Abstract
Impairment of the insulin-degrading enzyme (IDE) is associated with obesity and type 2 diabetes mellitus (T2DM). Here, we used 4-mo-old male C57BL/6 interleukin-6 (IL-6) knockout mice (KO) to investigate the role of this cytokine on IDE expression and activity. IL-6 KO mice displayed lower insulin clearance in the liver and skeletal muscle, compared with wild type (WT), due to reduced IDE expression and activity. We also observed that after 3-h incubation, IL-6, 50 and 100 ng ml−1, increased the expression of IDE in HEPG2 and C2C12 cells, respectively. In addition, during acute exercise, the inhibition of IL-6 prevented an increase in insulin clearance and IDE expression and activity, mainly in the skeletal muscle. Finally, IL-6 and IDE concentrations were significantly increased in plasma from humans, after an acute exercise, compared to pre-exercise values. Although the increase in plasma IDE activity was only marginal, a positive correlation between IL-6 and IDE activity, and between IL-6 and IDE protein expression, was observed. Our outcomes indicate a novel function of IL-6 on the insulin metabolism expanding the possibilities for new potential therapeutic strategies, focused on insulin degradation, for the treatment and/or prevention of diseases related to hyperinsulinemia, such as obesity and T2DM.
Similar content being viewed by others
Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine with several functions in different tissues1. Initially, IL-6 was described as an important factor of the immune system2. However, it has been shown that this cytokine also plays an important role in metabolic regulation3, especially on glucose homeostasis4.
Insulin is one of the most important hormones in glucose homeostasis, and its action depends on its secretion, the sensitivity of target tissues, and clearance. There are several studies regarding the effects of IL-6 on insulin sensitivity5,6 and secretion7,8; however, studies concerning its function on insulin clearance are scarce.
Insulin clearance occurs mainly in the liver, primarily by the action of insulin-degrading enzyme (IDE)9. This enzyme, a 110 kDa zinc-metalloprotease, was initially identified as an insulin degrading enzyme10. Subsequently, it was found that IDE also degrades other amyloidogenic peptides, such as amyloid β11. Impairment of IDE are closely related to the development of diseases, such as type 2 diabetes mellitus (T2DM)12,13 and Alzheimer’s disease (AD)14. Also, it is proposed that IDE malfunction could be the link between these two pathologies15.
Selective modulators of IDE activity could work as potential drugs for treatment of T2DM and AD16. While IDE activators have been proposed as AD therapies17, it is uncertain if activation of this enzyme would be the better therapeutic approach for T2DM. To the contrary, treatment with IDE inhibitors seems to potentiate insulin signaling18 and a fast and short pre-meal IDE inhibition could be useful for T2DM therapy19. However, despite increasing insulin signaling, acute IDE inhibition impaired glucose tolerance in mice, casting a shadow on the usefulness of the IDE inhibition for the treatment of T2DM20. Interestingly, IDE knockout (KO) mice display chronic hyperinsulinemia21 that, over time, induces a reduction of insulin receptor expression, leading to insulin resistance22. Also, downregulation of IDE, associated with hyperinsulinemia, is observed in obese and diabetic patients23,24, and rodents13,25. Therefore, we believe that finding molecules which are able to increase IDE function could be important for the development of new therapeutic strategies against diseases related to hyperinsulinemia, such as obesity and T2DM.
Some interventions25,26,27 and molecules28,29 can modulate the expression and activity of IDE. In this way, physical exercise increases IDE expression in the liver and skeletal muscle of rodents30, and this could explain the augmentation of insulin clearance observed in these rodents and humans31. It is also known that the ciliary neurotrophic factor (CNTF), a member of the IL-6 family of cytokines, can also modulate insulin clearance and IDE expression in the liver of Swiss mice and in HEPG2 cells32. Thus, we hypothesized that IL-6 likewise could have an effect on insulin degradation.
Here, we demonstrate that IL-6 deficient mice displayed reduced insulin clearance, probably due to lower IDE action in the liver and skeletal muscle. In addition, IL-6 incubation increased the expression of IDE in HEPG2 and C2C12 cells. We also found that, during acute endurance exercise, IL-6 mediated the increase of IDE expression and activity, mainly in the skeletal muscle, increasing insulin clearance, a phenomenon that may also occur in humans.
Results
IL-6 KO mice displayed altered metabolic parameters
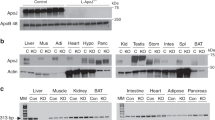
KO mice showed a significant reduction of IL-6 content in plasma, liver, and skeletal muscle, confirming the deficiency of this cytokine in these mice (Suppl Fig. 1). KO mice also displayed a reduction in the body and skeletal muscle weight, despite an augmented adiposity, compared with wild type (WT) mice. In addition, a decreased insulinemia and increased glycemia was observed in the KO group (Table 1).
