Abstract
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterized by defects in communication and social interactions, as well as stereotypic behaviors. Symptoms typically worsen with anxiety and stress. ASD occur in early childhood, often present with regression and have a prevalence of 1 out of 68 children. The lack of distinct pathogenesis or any objective biomarkers or reliable animal models hampers our understanding and treatment of ASD. Neurotensin (NT) and corticotropin-releasing hormone (CRH) are secreted under stress in various tissues, and have proinflammatory actions. We had previously shown that NT augments the ability of CRH to increase mast cell (MC)-dependent skin vascular permeability in rodents. CRH also induced NT receptor gene and protein expression in MCs, which have been implicated in ASD. Here we report that serum of ASD children (4–10 years old) has significantly higher NT and CRH levels as compared with normotypic controls. Moreover, there is a statistically significant correlation between the number of children with gastrointestinal symptoms and high serum NT levels. In Bull Terriers that exhibit a behavioral phenotype similar to the clinical presentation of ASD, NT and CRH levels are also significantly elevated, as compared with unaffected dogs of the same breed. Further investigation of serum NT and CRH, as well as characterization of this putative canine breed could provide useful insights into the pathogenesis, diagnosis and treatment of ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterized by impaired social interactions and communication, as well as stereotypic behaviors.1, 2, 3, 4 Recent results from the Centers of Disease Control in the USA indicate that 1 out of 68 children have ASD (http://www.cdc.gov/media/releases/2014/p0327-autism-spectrum-disorder.html). Fifty percent of such children regress at 2–3 years of age, often after a specific event such as reaction to vaccination, infection,5,6 trauma,7 environmental toxins8, 9, 10 or stress,11 implying the importance of some epigenetic triggers. In addition, many children exhibit gastrointestinal (GI) symptoms.12 Recent evidence suggests that ASD involve some defective immune responses in many ASD patients13, 14, 15, 16 and their relatives,17 as well as some neuroimmune component,18 especially allergic-like symptoms.19
In spite of various clues from genetic and environmental studies regarding the possible pathogenesis, their significance in the pathogenesis of autism is still unclear.20,21 As a result, the development of new drugs for ASD has been slow.22,23 Behavioral phenotyping of mice with mutations in certain genes relevant to neuropsychiatric disorders has led to some ‘mouse models’ of autism.24, 25, 26, 27, 28, 29, 30 Other mice develop defects in social interactions as a result of maternal ‘viral’ infections during gestation.6,31,32 There are also some possible spontaneous (noninduced) animal models of autism that include the jumping/somersaulting deer mice housed in restricted environments33 and caged parrots exhibiting various repetitive behaviors.34 However, these behaviors are more appropriately described as stereotypies or perhaps animal equivalents of obsessive-compulsive behavior rather than autism.35
Bull Terriers (BTs), like some other dog breeds, are highly inbred and some exhibit an almost breed-specific behavior of repetitive tail chasing.36 About 20% of BTs in one pedigree we examined was affected with either subclinical or clinical tail chasing, which is one of the most serious behavioral issues that plagues the breed (draft on file). Tail chasing, which involves spinning in tight circles is reminiscent of stereotypic spinning exhibited by autistic children and is similarly induced by high arousal.37 Other behavioral issues include aggression, seizures and ‘trancing’ (exhibiting a fixed stare and associated immobility). The frequent manifestation of the behavioral problem of tail chasing after some precipitating event, such as trauma or stress, suggests that epigenetic factors are also involved.37
Recently, we reported increased serum levels of the peptide neurotensin (NT) in a small sample of 3-year-old children with autistic disorder.38 NT is a vasoactive peptide isolated from the brain39 and is implicated in immunity.40 It is increased in the skin following acute stress, triggers skin mast cells (MCs) and increases skin vascular permeability in rodents through MC activation.41
We had previously shown that acute restraint stress in rats increases skin vascular permeability, an effect mimicked by intradermal injection of corticotropin-releasing hormone (CRH).42 We had also shown that NT augments the effect of stress43 and CRH.44 CRH also triggered MC-dependent vasodilation in the microvasculature of human skin.45 Interactions among CRH, NT, MCs and other cell types may represent the equivalent of the hypothalamic–pituitary–adrenal axis outside the brain.46
Here we show that NT and CRH are increased in children with ASD and in BTs who exhibit a phenotype similar to ASD. There is a strong correlation between the number of ASD children with GI symptoms and high serum NT levels. The present findings represent a possible pathway that contributes to the pathogenesis of ASD in at least a subphenotype of subjects. BTs may be a useful spontaneous model of ASD.
