Abstract
Novel chemical vapor deposition (CVD) process was successfully developed for the growth of Sr2Si5N8:Ce3+ phosphors with elevated luminescent properties. Metallic strontium was used as a vapor source for producing Sr3N2 vapor to react with Si3N4 powder via a homogeneous gas-solid reaction. The phosphors prepared via the CVD process showed high crystallinity, homogeneous particle size ranging from 8 to 10 μm, and high luminescence properties. In contrast, the phosphors prepared via the conventional solid-state reaction process exhibited relative low crystallinity, non-uniform particle size in the range of 0.5–5 μm and relatively lower luminescent properties than the phosphors synthesized via the CVD process. Upon the blue light excitation, Sr2−xCexSi5N8 phosphors exhibited a broad yellow band. A red shift of the emission band from 535 to 556 nm was observed with the increment in the doping amount of Ce3+ ions from x = 0.02 to x = 0.10. The maximum emission was observed at x = 0.06, and the external and internal quantum efficiencies were calculated to be 51% and 71%, respectively. Furthermore, the CVD derived optimum Sr1.94Ce0.06Si5N8 phosphor exhibited sufficient thermal stability for blue-LEDs and the activation energy was calculated to be 0.33 eV. The results demonstrate a potential synthesis process for nitride phosphors suitable for light emitting diodes.
Similar content being viewed by others
Introduction
Presently, blue emitting InGaN based white light emitting diodes (WLEDs) are gathering enormous attention for indoor and outdoor lighting industries because of their elevated brightness, extended lifetime, and eco-friendliness1,2,3. One of the most famous phosphors for solid state lighting is yellow-emitting Y3Al5O12:Ce3+ phosphor, which can be efficient excited by the InGaN chip. However, WLEDs based on this phosphor have certain disadvantages including a poor color rendering index (CRI) at long wavelengths2,3. For enhancing the CRI of WLEDs, nitrodosilicate materials which are suitable for ultraviolet (UV)/blue InGaN chip have been studied significantly owing to their long-wavelength emissions and good thermal stability4,5. Nitridosilicate compounds possess covalent and short Re–N chemical bonds in condensed structures with corner-sharing or edge-sharing SiN4 tetrahedral frameworks6. Such compact structures provide strong crystal fields and covalent nature for nitridosilicate phosphors. The above characteristics result in a relatively broad excitation range, long emission wavelengths, and low thermal quenching behavior7,8,9,10,11.
Among the nitridosilicate phosphors, M2Si5N8: Eu2+ (M = Ca, Sr, Ba) are considered to be the significant orange-red phosphors for white LEDs (WLEDs) because of the high quantum efficiency under blue excitation and high thermal stability12,13. Ce3+-doped Sr2Si5N8 has been reported as one of the potential yellow phosphors for WLEDs14. However, the research on the detailed structural and photoluminescence analysis including quantum efficiency, concentration quenching and thermal quenching behavior are still few. For the fabrication of industrial Sr2Si5N8 phosphors, the conventional solid-state reaction process is known to be the most common approach12,13,14,15,16. However, the high reaction temperatures (1350–1600 °C) exceed the melting point for one of the constituent materials, Sr3N2 (m.p. = 1030 °C)10,11,12,13,14. Excessive liquid phase during the reaction causes non-homogeneous reactions, resulting in phosphors with low quality and insufficient emission intensity. Furthermore, owing to the air-sensitive properties of Sr3N2, the mixing process of raw materials needs to be carried out in a glove box17,18. The use of glove boxes is relatively complicated, thereby increasing the cost for preparing phosphors. Therefore, the innovation of a low cost approach that can be able for the mass production of high quality phosphors is desirable.
To overcome the drawbacks in the traditional solid-state reaction method, the chemical vapor deposition (CVD) process was deliberately developed to prepare Sr2Si5N8-based phosphors in the present research. Metallic strontium was used as a vapor source for producing Sr3N2 vapor to react with other raw materials during the reaction process. Because of the gas-solid reaction rather than the conventional liquid-solid reaction, the CVD process is beneficial for increasing the homogeneity of reaction and improving the quality of phosphors. For producing yellow-light emission, Ce3+ ions were doped into Sr2Si5N8 host. The phosphors prepared using the proposed CVD and conventional solid-state reaction processes were compared with regard to crystallinity, particle morphology and luminescence properties. The yellow emitting Sr2Si5N8:Ce3+ and commercial red emitting Sr2Si5N8:Eu2+ phosphors were then combined with blue LED chips for the fabrication of WLEDs to demonstrate the industrial application of the CVD-derived phosphors. The conceptual mechanism of the CVD process for the synthesis of yellow-emitting Sr2Si5N8:Ce3+ phosphors and the relevant characteristics may open up a new path for the advancement of lighting industry.