IL-6 KO mice had impaired glucose, but not insulin, tolerance
IL-6 plays an important role on glucose metabolism4,33. Here, we observed an impaired glucose tolerance in KO mice (Fig. 1A and B), without a change in insulin tolerance (Fig. 1C and D), compared with WT mice. In addition, the Akt phosphorylation in the liver and skeletal muscle (Fig. 1E and F) was similar between KO and WT groups.
Blood glucose (A) and AUC of blood glucose (B) before (0 min) and 15, 30, 60 and 120 min after administration of 1 g kg−1 glucose, n = 6. Blood glucose (C) and AUC of blood glucose (D) before (0 min) and 5, 10, 15, 20, 25, 30 and 60 min after administration of 0.75 U kg−1 insulin, n = 6. Immunoblotting of AKT phosphorylated at serine 473 with (+) or without (−) administration of 10 U insulin per mouse, in the liver (E) and gastrocnemius muscle (F), n = 4. Plasma C-peptide (G), insulin (I) and C-peptide/insulin ratio (K), during ipGTT, and its respective AUCs (H,J and L), n = 4. Insulin secretion in isolated pancreatic islets stimulated with 2.8, 5.8, 11.2 and 22.4 mmol l−1 glucose (M). Total insulin content of islets (N), n = 7. Plasma insulin concentration (O) and AUC of plasma insulin (P), during ipITT, n = 6. WT, wild type mice; and KO, IL-6 knockout mice. Data are presented as the mean ± S.E.M. *p ≤ 0.05 vs WT.
IL-6 KO mice displayed decrease of insulin secretion and clearance
During the ipGTT, KO mice displayed decreased plasma C-peptide concentration (Fig. 1G and H), indicating impairment of insulin secretion. This observation was confirmed by a significant reduction in glucose-stimulated insulin secretion in isolated pancreatic islets from KO mice (Fig. 1M). Interestingly, the insulinemia was not reduced at the same extent than plasma C-peptide, during the ipGTT (Fig. 1I and J). Thus, the C-peptide/insulin ratio was reduced in the KO mice (Fig. 1K and L), indicating that insulin clearance was also decreased. A higher concentration of insulin was registered at 60 min of the ipITT, in the KO mice (Fig. 1O), indicating a reduction of insulin clearance in these mice, although no significantly difference was observed in the area under curve (AUC) of plasma insulin between groups (Fig. 1P).
IL-6 KO mice had reduced IDE expression and activity in the liver and skeletal muscle
IDE is the main enzyme responsible for insulin degradation9. Corroborating the reduced insulin clearance in the KO mice, IDE gene expression (Fig. 2A and B), protein content (Fig. 2C and D), and activity (Fig. 2E–H) were decreased in the liver and skeletal muscle, compared with WT mice.
mRNA expression of IDE in the liver (A) and gastrocnemius muscle (B), n = 6. Protein expression of IDE in the liver (C) and gastrocnemius muscle (D) and its representative immunoblottings images, n = 5. Kinetic of IDE activity assay in liver (E) and in gastrocnemius (F) of mice, n = 5–6. Fluorescent intensity at Ex/Em = 490/520 nm was continuously recorded, every 5 min, during 60 min. 5-FAM concentration was calculated using a standard curve and normalized per μg of total protein. IDE activity was calculated as described27 and normalized per μg of total protein in the liver (G) and gastrocnemius (H) of mice, n = 5–6. WT, wild type mice; and KO, IL-6 knockout mice. Data are presented as the mean ± S.E.M. *p ≤ 0.05 vs WT.
IL-6 increased IDE expression in HEPG2 and C2C12 cells
In the following set of experiments, HEPG2 and C2C12 cells were incubated, for 3-h, at different concentrations of IL-6. The IDE protein content was significantly increased in HEPG2 and C2C12 cells, at 50 and 100 ng ml−1 IL-6, respectively (Fig. 3A and B).
IL-6 inhibition impaired the increase on insulin clearance in mice, after an acute endurance exercise
Since a substantial amount of IL-6 is released in the plasma during skeletal muscle contractions (Supple Fig. 2), we investigated the role of this cytokine on insulin clearance, after acute exercise. During ipITT, we observed that inhibition of IL-6 increased insulin tolerance in EXE + TCZ (exercised mice treated with tocilizumab, a pharmacological IL-6 receptor neutralizing antibody), and this increase was bigger than in the exercised (EXE) mice, as judged by the AUC of blood glucose (Fig. 4A and B). Interestingly, the higher insulin tolerance was accompanied by higher levels of plasma insulin during the ipITT, indicating a lower insulin clearance in the EXE + TCZ mice, compared with EXE mice (Fig. 4C and D).
Blood glucose (A) and AUC of blood glucose (B) before and 5, 10, 15, 20, 25, 30 and 60 min after administration of 0.75 U kg−1 insulin. Plasma insulin (C) and AUC of plasma insulin (D) at 0, 5, 15, 30 and 60 min during ipITT. CTL, control mice; EXE, exercised mice; and EXE + TCZ, exercised mice treated with 2 mg kg−1 Tocilizumab 1-h before the exercise protocol. Data are presented as the mean ± S.E.M. n = 6. *p ≤ 0.05 vs CTL and #p ≤ 0.05 vs EXE.