Materials and methods
Fasting blood was obtained from 40 young Caucasian children (34 male and six female, 4–10 years of age) with diagnoses ranging across the entire ASD spectrum. Participants were evaluated as part of a clinical trial that was conducted at the Attikon General Hospital, Athens Medical School, Athens, Greece.47 Children were diagnosed with ASD on the basis of clinical assessment and corroborated by meeting the cutoff scores on both the DSM-IV-TR symptom list and the Autism Diagnostic Observation Schedule algorithm. They were medication-free before blood draw for at least 2 weeks for all psychotropic medications (4 weeks for fluoxetine or depot neuroleptics). The exclusion criteria were: (1) any medical condition likely to be etiological for ASD (for example, Rett syndrome, focal epilepsy, Fragile X syndrome or tuberous sclerosis), (2) any neurologic disorder involving pathology above the brain stem other than uncomplicated nonfocal epilepsy, (3) contemporaneous evidence or unequivocal retrospective evidence of probable neonatal brain damage, (4) any genetic syndrome involving the CNS even if the link with autism was uncertain, (5) clinically significant visual or auditory impairment even after correction, (6) any circumstances that might possibly account for the picture of autism (for example, severe nutritional or psychological deprivation), (7) mastocytosis (including urticaria pigmentosa), (8) history of upper airway diseases, (9) history of inflammatory diseases, (10) history of any allergies.47 This protocol was approved by Attikon Hospital Human Investigation Review Committee.
All blood samples were labeled only with a code number, as well as the age and sex of the respective subject. Serum was also collected from normally developing healthy children, unrelated to the ASD subjects, who were seen for routine health visits at the pediatric department of the Social Security Administration (IKA) polyclinic. All ASD and control blood samples were prepared immediately and serum was stored in −80 °C. Samples were then transported by the senior Author on dry ice to Boston for analysis.
Serum from unaffected (n=18) and affected BTs (n=14) and unaffected Labrador retriever dogs (n=6) was collected at various facilities accessible to the owners and was sent to the senior Author’s laboratory on dry ice. Information was also provided on age, sex and behavioral characteristics of all dogs. Phenotypic data were also collected from behavioral surveys of additional BTs (total 45 affected and 42 control) to more precisely examine behavioral differences between affected and unaffected dogs.
Extraction of serum peptides
The extraction of serum NT and CRH peptides was performed using a SEP-COLUMN containing 200 mg of C18 (Cat. No. RK-SEPCOL-1), buffer A (Cat. No. RK-BA-1) and buffer B (Cat. No. RK-BB-1; Phoenix Pharmaceuticals, Burlingame, CA, USA). A combination of a centrifugal concentrator (Savant Speedvac SVC 100H) and a lyophilizer (Edwards K4 Modulyo Freeze Dryer, West Sussex, England) was used for drying the samples after extraction. First, Speedvac was used to dry the samples for approximately 15 min to remove the organic layer. The remaining sample was snap-frozen in liquid nitrogen and freeze-dried overnight using a lyophilizer. The dried extracts were then reconstituted with 1 × assay buffer and phosphate-buffered saline for NT determination in human and dog serum samples, respectively.
Assessment of serum NT and CRH levels
Human and dog serum NT levels were determined with commercially available enzyme-linked immunosorbent assay kits (Phoenix Pharmaceuticals) and (TSZ Scientific, Framingham, MA, USA), respectively, according to the manufacturers’ protocol. Human and dog serum CRH levels were determined with commercially available enzyme-linked immunosorbent assay kit (Phoenix Pharmaceuticals).
