Abstract
To explore dendritic cells/tumor-derived endothelial cells (DC/EC) fusion cells are potent stimulators of T cells to impact tumor progression. ECs were isolated from mice hepatoma cell line (H22) Xenograft, and dendritic cells were isolated from bone marrow of BALB/c mice, then the isolated ECs were cultured and detected the endothelial surface expression of CD105 by flow cytometry. The endothelial characteristics of ECs were detected by tube formation assay and Dil-Ac-LDL uptake assay. After the fusion with polyethylene glycol (PEG), we used DCs, ECs, DCs mixed ECs as the control groups, DC/EC fusion cells as the experimental group, Secretion of IFN-α and IFN-γ was evaluated, T lymphocyte proliferation and cytotoxic T lymphocytes (CTL) were detected in vitro. In vivo, T lymphocyte induced by five groups was injected to detect the effect of tumor progression. Purified ECs (CD105+) took the function of endothelial cells, then successfully fused with DCs. The DC/EC fusion cells were functional in stimulating the proliferation of T cells, which produced IFN-α and IFN-γ. In vivo, T cells stimulated by DC/EC fusion cells effectively repressed tumor growth. The fusion cells, which was capable of stimulating T cells, is indispensable for antitumor immunity.
Similar content being viewed by others
Introduction
Dendritic Cells (DCs) are regarded as antigen-presenting cells, which has the capacity of eliciting primary immune responses1,2. DCs collect and dispose the antigens into peptides, then DCs present peptides in MHC classes I and II and costimulatory molecules for identification by T cells1,3,4.
Angiogenesis plays a significant role in promoting tumor progression. Tumor development beyond 1–2 mm is dependent on the formation of a functional blood supply system for nutrient delivery. Based on previous studies involving established cell lines or vessels, the blood vessels of tumor and those of normal tissues differ in permeability, composition of the basement membrane, extracellular matrix and cellular composition5,6,7. When compared to normal blood vessels, tumor vessels are tortuous, poor organizational characteristics, high permeability and inclined to leaky take the macromolecules of tumor microenvironment to blood circulation8,9. Normal vascular endothelial cells derived from embryos, while about 50% to 60% of tumor vascular endothelial cells derived from tumor stem cells, coexpress the specific antigens of tumor cells10,11. Tumor vascular endothelial cells can split proliferation continuously, however normal vascular endothelial cells could not12. CD105 has been suggested to be the most suitable marker available to quantify tumor angiogenesis, which was observed in tissues undergoing active angiogenesis, whereas absent in blood vessels within normal tissues13. In this study, the tumor vascular endothelial cells we selected all express CD105. Thus, it plays an essential role of anti-cancer therapy by targeting tumor endothelial cells.
Previously strategy is the fusion of DCs and tumor cells14,15,16. In this approach, tumor-derived endothelial Ags are delivered to DCs. Such as APCs, the fusion cells (DC/EC) function with the ability to migrate to draining lymph nodes, where they interact with T cells and induce effective antitumor immunity17,18,19. These results make clear that tumor-derived endothelial Ag presentation targeting activation of T cells is necessary in antitumor immunity.
Results
Purification and characterization of CD105+ cells
CD105+ cells were purified from H22 Xenografts by magnetic activated cell sorting (MACS)20, CD105+ and CD105− cells were separated from the single cell suspensions and analyzed the expression of CD105 by flow cytometry. The fractions of CD105 expression cells in CD105+ cells or CD105− cells of tumor Xenograft were 97.6 ± 1.4% and 0.3 ± 0.1%. These results indicate an excellent enrichment of CD105+ subpopulations by the magnetic cell separation (Fig. 1a). To compare the endothelial cell function of CD105+ cells with that of CD105− cells, tube formation assay and Dil-Ac-LDL uptake assay were detected. In Fig. 1b and c, CD105+ cells from tumor tissue showed Dil-Ac-LDL up-take and formation of endothelial tubes, respectively. These results suggested that CD105+ cells took of endothelial cell function.