IL-6 inhibition impaired IDE expression and activity in the skeletal muscle of mice, after an acute endurance exercise
We also evaluated the IDE expression and activity in exercised mice, treated with an IL-6 inhibitor. Corroborating the insulin clearance data, a single bout of exercise increased IDE gene expression in the liver and skeletal muscle (Fig. 5A and B), but IDE protein expression increased only in the skeletal muscle of EXE mice, compared with CTL mice (Fig. 5C and D). No alteration was found in IDE activity, after acute exercise (Fig. 5E–H). Interestingly, IL-6 inhibition prevents an increase in the expression of the IDE gene in liver and skeletal muscle (Fig. 5A and B), and IDE protein expression only in the skeletal muscle (Fig. 5C and D). We also observed reduced IDE activity in the skeletal muscle of EXE + TCZ mice, compared with EXE mice (Fig. 5F and H).
mRNA expression of IDE in the liver (A) and gastrocnemius muscle (B), n = 5–6. Protein expression of IDE in the liver (C) and gastrocnemius muscle (D) and its representative immunoblottings images, n = 5–6. Kinetic of IDE activity assay in liver (E) and gastrocnemius (F), n = 5. Fluorescent intensity at Ex/Em = 490/520 nm was continuously recorded, every 5 min, during 60 min. 5-FAM concentration was calculated using a standard curve and normalized per μg of total protein. IDE activity was calculated as described27 and normalized per μg of total protein in the liver (G) and gastrocnemius (H) of mice, n = 5. CTL, control mice; EXE, exercised mice; and EXE + TCZ, exercised mice treated with 2 mg kg−1 Tocilizumab 1-h before the acute exercise protocol. Data are presented as the mean ± S.E.M. *p ≤ 0.05 vs CTL and #p ≤ 0.05 vs EXE.
IL-6 was positively correlated with IDE protein expression and activity in the plasma of humans subjects, after an acute endurance exercise
Finally, we measured IL-6 concentration, IDE protein expression and activity in the plasma of human subjects, before and after acute exercise. Plasma IL-6 and IDE concentrations were significantly increased post-exercise, compared with pre-exercise group (Fig. 6A and B), and a positive correlation between these two plasmatic parameters was confirmed (Fig. 6E). Although the increase of IDE activity, in the post-exercise group, was not statistically significant compared with pre-exercise (Fig. 6C and D), this plasmatic parameter was also positively correlated with IL-6 concentration (Fig. 6F).
Plasma IL-6 concentration (A), n = 5. IDE protein expression and its representative immunoblotting image (B), n = 5. Kinetic of IDE activity assay in the plasma (C), n = 5. Fluorescent intensity at Ex/Em = 490/520 nm was continuously recorded, every 5 min, during 60 min. 5-FAM concentration was calculated using a standard curve and normalized per μg of total protein. Total IDE activity (D) was calculated as described27 and normalized per μg of total protein, n = 5. Data represent the mean ± S.E.M. *p ≤ 0.05 vs PRE-EXE. Correlation between plasma IL-6 concentration and IDE protein expression (E) or IDE activity (F), n = 10. The Pearson product moment correlation coefficient (r) was determined using the software GraphPad Prism 5. PRE-EXE, plasma samples of human subjects before acute endurance exercise; and POST-EXE, plasma samples of human subjects 3-h after acute exercise (30-min cycling at 70% of VO2peak).
Discussion
Impaired IDE expression and/or activity are closely related to diseases associated with hyperinsulinemia, such as obesity and T2DM12. Therefore, increasing the function of IDE could be a strategy for the treatment of these pathologies. Here, we found that exposure to IL-6 increased IDE protein expression in HEPG2 and C2C12 cells. We also observed that IL-6 KO mice displayed reduced insulin clearance, probably due to lower IDE function in the liver and skeletal muscle. Finally, we demonstrated that during acute exercise, IL-6 release seems to be necessary to increase IDE expression and activity, mainly in the skeletal muscle of mice, an effect that likely occurs also in humans.
IL-6 is an interesting cytokine with a dual effect on glucose homeostasis. Initially, it was thought that this cytokine has deleterious effects on glucose homeostasis by impairing the insulin signaling, leading to insulin resistance6. Later, it was found that the loss of IL-6, not only failed to protect against these deleterious effects, but also induced glucose intolerance leading to development of mature-onset obesity34. These data show that IL-6 is important for the maintenance of normal carbohydrate and lipid metabolism. Indeed, here, 4-mo-old IL-6 KO mice developed glucose intolerance (Fig. 1A and B) and increased fat pad weight (Table 1), although impairment in insulin signaling and overweight were not observed. These last alterations were registered in older (9-mo-old) IL-6 deficient mice34. Although young IL-6 KO mice do not have impaired insulin sensitivity (Fig. 1C and D), they display reduced glucose-stimulated insulin secretion (Fig. 1M), which could explain the glucose intolerance during ipGTT. These data suggest that the impairment of insulin secretion and/or clearance precede the onset of insulin resistance in IL-6 KO mice.
