exposure of early medical events were assessed in relation to either quantitative or qualitative discordance for ASD. Qualitative discordance was defined as only one twin within a pair meeting the diagnostic criteria of ASD, and quantitative discordance as at least one point intra-pair difference on the SRS-2 total score. In the first step of analyses, intra-pair medical event differences in a sample of qualitative ASD discordant twins was examined and compared with TD controls. In the second step of analyses, the association between identified medical events (exposure) and quantitative discordance for ASD (outcome) was assessed.
Participants
The study was approved by the National Swedish Ethical Review Board and all the participants and/or their legal guardians gave written informed consent. A total of 126 twin pairs (80 MZ, 46 DZ) were recruited from the ‘Roots of Autism and ADHD Twin Study in Sweden’ described elsewhere in detail.34
ASD discordant MZ twin sample
The first analysis step (exploratory) included 13 MZ twin pairs discordant for clinical ASD and 13 MZ typically developing (TD) control pairs (n=52) matched for sex (16 males and 10 females in each group). Among the twins with ASD, five had a comorbid diagnoses of ADHD. The total intelligence quotient (IQ) in the ASD discordant twin sample ranged from 40 to 121 (M=89.2), and 81 to 123 (M=98.1) in the TD control twins. A quantitative intra-pair difference was found for all but two of the behavioral measures, with the largest effect size for the SRS-2 total score (r=0.85, Z=−3.06, P=0.002), that was higher in the ASD twin, and the second largest for full-scale IQ (r=0.80, Z=−2.87, P=0.004), that was higher in the non-ASD twin. Effect sizes were calculated according to r=Z/√N. These intra-pair differences were not found in the 13 TD MZ pairs (Supplementary Tables 1 and 2).
Autistic trait discordant MZ and DZ twin sample
In the second step of analysis (confirmatory), we included 100 twin pairs quantitatively discordant for autistic traits, 54 MZ pairs and 46 DZ pairs. In the MZ group, 29 participants were assessed positive for ASD, 32 positive for ADHD and 13 were positive for both disorders. The equivalent numbers in the DZ groups were 23 participants with ASD, 35 with ADHD and 13 with ASD and ADHD combined. IQ ranged from 42 to 142 in the total sample, with a mean of 96.6 in the MZ group and a mean of 97.7 in the DZ group. The percentage of females was 44% in the MZ group and 46% in the DZ group (Table 1).
Zygosity
Zygosity was determined by genotyping of saliva or whole-blood derived DNA using Infinium Human-CoreExome chip (Illumina, San Diego, CA, USA). The estimating identity by descent was analyzed using the PLINK software35 after quality control and removal of SNPs with minor allele frequency less than 0.05 within the samples. All pairs of DNA samples showing π ⩾0.99 were considered as monozygotic pairs. For few pairs, a short tandem repeat kit (Promega Powerplex 21) or zygosity questionnaire (six pairs) was used.
Medical and developmental history
For the exploratory first step of analyses comparing qualitative ASD discordant MZ twins to TD MZ twin controls, detailed information on medical and developmental history with focus on the first 5 years of life was collected from questionnaires and medical records. Intraclass Correlation Coefficient (ICC) analysis36 showed good agreement between the questionnaire and medical record information. The agreement was moderate for cumulative load of early medical events (ICC=0.55, 95% confidence interval=0.22–0.75), substantial for dysregulation (ICC=0.76, 95% confidence interval=0.58–0.86) and almost perfect for birth weight (ICC=0.93, 95% confidence interval=0.88–0.96). Medical records comprised prenatal records, birth records, records of pediatric clinics and medical and psychiatric care unit documentation. Medical history in the total sample was assessed from a parent reported questionnaire. The 114 items of the questionnaire covered medical history events such as current diagnosis and medications, family situation at birth, family medical history, pre-, peri- and postnatal factors, child disease history and diagnostic tests. Most questions were yes/no questions with a request to specify for positive answers. In some cases, the questions on paper were followed up with an interview with clinical geneticists for clarifications. The questionnaire data and health care records for the subsample were reviewed independently by four researchers (TC, B-MA, AN, CW) with different clinical backgrounds (that is, child and adolescent psychiatrist, neuropediatrician, clinical geneticist and psychologist). Two of the researchers (B-MA, AN) were blinded for clinical diagnoses, hypotheses and planned analysis. Intra-pair differences for the frequency and age of onset for developmental alterations, medical complications and life events were noted. In the case of contradictory information, a decision-making hierarchy was applied on the basis of the presumed validity of the information sources. For example, birth weight data from the birth record was considered more valid than the same information from the questionnaire. Registered medical history events were coded binary (‘1’ for present, ‘0’ for not present in each individual; Supplementary Table 3). In addition, the medical history events were categorized according to the type of events (for example, delivery-related factors and minor and frequent infections) and summarized into ordinal data. All medical history events, that by the four researchers, were identified as differing within ASD discordant pairs (that is, present in only one twin in a pair), were added up to generate a cumulative load of early medical events for each individual (31 factors). To deal with missing data (NA), the sum of individual factors was divided with the total number of variables, subtracted with the individual number of missing variables according to the formula below.