(a) Flow cytometry plot data obtained using CD105+ antibody to quantify endothelial cells. (b) CD105+ cells take up acetylated LDL compared to CD105− cells and H22 cell lines. (c) CD105+ cells form capillary-like networks on Matrigel compared to CD105− cells and H22 cell lines. Scale bars = 100 μm in (b), =200 μm in c. ***P < 0.001.
Characterization of the DC/EC fusion cells
Immunofluorescence was used to assess efficiency of the fusions. In Fig. 2a, endothelial cells dyed CFSE, DCs dyed PKH26 and the fusion cells (DC/EC) expressed both of fluorescence. These findings demonstrate the formation of heterokaryons by fusing endothelial cells to DC successfully.
(a) ECs were stained with CFSE (Green), DCs were stained with PKH26 (Red) and the nuclear were stained with DAPI (Blue). Scale bars = 100 μm. (b) Cultured with DC only, EC only, DC mixed with EC, or DC/EC fusion, then the stimulated T cells were incubated with PKH-26-labled target ECs at the indicated effector-to-target cell (10:1, 20:1, 30:1), and then detected for lysis. (c) The proliferation of T cells was measured after cultured with DC only, EC only, DC mixed with EC, or DC/EC fusion. (d) The IFN-α and IFN-γ production of T cells were analyzed by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001.
Induction of endothelial/Fusion Cells (FCs)-specific CTL responses by four types of cell preparations
To investigate the Ag-specific CTL induction capacity by four types of cell preparations, cytotoxicity assays were performed. Incubation of the endothelial/FCs-stimulated T cells demonstrated selective lysis of the tumor endothelium, while there was no significant lysis of the DC only, EC only, DC mixed with EC (Fig. 2b). These results indicate that T cells stimulated by DC/EC fusion cells had selective lysis of tumor endothelium.
Stimulation of T cell proliferation compromised in fusion cells
To determine the ability of fusion cells about stimulating T cell proliferation, flow cytometry (Beckman Coulter Epics XL, USA) was applied. T cells co-cultured with DC/EC fusion cells proliferated vigorously, co-cultured of these T cells with DC only, EC only, DC mixed with EC resulted in proliferation of T cells, however, at a lower level (Fig. 2c).
IFN-α and IFN-γ production of T cells by four types of cell preparations
To compare the activation of T cells by four types of cell preparations, we detected IFN-α and IFN-γ production by ELISA. The group of DC/EC fusion cells were superior in producing IFN-α and IFN-γ in T cells. In contrast, however, there was little, if any, IFN-α and IFN-γ production in T cells co-cultured with cell suspensions of DC only, EC only, DC mixed with EC (Fig. 2d).
DC/EC fusion cells induce antitumor activity
To assess the induction of antitumor immunity, mice were immunized with irradiated DC, EC, DC mixed with EC or DC/EC fusion cells and then challenged intravenously with 2 × 105 viable H22 cells. In Fig. 3a, immunization of DC/EC fusion cells significantly decreased the tumorigenicity of H22 in BALB/c mice. The tumor volume and weight of the DC/EC fusion cells group was significantly decreased compare to the PBS group (Fig. 3b and c). These findings indicate that T cells induced by DC/EC fusion cells is effective in anti-tumor activity.
Cell proliferation, endothelial cell expression and cell apoptosis in H22 Xenograft
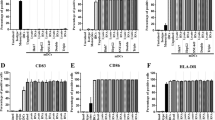
In Fig. 4a, the result showed that there was a significantly increase in apoptosis in DC/EC fusion groups when compared to other groups. We explored the function of activated T cells by immunization, which was confirmed by IHC staining using Ki-67 and CD105 antibody (Fig. 4b and c). T cells induced by DC/EC fusion cells led to low level of Ki-67 protein and CD105 protein expression compared to that by mixed with DC and EC, DC only, EC only or PBS. Importantly, we found no toxicity in heart, lung, liver or kidney tissues of mice injected with T cells induced by immunization (Fig. 4d). These data indicate that T cells induced by DC/EC fusion cells can enhance antitumor immunity.