The role of IL-6 on insulin secretion has been extensively studied, and there is substantial evidence that this cytokine increases insulin secretion7,8, and this increase seems to depend on the activation of the phospholipase C (PLC) - inositol triphosphate (IP3) dependent pathway8. However, further investigation demonstrated that IL-6 enhances insulin release also by increasing the glucagon-like peptide 1 (GLP-1) secretion from L cells and pancreatic α cells7.
While several studies have investigated the function of IL-6 on insulin secretion, its participation on insulin clearance was neglected. Here, we provide evidence that IL-6 must be essential to enhance insulin clearance probably by increasing IDE expression and activity in the liver and skeletal muscle. These are opposite effects from those reported for CNTF, a member of the IL-6 family of cytokines32. Although these two cytokines act through activation of glycoprotein 130 (gp 130), there are some peculiarities in their receptors35 that could explain these different effects on insulin clearance and IDE expression.
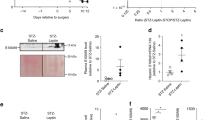
It is known that increase of adiposity is associated with decrease of IDE expression and insulin clearance, as observed in obesity25,36. Here, we show that IL-6 KO mice had an increased fat pad weight which could explain the reduced IDE expression and activity in these mice. However, to prove that this IDE modulation is an effect of IL-6 loss, we treated this KO mice with 200 ng IL-6 during 3 days before the experiments. Interestingly, IL-6 treatment restored IDE gene expression of KO mice, without any alteration in the fat pad weight (see Supple Fig. 3). These data, associated with the results from in vitro experiments, confirm the important role of IL-6 on IDE modulation.
During physical exercise, muscle contractions release and increase the concentration of IL-6 in the bloodstream, and this myokine37 modulates glucose homeostasis. Previous data suggest that the primary function of IL-6 during exercise is to maintain glucose turnover4. At the same time that IL-6 favors insulin to stimulate glucose uptake by the skeletal muscle38, it increases glucose production in the liver39 to sustain the high demand for glucose by the former tissue. Although controversial40, it has been reported that, during exercise, this myokine could improve insulin sensitivity41. However IL-6 has been found to increase insulin secretion after exercise7 whereas a reduction in plasma insulin was observed42. Here we proposed that IL-6 could also increase insulin clearance and IDE expression, induced by exercise, as reported30. Interestingly, we confirmed our hypothesis showing that inhibition of IL-6 signaling during acute endurance exercise blocks the augmentation of insulin clearance and IDE expression, mainly in the skeletal muscle. This phenomenon seems to be important to avoid a hazardous decrease of glycemia during exercise, making it more effective and safe.
Physical exercise is one of the first clinical approaches to prevent and treat metabolic diseases, such as obesity and T2DM. However, adherence to physical activity by obese and diabetic patients is very low43. Thus, finding alternatives for physical exercise would be useful for the prevention and treatment of these diseases. In this scenario, IL-6 has been suggested as a promising candidate44. Here, we found that, as in mice, IL-6 seemed to have the same effects on insulin clearance in humans after acute endurance exercise, since the concentration of this myokine positively correlated with IDE expression in the plasma of these subjects. Although considering IL-6 as a promising alternative to physical exercise, treatment with this cytokine may be not recommended due to its dual effects on glucose metabolism. Thus, find the mechanism activated by IL-6 during exercise, could help us to develope a safer exercise mimetic.
IL-6 may act on its target cells by canonical and non-canonical pathways. The canonical pathway is well-established and comprises the activation of the signal transducer and activator of transcription 3 (STAT3), which, in the nucleus, enhances the transcription of several genes45,46. The non-canonical pathway refers to the activation of the 5′-adenosine monophosphate-activated protein kinase (AMPK). Even though the non-canonical pathway remains poorly understood, it was reported that IL-6 directly activates AMPK through increasing cyclic adenosine monophosphate (cAMP) and AMP/ATP ratio in skeletal muscle of rats47. Since IL-6 KO mice show reduced activation of AMPK48, we speculate that this non-canonical pathway could be the mechanism whereby IL-6 increases IDE expression. Moreover, we previously demonstrated that the treatment with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) increased IDE expression in C2C12 cells30, corroborating our hypothesis. However, this data does not exclude the possible role of STAT3 on IDE expression. Therefore, further investigations are necessary to clarify this matter.
In summary, here we described a novel function of IL-6 on glucose homeostasis, which consists of increase insulin clearance, probably due to augmentation of IDE expression and activity in the liver and skeletal muscle. In addition, we demonstrated that, after acute endurance exercise, IL-6 mediated the increase of IDE, mainly in the skeletal muscle of mice, an effect that seems to occur also in humans. Our findings expand the possibilities for new potential therapeutic strategies, focused on insulin degradation, that could be used in the treatment and/or prevention of diseases related to hyperinsulinemia, such as obesity and T2DM.