The mean number of missing data in the sample was 1.7 (range 0–23). In addition to the binary code of intra-pair birth weight differences, the quantitative information (differences in grams) was used in a separate analysis of birth weight.
Statistical analysis
Statistics were computed using IBM SPSS 22 and the drgee package37 in R version 3.2.4. Owing to the sample size, nonparametric tests were used (that is, Wilcoxon signed-rank test for continuous and ordinal data and McNemar’s test for binary data) to assess the within-pair effect for the identified medical events in the qualitative discordant MZ subsample. All the tests in the first step of analyses were two-tailed. In the second step of analyses of the quantitative discordant sample, a conditional linear regression model, based on generalized estimating equations analysis38 was fitted. The conditional model estimates the within-pair effect, adjusting for all shared factors in twin pairs (for example, all genetic factors in MZ pairs).39 One-tailed tests were applied for the regression analyses. Autistic trait severity (SRS-2 total score) was used as outcome variable in the model. The model was adjusted for the following covariates: full-scale IQ, ADHD diagnoses (binary) and sex. In addition, the sample was split into zygosity groups comparing the within-pair effects in the MZ and DZ pairs.
Results
Early medical history in twins discordant for clinical ASD
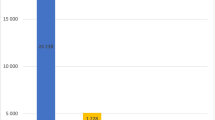
Single early medical factors, likely to be caused by NSE, that discriminated between twins in qualitative ASD discordant pairs were dysregulation during the first year of life (comprising feeding and sleeping problems, excessive crying and worrying; Z=−2.56, P=0.011) and birth weight (Z=−2.20, P=0.028) (Table 2). MZ twins with ASD and their non-ASD co-twins also differed for the cumulative load of early medical events (Z=−2.85, P=0.004; Table 2, Figure 1). None of these intra-pair differences were observed in the 13 TD MZ pairs (Figure 1).
Early medical history in autistic traits discordant twins
A single early medical factor, likely to be caused by NSE, that correlated with the extent of autistic traits was dysregulation during the first year of life (β=31.75, P=0.03). The association for birth weight and ASD traits was not significant, but showed a similar trend (β=−0.01, P=0.05; See Table 3). There was an association between the intra-pair differences in cumulative load of early medical events and autistic traits in the MZ pairs (β=78.18, P=0.002; Table 3). This effect indicates a three point intra-pair increase for autistic traits on the SRS-2 for every single medical event’s difference. All models were adjusted for full-scale IQ and ADHD diagnoses, with no significant gender effect found. The association between autistic traits and the cumulative load of early medical events hold also when excluding the dysregulation variable from the cumulative load score (β=54.42, P=0.005). No significant associations were seen in the DZ pairs (Table 3; Figure 2).
The association between intra-pair differences in SRS-2 total score and cumulative load of early medical events in MZ and DZ pairs. The graphs show a significant positive correlation (Pearsons’s R2) for MZ pairs (a) and a nonsignificant correlation for DZ pairs (b). DZ, dizygotic; MZ, monozygotic; SRS-2, Social Responsiveness Scale—2nd edition.
Discussion
In this study, we show that early medical events are associated with clinical ASD phenotypes and continuously distributed autistic traits. The latter was particularly true for early dysregulation and the cumulative load of a variety of early adverse medical events. Using co-twin modeling, we were able to control for genetic and SE factors, endorsing that the found associations were indeed driven by NSE factors within the MZ twin pairs. Our study show the novel finding of a cumulative environmental effect on ASD risk, when controlling for genetics. In addition, we were able to replicate earlier findings of early dysregulation as a precursor of behavioral problems and autistic traits. A previous large population-based study including over 4000 infants reported that early regulatory problems predict behavioral problems (that is, external, internal and attentional problems) later in life,40 and a Swedish clinical study found that regulatory issues were frequent in children later diagnosed with ASD.41 Our results advance these results because they demonstrate that an association between early regulation difficulties and ASD exists independent of genetic influences. Earlier studies were unable to differentiate genetic and environmental effects on these phenomena. In line with a previous community twin study,12 we found that an increased total load of early medical events is associated with autistic traits. Nevertheless, the associations identified in the current studies were substantially stronger.