(a) TUNEL expression in tumor tissues and quantitation of its expression. (b) Ki-67 expression in tumor tissues and quantitation of Ki-67 expression. (c) CD105 expression in tumor tissues. (d) HE staining of mice organs to determine toxicity of tissues. Scale bars = 100 μm in a, = 50 μm in b and d, = 200 μm in (c). **P < 0.01, ***P < 0.001.
Discussion
During the development of immune system, body can produce immune tolerance to autologous antigen, so in a healthy state, the body’s immune system will not produce an immune response to normal tissue. Previous study found that in situations where a tumor-associated antigen exists but remains unidentified, an approach may be needed for presentation of that antigen by a professional antigen-presenting cell (APC)21. Stimulus for DCs activation is in combination with Ags, mature DCs induce an effective anti-tumor immunity22,23,24,25,26,27. The DC/tumor fusion is an effective approach, because multiple tumor-associated Ags, including those known and unknown, are endogenously processed and presented by MHC class I and II pathways with the ability to migrate to draining lymph nodes28,29,30,31,32,33,34,35. In previous studies, DC-Tumor fusion have been found to have the effective treatment in carcinomas, lymphomas, and melanomas in mice14,36,37,38,39,40,41. These findings have recently been extended to the treatment and long-term survival of patients14,42,43,44,45. However, the fusion of DCs with tumor-derived endothelial cells and its function is largely unknown.
In this experimental setting, T cells are stimulated by four kinds of cell suspensions (DC only, EC only, DC mixed with EC, DC/EC fusion). The results demonstrate that T cells activated by DC/EC fusion cells are effective in protecting mice against tumor challenge. In this context, fusion of tumor-derived endothelial cells with DCs may result in heterokaryons that express the necessary MHC, DC/EC fusions as compared with mixed DC with EC, DC only or EC only, were sufficient to give rise to T cell proliferation, IFN-α, IFN-γ production and CTL responses. T cells treat neovascular antigens of tumor vascular endothelial cells as heterologous antigens (such as CD105). When these antigens are largely and stably presented to T cells by DCs, could activate the specific T cells, kill tumor vascular endothelial cells, but not target normal blood vessels21.
In conclusion, we have isolated and generated tumor-derived endothelial cells and DCs from tumor-bearing mice with success. Immunogenic cells created by DC/EC fusions have the capacity to greatly stimulate EC-specific T cells. Our findings that T cells activated by DCs fused with tumor-derived endothelial cells could induce higher levels of IFN-α and IFN-γ, as well as significantly reinforce CTL responses in vitro or in vivo, which may help in designing optimal strategies for the therapy and may improve DC/EC fusion-based vaccination strategies.
Materials and Methods
DC generation
DCs were obtained from bone marrow of BALB/c mice17. BALB/c mice were obtained from Vital River Company (Beijing, China) and were housed and cared for in accordance with the Federation of European Laboratory Animal Science Association guidelines, and all protocols were approved by the Animal Ethics Committee of Guangxi Medical University (Nanning, Guangxi, China). Briefly, bone marrow cells were flushed and cultured in RPMI 1640 medium supplemented with 20 ng/ml murine rGM-CSF (Sigma-Aldrich). After five days of culture, DCs were purified and harvested for fusion to endothelial cells.
Preparation of endothelial cells
Tumors were removed from BALB/c mice bearing H22 and placed in cold PBS solution with 50 units/ml heparin. Peripheral and necrotic tissues were excised and remaining tumor was minced by using a scalpel. Dissociation of 0.1 × 0.1 × 0.1 cm3 minced tissue was performed in a 37 °C enzyme cocktail of 10 mg collagenase type I, 20 ml DMEM, 2 ml FBS for 60 min of constant mixing with vortex. The cell suspension was passed through 80 mesh strainer, PBS solution washed, then the cells were resuspended in 100 μl 0.01 M PBS buffer. Single cells were magnetically labeled with anti-CD105 Microbeads (Miltenyi Biotec) in the dark at 4 °C for 30 min and applied to the prepared MS Column (Miltenyi Biotec). CD105+ cells bound to the beads were flushed out by applying the plunger supplied with the column. Then, the sorted CD105+ cells were cultured by Endothelial Cell Medium (ScienCell).