Methods
Animals
The 4-mo-old males C57BL/6 wild type (WT) and IL-6 knockout (KO) mice were maintained on a 12-h light/12-h dark cycle with controlled temperature and humidity during the entire experiment. All mice were allowed to feed (standard chow diet) and drink tap water ad libitum. After the experiments using WT and KO mice, other males C57BL/6 WT mice were distributed into three different groups: control (CTL), exercised (EXE) and exercised mice treated with 2 mg kg−1 tocilizumab (a pharmacological IL-6 receptor neutralizing antibody) 1-h before the exercise protocol (EXE + TCZ).
Maximum oxygen consumption (VO2 max) and acute endurance exercise protocol in mice
Before the acute exercise protocol, we measured the VO2 max in all mice from the CTL, EXE and EXE + TCZ groups, as described before30. After 10 days, mice from EXE and EXE + TCZ groups were submitted to a single bout of exercise on a treadmill inclined at 25° for 3-h at 60–70% of VO2 max. All measurements made in these mice were performed immediately after the acute exercise.
Intraperitoneal glucose and insulin tolerance tests (ipGTT and ipITT)
The ipGTT and ipITT was performed as previously described27. The blood glucose concentration was measured by tail bleed using glucose strips on Accu-Chek Performa II glucometer (Roche, Sao Paulo, Brazil). During the ipGTT, blood samples were collected from the tail before (0 min) and 15 and 30 min after glucose administration. The plasma samples was obtained by centrifugation (1100 g for 15 min at 4 °C) and was stored at −80 °C for posterior insulin and C-peptide quantification.
In vivo insulin clearance test
During the ipITT, blood samples were collected before (0 min) and 15, 30 and 60 min after insulin administration to determine the plasma insulin concentrations25.
Plasma C-peptide and insulin concentration
Plasma C-peptide was measured by Rat/Mouse C-Peptide 2 ELISA Kit (Cat. EZRMCP2-21K, EMD Millipore), following the manufacturer’s instructions, and plasma insulin concentration was determined by radioimmunoassay (RIA), as described49.
Tissue samples
The mice were killed in a CO2-saturated chamber followed by beheading. Liver and gastrocnemius skeletal muscle samples from the mice were collected, snap-frozen in liquid nitrogen, and stored at −80 °C for subsequent experiments (RT-PCR Real time, Western blot and IDE activity). The pancreatic islets isolation was performed as previously described50.
Glucose-stimulated insulin secretion in pancreatic islets
The islets were pre-incubated for 1-h in Krebs-Henseleit buffer solution (KHBS) containing 0.5 g l−1 bovine serum albumin (BSA) and 5.6 mmol l−1 glucose (95% O2, 5% CO2, pH 7.4, 37 °C). Subsequently, we incubate five islets per well for an additional hour in the KHBS containing 2.8, 5.6, 11.2 or 22.4 mmol l−1 glucose. The supernatant was collected to evaluate the insulin secretion, and the islets were homogenized in an alcohol/acid solution to measure the total insulin content by RIA.
Real time RT- PCR
Tissues samples of liver and skeletal muscle was homogenized in 1 ml TRIzol® Reagent (Cat. 15596026, Invitrogen™, Thermo Fisher Scientific Inc, Waltham, MA, USA), and the total mRNA was extracted following the manufacturer’s instructions. To prepare the cDNA, we used 1 μg of total mRNA and High-Capacity cDNA Reverse Transcription Kit (Cat. 4368814, Applied Biosystems™, Thermo Fisher Scientific Inc, Waltham, MA, USA). Real time PCR was performed on 7500 Fast Real-time PCR System (Applied Biosystems™) using Fast SYBR® Green Master Mix (Cat. 4385612, Applied Biosystems™). Mice IL-6 and IDE gene expression was measured and GAPDH was used as housekeeping gene (IL-6 forward 5′-CACGGCCTTCCCTACTTCAC-3′ and reverse 5′-GGTCTGTTGGGAGTGGTATC-3′; IDE forward 5′-CTGTGCCCCTTGTTTGATGC-3′ and reverse 5′-GTTCCCCGTAGCCTTTTCCA-3′; GAPDH forward 5′-CCTGCACCACCAACTGCTTA-3′ and reverse 5′-GCCCCACGGCCATCACGCCA-3′). Real-time PCR data were analyzed using the 7500 Software version 2.0.5 (Applied Biosystems™).