Statistical analysis
Before analysis, the data were validated and inspected for outliers. The results are presented both as scattergrams and box plots. In scattergrams, the symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% confidence intervals for each group. In box plots, the horizontal line in the box separates the 2.5 and 97.5 percentiles and the bars show standard deviation. Normality of distribution was checked with the Shapiro–Wilk’s test. Comparisons between two different groups were performed using Mann–Whitney U-tests. For the analyses, we used the GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA, USA). Significance of comparisons between healthy subjects and subjects with ASD is denoted by P<0.05. Any association between serum levels of CRH and NT were examined using Spearman’s rank correlation. Fisher’s exact test was performed to assess whether there is any relationship between the number of ASD children with GI symptoms and high serum NT levels and whether it is more than expected by chance. The two-sided P-value <0.05 was considered statistically significant.
Results
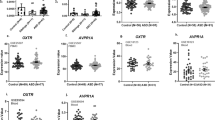
Serum NT levels are significantly (P=0.02) elevated (0.40±0.56 ng ml−1) in children, (n=38, 4–10 years old) with ASD as compared with normotypic (n=13, 4–10 years old) controls (0.03±0.03 ng ml−1; Figure 1a). Specifically, there were two clusters of ASD children with low and high serum NT levels indicating two subgroups. Low NT levels are those below the mean, whereas high NT levels are those above the mean.
(a) Comparison of serum NT levels in normal and ASD children (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% confidence intervals (CI) for each group (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. (b) Correlation of number of ASD children with GI symptoms and high serum NT levels (P=0.0023, Fisher’s exact test). Low NT levels are those below the mean, while high NT levels are those above the mean. ASD, autism spectrum disorder; GI, gastrointestinal; NT, neurotensin.
There is a strong correlation between the number of ASD children with GI symptoms and high serum NT levels (Figure 1b, P=0.0023).
Serum CRH levels are significantly (P<0.0001) elevated (0.41±0.19 ng ml−1) in children (n=38, 4–10 years old) with ASD as compared with normotypic (n=13, 4–10 years old) controls (0.21±0.08 ng ml−1; Figure 2).
Serum levels of CRH in normal and ASD children (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% CI for each group (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. ASD, autism spectrum disorder; CI, confidence interval; CRH, corticotropin-releasing hormone.
Serum NT levels are also significantly (P<0.0001) elevated (0.11±0.03 ng ml−1) in tail-chasing (Figure 3) BT dogs (n=14, 1–15 years old) as compared with unaffected (n=18, 6.5 months–11 years old) BT (0.07±0.02 ng ml−1; Figure 3). There was no difference between Labrador retriever and unaffected BT controls (Figure 4a), but there was still a significant difference between Labrador retriever (dog) controls and affected BTs (Figure 4b).
Serum levels of NT in unaffected and affected (autistic-like) Bull Terriers (BTs). (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% CI for each group. (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. CI, confidence interval; NT, neurotensin.
(a) Comparison of serum NT levels in control Labrador retriever (LAB) dogs and unaffected BTs. (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% CI for each group. (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. (b) Comparison of serum NT levels between control LAB dogs and affected BTs. (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% CI for each group. (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. BT, Bull Terrier; CI, confidence interval; NT, neurotensin.
It should be noted that human serum NT levels range from 0.004 to 1.63 ng ml−1, whereas those of dogs are lower, ranging from 0.05 to 0.2 ng ml−1.
Serum CRH levels are also significantly (P=0.002) elevated (0.36±0.06 ng ml−1) in affected BT dogs (n=14, 1–15 years old) as compared with (0.24±0.12 ng ml−1) unaffected BT controls (n=18, 6.5 months–11 years old; Figure 5).
Serum levels of CRH in unaffected and affected (autistic-like) Bull Terriers (BTs). (i) Symbols represent individual data points, the long horizontal lines represent the mean and the shorter ones show the 95% CI for each group. (ii) Data are presented as box plots with the horizontal line in the box separating the 2.5 and 97.5 percentiles and the bars showing standard deviation. CI, confidence interval; CRH, corticotropin-releasing hormone.