Our study partly supports previous results indicating low birth weight to be associated with ASD risk.20 We found a trend for intrauterine growth to be associated with clinical ASD and autistic traits. However, although earlier studies deemed low birth weight a consequence of genetic factors, our data indicate environmental factors as cause. In addition to highlighting NSE factors causing early developmental adversity in ASD, our results also suggest early adversity factors being of potential relevance as early red flags for autism risk evaluation. Although symptoms of ASD emerge as early as 12 months of age,6 and ASD can quite reliably be diagnosed between 24 and 36 months of age, many children are not diagnosed until the age of 5 or 6 years. This is unfortunate as early detection is a prerequisite for early intervention,42 which is associated with better outcomes.43 The screening tools available for ASD before the age of 24 months are limited in sensitivity and specificity,43 and as the condition is exclusively behaviorally defined, the tools do not include early medical features. Our data indicate that taking into account the cumulative load of early medical factors might strengthen or discourage a suspicion of ASD, at least in a minority of cases. However, further research should explore how these general risk factors could be practically useful for identifying a specific condition such as ASD.
Our study harbors some noteworthy strengths. For instance, although there is a large phenotypic as well as etiological overlap between ASD, intellectual disability and ADHD,44 few ASD studies have taken comorbid diagnoses into consideration, either excluding comorbidity, not assessing it at all or limiting the ASD sample to those individuals without intellectual disabilities. This study controlled for the effects of IQ as well as diagnosis of ADHD, indicating that results are specific to ASD. Interestingly, findings were not limited to clinical ASD phenotypes, but equally valid for autistic traits, providing further support for the notion that ASD forms the extreme end of a trait continuum.2, 3, 4 The importance of investigating mental health from a quantitative perspective is endorsed by the American National Institutes of Health Research Domain Criteria approach, attempting to classify mental disorders incorporating multiple dimensional outcomes rather than single categorical diagnoses. A growing body of literature supports that several currently exclusively behavioral defined psychiatric diagnoses could be enriched and clarified by multidimensional information, and a medical and environmental factor association specifier has been added to the ASD diagnostic criteria in the fifth edition of DSM.45, 46 In this regard, our findings endorse that information on the cumulative load of early medical events might qualify to be specified in the context of ASD assessment.
This study has several limitations that need to be addressed. First, the cumulative load of early medical events, as well as dysregulation, might not be primary or directly acting environmental risk factors for ASD, such as maternal valproate intake. These factors might be secondary (or tertiary), although they precede an ASD diagnosis, as we in this study are unable to draw firm conclusions about the possible primary factors. Second, we cannot finally exclude that possible selection and confirmation bias, and reverse causation limit the generalizability of our conclusions. However, as we use medical records as a source of information, we assume to have collected reliable data of a child’s medical history, hence eliminating recall bias. Two of the abstractors of the medical records were unblinded to the diagnosis of the twins. Although, there were no systematic differences between the data abstracted by the blinded vs the non-blinded clinicians, and such a bias is unlikely for the autistic traits analyses, some degree of confirmation bias cannot be ruled out. Third, although we stress here the significance of NSE, as we apply a high genetic control, rare post-twining de novo mutations are acknowledged and may contribute to a minority of cases of discordance. Fourth, this study is unable to weight the different factors summing up to the cumulative load, making our approach crude. Fifth, one might consider the sample size of the ASD discordant pairs included in the first step of the study to be small. However, MZ twin designs offer a high degree of control for confounders, and our ASD discordant sample actually represents a rather large minority of those discordant pairs in Sweden.47 Sixth, despite the advantages of twin studies, twins might not always be representative of the general population, making cross-validation of twin findings in singletons desirable for confirm generalizability. For example, a factor such as shared or non-shared placenta, could have a MZ twin-specific NSE effect that we have not been able to control for in this study. Nevertheless, twinness in itself has not been shown to be a risk factor for ASD,48 nor autistic traits.49
In summary, we found (i) that the load of adverse early medical events is associated with autistic traits and ASD; (ii) a link between autistic traits, ASD and early dysregulation; (iii) that these early medical events are likely to be driven by NSE; and (iv) that the cumulative load of early medical events could be informative in the early detection of ASD. Our findings require an independent follow-up in epidemiological samples, for confirmation of the results and to investigate the specificity with regard to other neurodevelopmental disorders.