Western Blot
For protein extraction, tissue samples of liver and skeletal muscle, were homogenized in a lysis buffer containing 10 mmol l−1 EDTA, 100 mmol l−1 Tris base, 100 mmol l−1 sodium pyrophosphate, 100 mmol l−1 sodium fluoride, 10 mmol l−1 sodium orthovanadate, 2 mmol l−1 Phenylmethylsulfonyl fluoride, 1% Triton X-100 and 1 μg ml−1 aprotinin. Protein concentration of the samples was determined using Bradford reagent (Cat. 500-0006 N, BioAgency Biotecnologia, São Paulo, Brazil). After, 30 μg protein samples were homogenized and boiled (5 min at 100 °C) in a Laemmli buffer, applied on 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and transferred to nitrocellulose membranes. These membranes were blocked in a Tris-buffered saline (10 mmol l−1 Tris base, 150 mmol l−1 NaCl and 0.25% (vol./vol.) of Tween 20) containing 5% (wt./vol.) BSA for 1-h at room temperature. After, the membranes were incubated overnight at 4 °C with primary antibodies (anti-phospho-Aktser473, Santa Cruz Biotechnology cat. sc-7985; anti-IDE, Abcam cat. ab32216; anti-GAPDH, Sigma cat. G9545). Bands detection was performed by chemiluminescence (SuperSignal West Fento, Pierce Biotechnology Inc., Rockford, IL, USA) after incubation with an appropriated horseradish peroxidase-conjugated secondary antibody, and the bands were visualized using the Amersham Imager 600 (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Band intensities were analyzed using ImageJ software (National Institutes of Health, Maryland, USA).
HEPG2 and C2C12 cells culture
HEPG2 (a human liver carcinoma cell line) and C2C12 (a mouse myoblast cell line) cells were culture as described before30. For the differentiation of C2C12 cells, we used DMEM high glucose containing 2% (vol./vol.) horse serum, and we culture it in this medium for 5 days. HEPG2 and differentiated C2C12 cells were incubated at 0, 10, 50 or 100 ng ml−1 IL-6 (Cat. PMC0061, Gibco™, Thermo Fisher Scientific Inc, Waltham, MA, USA) for 3-h. The cells were collected in trypsin/EDTA solution, washed with phosphate-buffered saline (PBS), and then homogenized in a urea anti-protease/anti-phosphatase buffer, for subsequent analysis of IDE protein expression.
Plasma samples of human subjects
Plasma samples of human subjects were obtained from a previous study, and for all detailed information about this subjects (untrained healthy male) and experimental procedures, see ref. 51. The exercise protocol comprise a standardized warm-up period following by 30-min of cycling, at 70% of VO2peak. Blood samples were collected before (PRE-EXE) and 3-h after (POST-EXE) the exercise session for posterior analysis of IL-6 concentration, IDE protein expression and activity.
IDE activity assay
IDE activity, from tissues and plasma samples, was measured by SensoLyte® 520 IDE Activity Assay Kit (Cat. AS-72231, AnaSpec, Fremont, CA, USA), following the manufacturer’s recommendations. The total IDE activity was calculated as previously described27, using this equation:

A1 is the concentration of 5-FAM at 60 min and A0 at 0 min; T is the total time of the assay (60 min); V is the volume of samples, and D is the dilution. The 5-carboxyfluorescein (5-FAM, the product of the enzyme reaction) concentration and the total IDE activity were normalized per μg of total protein, which was determined using Bradford reagent.
Quantification of plasma IL-6 concentration
Plasma IL-6 concentrations were measured using Mouse IL-6 ELISA Kit (Cat. EZMIL6, Merck Millipore, Darmstadt, Germany) and Human IL-6 Quantikine® ELISA Kit (Cat. D6050, R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Statistics
For the statistical analysis of two groups we used the Student’s t-test and for three or more groups, we performed the one-way ANOVA using the unpaired Tukey’s post-hoc test. The data are presented as the mean ± standard error mean (SEM) and all data were considered significantly different if p ≤ 0.05. The Pearson product moment correlation coefficient (r) was determined to examine the relationship between IL-6 concentration and IDE protein expression or activity in the plasma of human subjects.
Study approval
All experimental procedures made in mice and humans subjects were approved by the local ethics committee (approval numbers 3087-1 and 848.145). The animal experiments were conducted following to the last revision of the National Institutes of Health (NIH) guide for the care and use of laboratory animals. In the case of humans, the experimental procedures were explained to all subjects, who provided written informed consent before the study that was conducted according to the latest revision of the Declaration of Helsinki51.
Additional Information
How to cite this article: Kurauti, M. A. et al. Interleukin-6 increases the expression and activity of insulin-degrading enzyme. Sci. Rep. 7, 46750; doi: 10.1038/srep46750 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kishimoto, T. IL-6: from its discovery to clinical applications. Int Immunol 22, 347–352, doi: 10.1093/intimm/dxq030 (2010).
Hirano, T. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76, doi: 10.1038/324073a0 (1986).
Pal, M., Febbraio, M. A. & Whitham, M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol 92, 331–339, doi: 10.1038/icb.2014.16 (2014).
Febbraio, M. A., Hiscock, N., Sacchetti, M., Fischer, C. P. & Pedersen, B. K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643–1648 (2004).