There is no significant correlation between serum CRH and NT either in children with ASD or in the affected BTs. There is also no significant correlation between serum CRH or NT and severity of symptoms in children with ASD. There is a possibility that high NT serum levels in ASD children with GI symptoms may reflect GI pathology only. However, no such information is available for the affected BTs, in whom the serum NT values are also more evenly distributed.
Discussion
Our present findings that serum NT is increased in children (4–10 years old) on the entire ASD spectrum confirm and expand our previous report of increased NT in 3-year-old children with autistic disorder (105.6±31.5 pg ml−1).38 The present values in affected children (4–10 years old) on the entire ASD spectrum are higher (403±558 pg ml−1) than the younger children reported before even though the serum NT values in the control children are similar (60.5±6 pg ml−1 vs 32.5±31.5 pg ml−1, respectively). Serum NT and CRH levels did not correspond to the severity of ASD symptoms. It would be interesting to correlate serum CRH levels and extent of stress in ASD children in future studies; however, this would be difficult to document since most children with ASD are nonverbal.
In mammals, NT was originally isolated from brain.39 The highest concentration of NTR reported in the brain is in regions48,49 suspected to be affected in most children with ASD. NT-producing cells are distributed along the length of the duodenum, jejunum and ileum and can release NT from cytoplasmic granules in response to several stimuli, such as fat and bacterial products.50 Most of the evidence that links NT to the pathophysiology of GI disorders is based on animal models. Nevertheless, recent results from normal or diseased human colon implicate NT in human disease.50
NT may induce neurotoxicity directly by activating NMDA receptors or inducing migration on microglia.51,52 NT also stimulates rodent MCs to secrete histamine and elevates histamine plasma levels through NTR.53 Activation of MCs leads to release of multiple mediators with potent vasodilatory, inflammatory and nociceptive properties54 through which they participate in acute and delayed hypersensitivity reactions in the skin.55 In addition, MCs are critical for innate and acquired immunity, as well as for inflammatory processes.54,56,57 Our finding that CRH is also increased in both ASD children and affected BTs may explain the behavioral sensitivity of these subjects to stress.11 We had previously reported that NT augments the ability of CRH to increase skin vascular permeability in rodents.44 NT also induces CRHR-1 gene expression on MCs58 and may mediate the effect of stress on immunity by at least targeting MCs,59 as part of a ‘brain-skin’ connection.60 The possible role of CRH in skin61 and GI62 diseases was reviewed recently.
We also show a strong correlation between the number of ASD children with GI symptoms and high serum NT levels. This finding is important because many children with ASD have GI symptoms12 and may implicate NT in at least a subphenotype of children with ASD. Nevertheless, the high serum NT levels in these children may simply reflect GI pathology.
CRH is typically secreted from the hypothalamus under stress and activates the hypothalamic–pituitary–adrenal axis. However, CRH and CRHR gene and peptide expression have been documented in human skin63 and gut.64 Stress induces local release of CRH in the skin and the gut,64 and stimulates degranulation of MCs.43 MCs are located close to CRH-positive nerve endings in the brain,65 as well as the gut.66 CRH can be released from skin cells,67 immune cells68 and MCs.69 Human MCs also express mRNA and protein for CRHR-1, activation of which induces selective release of VEGF.70 These findings have led to the proposal that the skin may have its own equivalent of the hypothalamic–pituitary–adrenal axis.46 The high serum CRH found in ASD children may explain why they cannot tolerate stress and how stress worsens allergic diseases in general.71, 72, 73, 74
Our new demonstration that NT and CRH are significantly increased in ‘affected’ tail-chasing BTs supports the premise that these dogs could represent at least an autism endophenotype.75 ASD is heterogeneous and there are likely subsets of ASD individuals.19,76 Our results also suggest the presence of two clusters of children with low and high serum NT levels (low NT levels are the levels below the mean, whereas high NT levels are those above the mean) indicating at least two subgroups.