References
de Schipper E, Lundequist A, Coghill D, de Vries PJ, Granlund M, Holtmann M et al. Ability and disability in autism spectrum disorder: a systematic literature review employing the international classification of functioning, disability and health-children and youth version. Autism Res 2015; 8: 782–794.
Robinson EB St, Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet 2016; 48: 552–555.
Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 2015; 72: 415–423.
Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry 2006; 45: 691–699.
Lai MC, Lombardo MV, Baron-Cohen S . Autism. Lancet 2014; 383: 896–910.
Volkmar F, Chawarska K, Klin A . Autism in infancy and early childhood. Annu Rev Psychol 2005; 56: 315–336.
Charman T, Gotham K . Measurement Issues: screening and diagnostic instruments for autism spectrum disorders - lessons from research and practice. Child Adolesc Ment Health 2013; 18: 52–63.
Zwaigenbaum L, Bauman ML, Stone WL, Yirmiya N, Estes A, Hansen RL et al. Early identification of autism spectrum disorder: recommendations for practice and research. Pediatrics 2015; 136 (Suppl 1): 10–40.
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A . The familial risk of autism. JAMA 2014; 311: 1770–1777.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68: 1095–1102.
Ronald A, Hoekstra RA . Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet 2011; 156B: 255–274.
Ronald A, Happe' F, Dworzynski K, Bolton P, Plomin R . Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Dev 2010; 81: 166–182.
Gardener H, Spiegelman D, Buka SL . Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009; 195: 7–14.
Gardener H, Spiegelman D, Buka SL . Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011; 128: 344–355.
Mandy W, Lai MC . Annual Research Review: the role of the environment in the developmental psychopathology of autism spectrum condition. J Child Psychol Psychiatry 2016; 57: 271–292.
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M . Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016; 165: 1762–1775.
Bolton P, Murphy M, MacDonald H, Whitlock B, Pickles A, Rutter M . Obstetric complications in autism: consequences or causes of the condition? J Am Acad Child Adolsc Psychiatry 1997; 36: 272–281.
Grafodatskaya D, Chung B, Szatmari P, Weksberg R . Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry 2010; 49: 794–809.
Crawford S . On the origins of autism: The Quantitative Threshold Exposure hypothesis. Med Hypotheses 2015; 85: 798–806.
Losh M, Esserman D, Anckarsater H, Sullivan PF, Lichtenstein P . Lower birth weight indicates higher risk of autistic traits in discordant twin pairs. Psychol Med 2012; 42: 1091–1102.
Vitaro F, Brendgen M, Arseneault L . The discordant MZ-twin method: one step closer to the holy grail of causality. Int J Behav Dev 2009; 33: 376–382.
Lord C, Rutter M, le Couteur A . Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmetnal disorders. J Autism Dev Disord 1994; 24: 659–685.
Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S . Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I). Western Psychological Services: Torrance, 2012.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
Kooij JJS . Diagnostic Interview for ADHD in Adults 2.0 (DIVA 2.0) Diagnostic Assessment and Treatment. Pearson Assessment and Information BV: Amsterdam, 2010.
Wechsler D . WISC-IV Technical and Interpretive Manual. Pearson: San Antonio, TX, USA, 2003.
Wechsler D . WAIS-IV Technical and Interpretative Manual. Pearson: San Antonio, TX, USA, 2008.
Roid GH, Miller LJ . Leiter International Performance Scale-Revised: Examiner's Manual. Stoelting: Wood Dale, IL, USA, 1997.
Dunn LM, Dunn LM . Peabody Picture Vocabulary Test-Revised. American Guidance Service: Circle Pines, MN, USA, 1981.
Harrison PL, Oakland T . Adaptive Behavior Assessment System, 2nd edn. Western Psychological Services: Los Angeles, CA, USA, 2003.
Constantino JN . Social Responsivness Scale (SRS). Western Psychological Services: Los Angeles, CA, USA, 2005.
Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 2003; 33: 427–433.
Bölte S, Poustka F, Constantino JN . Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res 2008; 1: 354–363.
Bölte S, Willfors C, Berggren S, Norberg J, Poltrago L, Mevel K et al. The roots of autism and ADHD twin study in Sweden (RATSS). Twin Res Hum Genet 2014; 17: 164–176.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Cicchetti DV . Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994; 6: 284–290.
Zetterqvist J, Vansteelandt S, Pawitan Y, Sjolander A . Doubly robust methods for handling confounding by cluster. Biostatistics 2016; 17: 264–276.