Nieto-Vazquez, I., Fernández-Veledo, S., de Alvaro, C. & Lorenzo, M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes 57, 3211–3221, doi: 10.2337/db07-1062 (2008).
Senn, J. J., Klover, P. J., Nowak, I. A. & Mooney, R. A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51, 3391–3399 (2002).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17, 1481–1489, doi: 10.1038/nm.2513 (2011).
Suzuki, T. et al. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes 60, 537–547, doi: 10.2337/db10-0796 (2011).
Duckworth, W. C., Bennett, R. G. & Hamel, F. G. Insulin degradation: progress and potential. Endocrine reviews 19, 608–624 (1998).
Tomizawa, H. H. & Halsey, Y. D. Isolation of an insulin-degrading enzyme from beef liver. J Biol Chem 234, 307–310 (1959).
Farris, W. et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo . Proc Natl Acad Sci USA 100, 4162–4167, doi: 10.1073/pnas.0230450100 (2003).
Karamohamed, S. et al. Polymorphisms in the insulin-degrading enzyme gene are associated with type 2 diabetes in men from the NHLBI Framingham Heart Study. Diabetes 52, 1562–1567 (2003).
Fakhrai-Rad, H. et al. Insulin-degrading enzyme identified as a candidate diabetes susceptibility gene in GK rats. Hum Mol Genet 9, 2149–2158 (2000).
Bertram, L. et al. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science 290, 2302–2303, doi: 10.1126/science.290.5500.2302 (2000).
Mittal, K., Mani, R. J. & Katare, D. P. Type 3 Diabetes: Cross Talk between Differentially Regulated Proteins of Type 2 Diabetes Mellitus and Alzheimer’s Disease. Sci Rep 6, 25589, doi: 10.1038/srep25589 (2016).
Pivovarova, O., Höhn, A., Grune, T., Pfeiffer, A. F. & Rudovich, N. Insulin-degrading enzyme: new therapeutic target for diabetes and Alzheimer’s disease? Ann Med, 1–11, doi: 10.1080/07853890.2016.1197416 (2016).
Leissring, M. A. et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003).
Leissring, M. A. et al. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS One 5, e10504, doi: 10.1371/journal.pone.0010504 (2010).
Maianti, J. P. et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature 511, 94–98, doi: 10.1038/nature13297 (2014).
Deprez-Poulain, R. et al. Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice. Nat Commun 6, 8250, doi: 10.1038/ncomms9250 (2015).
Abdul-Hay, S. O. et al. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One 6, e20818, doi: 10.1371/journal.pone.0020818 (2011).
Kanety, H., Moshe, S., Shafrir, E., Lunenfeld, B. & Karasik, A. Hyperinsulinemia induces a reversible impairment in insulin receptor function leading to diabetes in the sand rat model of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA 91, 1853–1857 (1994).
Fawcett, J., Sang, H., Permana, P. A., Levy, J. L. & Duckworth, W. C. Insulin metabolism in human adipocytes from subcutaneous and visceral depots. Biochem Biophys Res Commun 402, 762–766, doi: 10.1016/j.bbrc.2010.10.104 (2010).
Sun, J. et al. Serum insulin degrading enzyme level and other factors in type 2 diabetic patients with mild cognitive impairment. Curr Alzheimer Res(2016).
Brandimarti, P. et al. Cafeteria diet inhibits insulin clearance by reduced insulin-degrading enzyme expression and mRNA splicing. J Endocrinol 219, 173–182, doi: 10.1530/JOE-13-0177 (2013).
Castell-Auví, A. et al. The effects of a cafeteria diet on insulin production and clearance in rats. Br J Nutr 108, 1155–1162, doi: 10.1017/S0007114511006623 (2012).
Kurauti, M. A. et al. Acute exercise restores insulin clearance in diet-induced obese mice. J Endocrinol, doi: 10.1530/JOE-15-0483 (2016).
Wei, X. et al. Regulation of insulin degrading enzyme activity by obesity-associated factors and pioglitazone in liver of diet-induced obese mice. PLoS One 9, e95399, doi: 10.1371/journal.pone.0095399 (2014).
Hamel, F. G., Upward, J. L. & Bennett, R. G. In vitro inhibition of insulin-degrading enzyme by long-chain fatty acids and their coenzyme A thioesters. Endocrinology 144, 2404–2408 (2003).
Kurauti, M. A. et al. Acute Exercise Improves Insulin Clearance and Increases the Expression of Insulin-Degrading Enzyme in the Liver and Skeletal Muscle of Swiss Mice. PLoS One 11, e0160239, doi: 10.1371/journal.pone.0160239 (2016).
Tuominen, J. A., Ebeling, P. & Koivisto, V. A. Exercise increases insulin clearance in healthy man and insulin-dependent diabetes mellitus patients. Clin Physiol 17, 19–30 (1997).