Flow Cytometry
For flow cytometry, cells were stained at the concentration of 1 × 106 cells per 95 μl buffer and 5 μl phycoerythrin-conjugated anti-CD105 (ebioscience) at 4 °C for 30 min before flow cytometry analysis. All data were analyzed by EXPO32 Softwear.
Tube Formation Assay
To analyze the tube formation ability of CD105+ cells, 100 μl/well of growth factor-reduced Matrigel (BD Bioscience) was laid into 96-well plates to solidify. Cells were seeded into 96-well plates. After 6 h, the tube formation was assessed with microscopy20.
Dil-Ac-LDL Uptake Assay
CD105+ cells were plated into the 6-well plates at 5 × 104 cells/dish. At 75% confluence, the culture medium was replaced by the serum-free DMEM for 24 h, followed by incubation with 2 μg/ml Dil-Ac-LDL for 5 h in incubator. Then cells were washed and fixed with paraformaldehyde (4 °C, 30 min), followed by DAPI staining for 3 min. The Dil-Ac-LDL uptake was assessed with microscopy20.
Fusion of DCs with endothelial cells (DC/EC)
DCs were incubated with ECs for 5 min at a ratio of 10:1 in serum-free medium, which contained 50% polyethylene glycol. Then, culture medium was added to dilute the polyethylene glycol slowly. After washing, the cells were cultured in RPMI 1640 medium (10% FBS, 500 U/ml GM-CSF) for 7–14 days.
Cell proliferation assay
The effects of T cells co-cultured with DC only, EC only, DC mixed with EC, or DC/EC fusion cells on cells proliferation was examined at stimulate cells : T cells (S : T cells) ratios by flow cytometry (Beckman Coulter Epics XL, USA).
Cytotoxicity assays
T cells were stimulated and harvested, which were treated as effector cells in CTL assays46,47. The effects of T cells co-cultured with DC only, EC only, DC mixed with EC and DC/EC fusion cells for 5 hours, respectively. After T cells stimulation, 2 × 104 PKH-26 (Sigma-Aldrich) labeled-target ECs were cultured with T cells (37 °C, 5 h). Cytotoxicity assays were examined at the indicated effector-to-target cell (E:T) ratios by flow cytometry (Beckman Coulter Epics XL, USA).
ELISA
In order to assess the production of IFN-α or IFN-γ in T cells, DC only, EC only, DC mixed with EC, or DC/EC fusion cells were washed twice and cocultured with T cells (1 × 105 cells) at a ratio of 1:10 in 48-well plates at 37 °C for 3 days in the absence of IL-2. T cells were purified and cultured in the low-dose of IL-2 (10 U/ml) for 3 days. The test supernatants from these samples were collected and tested for IFN-α and IFN-γ production by ELISA (BD Pharmingen) according to manufacturer’s instructions.
Proliferation assay (In vivo)
BALB/c mice (Four weeks old, female) were purchased from Guangxi Laboratory Animal Center (Nanning, China). All animal care and experimental procedures were according to guidelines of the Institutional Animal Care and Use Committee of Guangxi Medical University. Left flanks of ten mice were implanted subcutaneously with 1 × 105 H22 (mice hepatoma cell lines) suspended in 0.01 M PBS. Tumor volumes were calculated by the formula. 0.52 × a (length) × b (width)2 in millimeters. The animals were sacrificed and the tumors were excised after 40 days.