A study of 333 BT dogs, comparing those affected with tail chasing disorder to normal controls37 produced intriguing parallels between the dogs’ condition and ASD (for a subset Table 1). Those BTs that exhibit tail chasing tend to be: (a) male; (b) are affected as early as 8 weeks of age; (c) are triggered by arousal or frustration in response to identifiable psychological, physiological or environmental events; (d) cannot handle stress; (e) have disabilities in social interaction; (f) find it difficult to communicate (affected BTs are relatively unresponsive to human signals); (g) have learning disabilities (affected dogs can be impossible to train); (h) exhibit repetitive behavior patterns, such as flank sucking and circling; (i) show fixations/fascinations with objects; (j) exhibit self-injurious behavior (as a result of tail chasing; Supplementary Figure 1); and (k) display aggressive behavior (both mild owner-directed aggression and explosive aggression), extreme explosive aggressive behavior (episodic dyscontrol or putatively a partial seizure event). They also exhibit trancing (an absence seizure-like behavior exhibiting a fixed stare and associated immobility) significantly more frequently than controls.37 Affected BTs are also more prone to environmental, social and situational phobias that makes high serum CRH levels relevant.37 Many owners of BTs in the aforementioned study reported that their dogs were ‘socially withdrawn’ and some spontaneously used the term ‘autistic’ to describe their personality.
Preliminary results we have obtained with colleagues from a genome-wide association study of a population of tail-chasing BTs and controls conducted at the National Human Genome Research Institute suggest that some genetic influence may contribute to the phenotype of tail-chasing BT. Interestingly, a cadherin 8 gene abnormality was reported as conferring susceptibility to autism,77 and single-nucleotide polymorphisms between cadherin 9 and 10 have also revealed strong association signals with autism risk.78
This is the first time that serum NT and CRH are shown to be significantly increased in both young children on the ASD spectrum and in BTs affected with a phenotype reminiscent of autism. This dog breed may be useful in the investigation of the diagnosis, pathogenesis and treatment of ASD. NTR and/or CRHR-1 antagonists, as well as MC blockers, could provide possible therapeutic approaches for stress-induced ASD. For instance, the natural flavonoid luteolin79 is known to inhibit mast cell-mediated allergic inflammation,80 and a dietary supplement containing luteolin was recently reported to significantly benefit children with ASD.47
References
Fombonne E . Epidemiology of pervasive developmental disorders. Pediatr Res 2009; 65: 591–598.
Johnson CP, Myers SM . Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007; 120: 1183–1215.
McPartland J, Volkmar FR . Autism and related disorders. Handb Clin Neurol 2012; 106: 407–418.
Abrahams BS, Geschwind DH . Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008; 9: 341–355.
Hornig M, Weissenbock H, Horscroft N, Lipkin WI . An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA 1999; 96: 12102–12107.
Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH . Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA 2012; 109: 12776–12781.
Blenner S, Reddy A, Augustyn M . Diagnosis and management of autism in childhood. BMJ 2011; 343: d6238.
Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M . How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. NeuroToxicol 2008; 29: 190–201.
Herbert MR . Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol 2010; 23: 103–110.
Rossignol DA, Genuis SJ, Frye RE . Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 2014; 4: e360.
Lanni KE, Schupp CW, Simon D, Corbett BA . Verbal ability, social stress, and anxiety in children with autistic disorder. Autism 2012; 16: 123–138.
Buie T, Campbell DB, Fuchs GJ III, Furuta GT, Levy J, Vandewater J et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010; 125 (Suppl 1): S1–S18.
Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol 2005; 33: 195–201.
Rossignol DA, Frye RE . A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 2012; 17: 389–401.
Goines P, Van de Water J . The immune system's role in the biology of autism. Curr Opin Neurol 2010; 23: 111–117.
Tete' S, Varvara G, Murmura G, Saggini A, Maccauro G, Rosati M et al. Impact of immunity in autism spectrum disorders. Eur J Inflamm 2013; 11: 23–31.
Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009; 124: 687–694.
Theoharides TC, Kempuraj D, Redwood L . Autism: an emerging 'neuroimmune disorder' in search of therapy. Expert Opin Pharmacother 2009; 10: 2127–2143.
Theoharides TC . Is a subtype of autism ‘allergy of the brain’? Clin Ther 2013; 35: 584–591.
Gadad BS, Hewitson L, Young KA, German DC . Neuropathology and animal models of autism: genetic and environmental factors. Autism Res Treat 2013; 2013: 731935.