Zorn CJW . Generalized estimating equation models for correlated data: a review with applications. Am J Polit Sci 2001; 45: 470–490.
Neuhaus JM, McCulloch E . Separating between- and within-cluster covariate effects by using conditional and partitioning methods. J R Stat Soc 2006; 68 (Part 5): 859–872.
Santos IS, Matijasevich A, Capilheira MF, Anselmi L, Barros FC . Excessive crying at 3 months of age and behavioural problems at 4 years age: a prospective cohort study. J Epidemiol Community Health 2015; 69: 654–659.
Barnevik Olsson M, Carlsson LH, Westerlund J, Gillberg C, Fernell E . Autism before diagnosis: crying, feeding and sleeping problems in the first two years of life. Acta Paediatr 2013; 102: 635–639.
Filipek PA, Pasquale AJ, Baranek GT, Cook ETJ, Dawson G, Gordon B et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord 1999; 29: 439–483.
Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D et al. Early intervention for children with autism spectrum disorder under 3 years of age: recommendations for practice and research. Pediatrics 2016; 136: S60–S81.
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G . Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47: 921–929.
American Psychiatric Association Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing: Arlington, VA, USA, 2013.
Kozak MJ, Cuthbert BN . The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology 2016; 53: 286–297.
Lichtenstein P, Carlström E, Rastam M, Gillberg C, Anckarsater H . The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry 2010; 167: 1357–1363.
Hallmayer J, Glasson EJ, Bower C, Petterson B, Croen L, Grether J et al. On the twin risk in autism. Am J Hum Genet 2002; 71: 941–946.
Curran S, Dworzynski K, Happe F, Ronald A, Allison C, Baron-Cohen S et al. No major effect of twinning on autistic traits. Autism Res 2011; 4: 377–382.
Acknowledgements
We express our sincere gratitude to all twins and parents who have participated in this research. We also thank Kerstin Andersson, Elodie Cauvet, Christina Coco, Andreas Fällman, Martin Hammar, Johanna Ingvarsson, Johan Isaksson, Therese Lindström, Anna Lövgren, Katell Mevel, Lynnea Myers, Soheil Mahdi, Janina Neufeld, Lina Poltrago, Anna Råde, Annelies V’ant Westeinde, Elin Vahlgren, and Eric Zander for their valuable contribution to the work presented in this study. Genotyping was performed by the SNP&SEQ Technology Platform in Uppsala (www.genotyping.se). The facility is part of the National Genomics Infrastructure (NGI) Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. The study was funded by the Swedish Research Council, Vinnova, Formas, FORTE, the Swedish Brain foundation (Hjärnfonden), Stockholm Brain Institute, Autism and Asperger Association Stockholm, Queen Silvia Jubilee Fund, Solstickan Foundation, PRIMA Child and Adult Psychiatry, the Pediatric Research Foundation at Astrid Lindgren Children’s Hospital, the Swedish Foundation for Strategic Research, Jerring Foundation, the Swedish Order of Freemasons, Kempe-Carlgrenska Foundation, Sunnderdahls Handikappsfond, The Jeansson Foundation, the Innovative Medicines Initiative Joint Undertaking (grant agreement number 115300), which comprises financial contribution from the European Union’s Seventh Framework Programme (FP7 /2007 – 2013) and in-kind contributions from companies belonging to the European Federation of Pharmaceutical Industries and Associations (EU-AIMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SBö has in the last 3 years acted as a consultant or lecturer for Shire, Roche, Medice, Eli Lilly, Prima Psychiatry, Kompetento, Expo Medica, System Analytic, Prophase and GLG Consultation, and receives royalties from Kohlhammer, UTB and Hogrefe/Huber publishers. The remaining authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Willfors, C., Carlsson, T., Anderlid, BM. et al. Medical history of discordant twins and environmental etiologies of autism. Transl Psychiatry 7, e1014 (2017). https://doi.org/10.1038/tp.2016.269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.269
This article is cited by
-
Nonshared environmental factors in the aetiology of autism and other neurodevelopmental conditions: a monozygotic co-twin control study
Molecular Autism (2022)
-
Association of cumulative early medical factors with autism and autistic symptoms in a population-based twin sample
Translational Psychiatry (2022)
-
The association between ADHD and physical health: a co-twin control study
Scientific Reports (2020)
-
On the Nature of Monozygotic Twin Concordance and Discordance for Autistic Trait Severity: A Quantitative Analysis
Behavior Genetics (2020)
-
The Association Between Somatic Health, Autism Spectrum Disorder, and Autistic Traits
Behavior Genetics (2020)