Immunohistochemistry
In order to quench endogenous peroxidase activities, 4 μm-thick tumor sections were made and incubated with 3% hydrogen peroxide. Heat mediation in citrate buffer (pH 6.0) was used to retrieve antigen. After blocking with 10% goat serum, the slides was incubated with primary antibody. Then, the samples were incubated with the antibodies against Ki-6748,49 (Abcam) or CD1059 (Abcam) overnight in a humidified container at 4 °C. 0.01 M PBS without primary antibodies was applied as the negative control. Immunohistochemical staining was performed with DAB and were counterstained with hematoxylin. Instead of adding antibody, TUNEL reaction mixture (R&D Systems, Minneapolis, MN) was then added to tumor sections, followed by incubation in a humidified chamber at 37 °C for 60 min and DAPI staining for 3 min.
Statistical analysis
Quantitative data were expressed as mean ± SD. One-way analysis of variance was used to determine significance. The difference was significant when P values were < 0.05.
Additional Information
How to cite this article: Huang, Y. et al. Fusions of Tumor-derived Endothelial Cells with Dendritic Cells Induces Antitumor Immunity. Sci. Rep. 7, 46544; doi: 10.1038/srep46544 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ardouin, L. et al. Broad and Largely Concordant Molecular Changes Characterize Tolerogenic and Immunogenic Dendritic Cell Maturation in Thymus and Periphery. Immunity 45, 305–318 (2016).
Anguille, S. et al. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacological Reviews 67, 731–753 (2015).
Carmi, Y. et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 521, 99–104 (2015).
Alatrash, G., Daver, N. & Mittendorf, E. A. Targeting Immune Checkpoints in Hematologic Malignancies. Pharmacol Rev 68, 1014–1025 (2016).
Anders, K. et al. Oncogene-targeting T cells reject large tumors while oncogene inactivation selects escape variants in mouse models of cancer. Cancer Cell 20, 755–767 (2011).
Barclay, A. N. & Van den Berg, T. K. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32, 25–50 (2014).
Bengsch, B. et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity 45, 358–373 (2016).
Latham, S. L. et al. Immuno-analysis of microparticles: probing at the limits of detection. Scientific Reports 5, 16314 (2015).
Kitajima, Y. et al. Estrogen deficiency heterogeneously affects tissue specific stem cells in mice. Scientific Reports 5, 12861 (2015).
Ricci-Vitiani, L. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010).
Cheng, L. et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153, 139–152 (2013).
Wang, R. et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468, 829–833 (2010).
Bellone. Abnormal expression of Endoglin and its receptor complex (TGF-β1 and TGF-β receptor II) as early angiogenic switch indicator in premalignant lesions of the colon mucosa. International Journal of Oncology 37 (2010).
Choi, B. et al. Effective Delivery of Antigen-Encapsulin Nanoparticle Fusions to Dendritic Cells Leads to Antigen-Specific Cytotoxic T Cell Activation and Tumor Rejection. ACS Nano 10, 7339–7350 (2016).
Chen, F. et al. Engineering of hollow mesoporous silica nanoparticles for remarkably enhanced tumor active targeting efficacy. Scientific Reports 4, 5080 (2014).
Bhattacharya, N. et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity 45, 641–655 (2016).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Han, J. et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Scientific Reports 5, 11483 (2015).
Blackburn, J. S. et al. Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation. Cancer Cell 25, 366–378 (2014).
Mao, Q. et al. A novel method for endothelial cell isolation. Oncology Reports (2015).
Gong, J., Chen, D., Kashiwaba, M. & Kufe, D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 3, 558–561 (1997).
Tong, L. et al. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Scientific Reports 6, 21642 (2016).
Slevin, M. et al. Monomeric C-reactive protein–a key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Scientific Reports 5, 13281 (2015).
Pulendran, B. The varieties of immunological experience: of pathogens, stress, and dendritic cells. Annual Review of Immunology 33, 563–606 (2015).
Brechmann, M. et al. A PP4 holoenzyme balances physiological and oncogenic nuclear factor-kappa B signaling in T lymphocytes. Immunity 37, 697–708 (2012).
Muranski, P. et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011).
Narayan, N. et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492, 199–204 (2012).
Garcia, J. et al. Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: The influence of tissue source and inflammatory stimulus. Scientific Reports 6, 24295 (2016).