Huguet G, Ey E, Bourgeron T . The genetic landscapes of autism spectrum disorders. Annu Rev Genomics Hum Genet 2013; 14: 191–213.
Ghosh A, Michalon A, Lindemann L, Fontoura P, Santarelli L . Drug discovery for autism spectrum disorder: challenges and opportunities. Nat Rev Drug Discov 2013; 12: 777–790.
Ecker C, Spooren W, Murphy D . Developing new pharmacotherapies for autism. J Intern Med 2013; 274: 308–320.
Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res 2007; 176: 4–20.
Crawley JN . Medicine. Testing hypotheses about autism. Science 2007; 318: 56–57.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN . Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 2008; 7: 152–163.
Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res 2008; 1: 147–158.
Silverman JL, Tolu SS, Barkan CL, Crawley JN . Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2009; 35: 976–989.
Banerjee S, Riordan M, Bhat MA . Genetic aspects of autism spectrum disorders: insights from animal models. Front Cell Neurosci 2014; 8: 58.
Wohr M, Scattoni ML . Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav Brain Res 2013; 251: 5–17.
Boksa P . Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 2010; 24: 881–897.
Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K . Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2012; 2: e98.
Lewis MH, Tanimura Y, Lee LW, Bodfish JW . Animal models of restricted repetitive behavior in autism. Behav Brain Res 2007; 176: 66–74.
Garner JP, Meehan CL, Mench JA . Stereotypies in caged parrots, schizophrenia and autism: evidence for a common mechanism. Behav Brain Res 2003; 145: 125–134.
Uchida Y, Dodman N, DeNapoli J, Aronson L . Characterization and treatment of 20 canine dominance aggression cases. J Vet Med Sci 1997; 59: 397–399.
Dodman NH, Bronson R, Gliatto J . Tail chasing in a bull terrier. J Am Vet Med Assoc 1993; 202: 758–760.
Moon-Fanelli AA, Dodman NH, Famula TR, Cottam N . Characteristics of compulsive tail chaing and associated risk factors in bull terriers. J Am Vet Med Assoc 2010; 238: 883–889.
Angelidou A, Francis K, Vasiadi M, Alysandratos K-D, Zhang B, Theoharides A et al. Neurotensin is increased in serum of young children with autistic disorder. J Neuroinflam 2010; 7: 48.
Carraway R, Leeman SE . The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 1973; 248: 6854–6861.
Mustain WC, Rychahou PG, Evers BM . The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes 2011; 18: 75–82.
Cochrane DE, Boucher W, Bibb P . Neurotensin stimulates histamine release in in vivo skin 'blisters' in rats: an effect inhibited by cromolyn or somatostatin. Int Arch Allergy Appl Immunol 1986; 80: 225–230.
Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology 1998; 139: 403–413.
Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC . Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin and substance P: a link to neurogenic skin disorders. Brain Behav Immunity 1999; 13: 225–239.
Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC . Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA 2006; 103: 7759–7764.
Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM . Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab 2003; 88: 5427–5432.
Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J . Key role of CRF in the skin stress response system. Endocr Rev 2013; 34: 827–884.
Taliou A, Zintzaras E, Lykouras L, Francis K . An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther 2013; 35: 592–602.
Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC . Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology 2000; 39: 1430–1442.
Goedert M, Lightman SL, Mantyh PW, Hunt SP, Emson PC . Neurotensin-like immunoreactivity and neurotensin receptors in the rat hypothalamus and in the neurointermediate lobe of the pituitary gland. Brain Res 1985; 358: 59–69.
Zhao D, Pothoulakis C . Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides 2006; 27: 2434–2444.
Martin S, Vincent JP, Mazella J . Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J Neurosci 2003; 23: 1198–1205.
Ghanizadeh A . Targeting neurotensin as a potential novel approach for the treatment of autism. J Neuroinflammation 2010; 7: 58.
Carraway R, Cochrane DE, Lansman JB, Leeman SE, Paterson BM, Welch HJ . Neurotensin stimulates exocytotic histamine secretion from rat mast cells and elevates plasma histamine levels. J Physiol 1982; 323: 403–414.
Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B et al. Mast cells and inflammation. Biochim Biophys Acta 2010; 1822: 21–33.
Metz M, Maurer M . Innate immunity and allergy in the skin. Curr Opin Immunol 2009; 21: 687–693.
Kinet JP . The essential role of mast cells in orchestrating inflammation. Immunol Rev 2007; 217: 5–7.
Frydas S, Varvara G, Murmura G, Saggini A, Caraffa A, Antinolfi P et al. Impact of capsaicin on mast cell inflammation. Int J Immunopathol Pharmacol 2013; 26: 597–600.
Alysandratos KD, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H et al. Neurotensin and CRH interactions augment human mast cell activation. PLoS One 2012; 7: e48934.
Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P . Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci 2004; 25: 563–568.
Paus R, Theoharides TC, Arck PC . Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends Immunol 2006; 27: 32–39.
Slominski A . On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol 2009; 160: 229–232.
Larauche M, Kiank C, Tache Y . Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 2009; 60 (Suppl 7): 33–46.
Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz J, Wortsman J . Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 2004; 145: 941–950.
Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC . Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol 2003; 130: 224–231.
Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC . Morphological and functional demonstration of rat dura mast cell-neuron interactions in vitro and in vivo. Brain Res 1999; 849: 1–15.
Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J . Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology 1989; 97: 575–585.
Donelan J, Marchand J, Kempuraj D, Papadopoulou N, Theoharides TC . Perifollicular and perivascular mouse skin mast cells express corticotropin-releasing hormone receptor. J Inv Dermatol 2006; 126: 929–932.
Karalis K, Louis JM, Bae D, Hilderbrand H, Majzoub JA . CRH and the immune system. J Neuroimmunol 1997; 72: 131–136.
Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology 2004; 145: 43–48.
Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol 2005; 174: 7665–7675.
Theoharides TC, Cochrane DE . Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol 2004; 146: 1–12.
Wright RJ, Cohen RT, Cohen S . The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol 2005; 5: 23–29.
Slattery MJ . Psychiatric comorbidity associated with atopic disorders in children and adolescents. Immunol Allergy Clin North Am 2005; 25: 407–420, viii.
Stauder A, Kovacs M . Anxiety symptoms in allergic patients: identification and risk factors. Psychosom Med 2003; 65: 816–823.
Sacco R, Curatolo P, Manzi B, Militerni R, Bravaccio C, Frolli A et al. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res 2010; 3: 237–252.
Goh S, Dong Z, Zhang Y, Dimauro S, Peterson BS . Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry 2014; 71: 665–671.
Pagnamenta AT, Khan H, Walker S, Gerrelli D, Wing K, Bonaglia MC et al. Rare familial 16q21 microdeletions under a linkage peak implicate cadherin 8 (CDH8) in susceptibility to autism and learning disability. J Med Genet 2010; 48: 48–54.
Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009; 459: 528–533.
Middleton EJ, Kandaswami C, Theoharides TC . The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 2000; 52: 673–751.
Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P et al. Luteolin inhibits mast cell-mediated allergic inflammation. J Biol Regul Homeost Agents 2013; 27: 955–959.
Acknowledgements
This study was supported in part by the Autism Research Institute, National Autism Association and Theta Biomedical Consulting and Development (Brookline, MA, USA). We thank Dr AK Theoharides for collecting the human normotypic serum samples and Mrs Smaro Panagiotidou for her word processing skills.
DISCLAIMER
TCT is the inventor of US patent No. 8,268,365 covering the diagnosis and treatment of diseases involving brain inflammation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Tsilioni, I., Dodman, N., Petra, A. et al. Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing Bull Terriers with a phenotype similar to autism. Transl Psychiatry 4, e466 (2014). https://doi.org/10.1038/tp.2014.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.106
This article is cited by
-
Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β
Journal of Neuroinflammation (2018)
-
Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities
Translational Psychiatry (2018)
-
αT-catenin in restricted brain cell types and its potential connection to autism
Journal of Molecular Psychiatry (2016)
-
Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders
Translational Psychiatry (2016)
-
Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6
Translational Psychiatry (2015)