Rezende, L. F., Santos, G. J., Santos-Silva, J. C., Carneiro, E. M. & Boschero, A. C. Ciliary neurotrophic factor (CNTF) protects non-obese Swiss mice against type 2 diabetes by increasing beta cell mass and reducing insulin clearance. Diabetologia 55, 1495–1504, doi: 10.1007/s00125-012-2493-5 (2012).
Glund, S. et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56, 1630–1637, doi: 10.2337/db06-1733 (2007).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8, 75–79, doi: 10.1038/nm0102-75 (2002).
Kraakman, M. J. et al. Targeting gp130 to prevent inflammation and promote insulin action. Diabetes Obes Metab 15 Suppl 3, 170–175, doi: 10.1111/dom.12170 (2013).
Erdmann, J. et al. Weight-dependent differential contribution of insulin secretion and clearance to hyperinsulinemia of obesity. Regul Pept 152, 1–7, doi: 10.1016/j.regpep.2008.10.008 (2009).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8, 457–465, doi: 10.1038/nrendo.2012.49 (2012).
Carey, A. L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697, doi: 10.2337/db05-1404 (2006).
Blumberg, D., Hochwald, S., Brennan, M. F. & Burt, M. Interleukin-6 stimulates gluconeogenesis in primary cultures of rat hepatocytes. Metabolism 44, 145–146 (1995).
Mooney, R. A. Counterpoint: Interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol (1985) 102, 816–818, discussion 818-819, doi: 10.1152/japplphysiol.01208a.2006 (2007).
Benrick, A., Wallenius, V. & Asterholm, I. W. Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol 97, 1224–1235, doi: 10.1113/expphysiol.2012.065508 (2012).
Wolsk, E., Mygind, H., Grøndahl, T. S., Pedersen, B. K. & van Hall, G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab 299, E832–840, doi: 10.1152/ajpendo.00328.2010 (2010).
Qiu, S. H., Sun, Z. L., Cai, X., Liu, L. & Yang, B. Improving patients’ adherence to physical activity in diabetes mellitus: a review. Diabetes Metab J 36, 1–5, doi: 10.4093/dmj.2012.36.1.1 (2012).
Paula, F. M. et al. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J 29, 1805–1816, doi: 10.1096/fj.14-264820 (2015).
Heinrich, P. C., Behrmann, I., Müller-Newen, G., Schaper, F. & Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334(Pt 2), 297–314 (1998).
Darnell, J. E. STATs and gene regulation. Science 277, 1630–1635 (1997).
Kelly, M., Gauthier, M. S., Saha, A. K. & Ruderman, N. B. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58, 1953–1960, doi: 10.2337/db08-1293 (2009).
Kelly, M. et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 320, 449–454, doi: 10.1016/j.bbrc.2004.05.188 (2004).
Rezende, L. F. et al. Ciliary neurotrophic factor promotes survival of neonatal rat islets via the BCL-2 anti-apoptotic pathway. J Endocrinol 195, 157–165, doi: 10.1677/JOE-07-0016 (2007).
Boschero, A. C. et al. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol 268, E336–342 (1995).
Conceição, M. S. et al. Attenuated PGC-1α Isoforms following Endurance Exercise with Blood Flow Restriction. Med Sci Sports Exerc 48, 1699–1707, doi: 10.1249/MSS.0000000000000970 (2016).
Acknowledgements
The authors thank Marise M. C. Brunelli, Jheynifer C. Souza, Monica P. Naru and Bil for technical assistance, and Bridgett A. Bollin for providing language help. This study was supported by the Fundação de Amparo e Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2014/24719-7 and 2015/12611-0). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.A.K., J.M.C.J. and L.F.R. contributed to research design and M.A.K., J.M.C.J., S.M.F., G.J.S., C.H.G.S., G.D.T., M.P.T.C.M. and C.R.C. conducted the experiments and acquired data. A.C.B. and E.M.C. provided all reagents. M.A.K. contributed to data analysis and M.A.K. and J.M.C.J. to data interpretation. M.A.K., J.M.C.J. and A.C.B. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kurauti, M., Costa-Júnior, J., Ferreira, S. et al. Interleukin-6 increases the expression and activity of insulin-degrading enzyme. Sci Rep 7, 46750 (2017). https://doi.org/10.1038/srep46750
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46750
This article is cited by
-
Diabetes-induced male infertility: potential mechanisms and treatment options
Molecular Medicine (2024)
-
Causal effect of interleukin (IL)-6 on blood pressure and hypertension: A mendelian randomization study
Immunogenetics (2024)
-
Diabetes as a potential compounding factor in COVID-19-mediated male subfertility
Cell & Bioscience (2022)
-
Whey protein sweetened with Stevia rebaudiana increases insulin-degrading enzyme, but not carcinoembryonic antigen-related cell adhesion molecule 1 expression in the liver from resistance-trained rats
Nutrire (2022)
-
Sirt3 deficiency induced down regulation of insulin degrading enzyme in comorbid Alzheimer’s disease with metabolic syndrome
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.