Zou, L. et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Scientific Reports 3, 2243 (2013).
Vereb, Z. et al. Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Scientific Reports 6, 26227 (2016).
Kalos, M. & June, C. H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 39, 49–60 (2013).
Hinrichs, C. S. & Restifo, N. P. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol 31, 999–1008 (2013).
Maliar, A. et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143, 1375–1384 e1371–1375 (2012).
Palazon, A. et al. The HIF-1alpha hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov 2, 608–623 (2012).
Methner, A. & Zipp, F. Multiple sclerosis in 2012: Novel therapeutic options and drug targets in MS. Nat Rev Neurol 9, 72–73 (2013).
Das, P. et al. Phosphorylation of Nonmuscle myosin II-A regulatory light chain resists Sendai virus fusion with host cells. Scientific Reports 5, 10395 (2015).
Dudda, J. C. et al. MicroRNA-155 is required for effector CD8 + T cell responses to virus infection and cancer. Immunity 38, 742–753 (2013).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Linnemann, C. et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 19, 1534–1541 (2013).
Escobar, G. et al. Genetic engineering of hematopoiesis for targeted IFN-alpha delivery inhibits breast cancer progression. Sci Transl Med 6, 217ra213 (2014).
Hossain, D. M. et al. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 39, 1057–1069 (2013).
Abhishek, D., Garg, L. V ., Carolien, Koks, Tina, Verschuere, Louis, Boon, Stefaan, W. & Van Gool, P. A. Dendritic cell vaccines based on imm Source Sci Transl Med SO 2016 Mar 2 8 328 328ra27[PMIDT26936504].pdf. Immunotherapy (2016).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 31, 71–75 (2013).
Kobayashi, E. et al. A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat Med 19, 1542–1546 (2013).
Kong, K. F. et al. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat Immunol 15, 465–472 (2014).
Leventhal, D. S. et al. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity 44, 847–859 (2016).
Menager, M. M. & Littman, D. R. Actin Dynamics Regulates Dendritic Cell-Mediated Transfer of HIV-1 to T Cells. Cell 164, 695–709 (2016).
Wang, S. et al. A light-controlled switch after dual targeting of proliferating tumor cells via the membrane receptor EGFR and the nuclear protein Ki-67. Scientific Reports 6, 27032 (2016).
Wang, R. X., Chen, S., Jin, X. & Shao, Z. M. Value of Ki-67 expression in triple-negative breast cancer before and after neoadjuvant chemotherapy with weekly paclitaxel plus carboplatin. Scientific Reports 6, 30091 (2016).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81572994, 81460432); Guangxi Natural Science Foundation (2015GXNSFDA139017); Guangxi Science and Technology Research and Technology Development Project (15104001-7); Project for Innovative Research Team in Guangxi Natural Science Foundation (2015GXNSFFA139001) and the University Scientific Research Project of Guangxi Department of Education (YB2014057).
Author information
Authors and Affiliations
Contributions
Y.H., Q.M., X.L. and Y.Z. designed the experiments. Y.H., Q.M. and J.H. prepared the fusion cells. J.S., Y.P., W.L. and Z.H. performed the experiments. Y.H., Q.M., J.H. and S.Z. analyzed the data. Y.H., Q.M., X.L. and Y.Z. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, Y., Mao, Q., He, J. et al. Fusions of Tumor-derived Endothelial Cells with Dendritic Cells Induces Antitumor Immunity. Sci Rep 7, 46544 (2017). https://doi.org/10.1038/srep46544
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46544
This article is cited by
-
A novel CTLA-4 blocking strategy based on nanobody enhances the activity of dendritic cell vaccine-stimulated antitumor cytotoxic T lymphocytes
Cell Death & Disease (2023)
-
Targeted repair of heart injury by stem cells fused with platelet nanovesicles
Nature Biomedical Engineering (2018)
-
SDF 1-alpha Attenuates Myocardial Injury Without Altering the Direct Contribution of Circulating Cells
Journal of Cardiovascular Translational Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.