Results and Discussions
Phase identification and structure of Ce3+-doped Sr2Si5N8
Sr2−xCexSi5N8 (x = 0.02–0.10) phosphors were prepared via the CVD process (method A). Figure 1 display XRD patterns of Sr1.94Ce0.06Si5N8 synthesized at various temperatures ranging from 1400 °C to 1600 °C. After annealing the precursors at 1400 °C, the compound of SrSiN2 was found to form. When the calcination temperature was increased from 1400 °C to 1500 °C, Sr2Si5N8 structure with low crystallinity was found without any trace of SrSiN2. Further increasing the annealing temperature to 1600 °C resulted in the formation of the Sr2Si5N8 structure with pure phase and high crystallinity. The recorded diffraction patterns of Sr1.94Ce0.06Si5N8 matched well with the standard pattern (ICDD No. 85-0101).
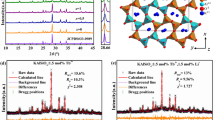
Figure 2(a) presents the rietveld XRD pattern of Sr1.94Ce0.06Si5N8 synthesized via the CVD method at 1600 °C. The solid curve indicates the simulated diffraction data, the “×” marks represent the experimental diffraction data, the straight bars indicate the positions of simulated diffraction patterns, and the dotted line denotes the deviation between the simulated and experimental values. The calculated Rp and wRp parameters were converged to reliable values of 0.0481 and 0.0643, respectively. The refinement results confirmed that Sr1.94Ce0.06Si5N8 belongs to the orthorhombic crystal system and the space group of Pmn21 (no. 176). Table 1 lists the as-estimated lattice parameters of Sr1.94Ce0.06Si5N8. The calculated lattice parameters were a = 5.7100 Å, b = 6.8202 Å and c = 9.3349 Å, and the crystal size was 109.3 nm. The inset of Fig. 2(a) displays the SAED pattern of Sr1.94Ce0.06Si5N8 measured from HRTEM. The lattice plane (013) of Sr2Si5N8 can be identified from the diffraction spots in the SAED pattern. The sharp diffraction spots indicate the high crystallinity of the as-prepared phosphors.
(a) Refinement pattern of observed (×) and calculated (solid line) X-ray diffraction patterns, difference profile (dot line), and positions of all the reflections (vertical bars) for Sr1.94Ce0.06Si5N8 phosphors prepared via the CVD process at 1600 °C. Inset: SAED pattern of Sr1.94Ce0.06Si5N8 phosphors. (b) Structural representation and (c) coordination environment of Sr2+ sites for Sr1.94Ce0.06Si5N8 phosphors.
From the refinement parameters, the crystal structure of Sr1.94Ce0.06Si5N8 was drawn via the VESTA software and shown in Fig. 2(b)19. From this figure, it is shown that Sr2+ ions are assembled in the channels formed via Si6N6 rings along the [100] orientation. Figure 2(c) indicates that there are two kinds of Sr2+ sites, Sr1 and Sr2 with the coordination numbers of 8 and 10, respectively. Both Sr2+ sites are occupied by Ce3+ ions. Furthermore, the mean distance of Sr1–N (2.865 Å) is smaller than that of Sr2–N (2.928 Å). Therefore, Ce3+ ions locating at the Sr1 sites is considered to experience stronger crystal field strength than those occupying the Sr2 site. The different crystal field strength between the two sites cause different luminescent properties, as shown in the later section.
As the doping concentration of Ce3+ ions in Sr2−xCexSi5N8 phosphors was increased from x = 0 to x = 0.10, all XRD patterns were identified to be Sr2Si5N8 phase without any impurity phases. A small peak shift was observed with the doping of Ce3+ ions. Figure 3 shows the shift of the XRD peak for Sr2−xCexSi5N8 (x = 0.02–0.10). As the doping of Ce3+ ions increased, the (113) peak shifted to high diffraction angles. The ionic radius of Ce3+ ion (115 pm) is smaller than that of Sr2+ ion (132 pm). Therefore, the lattice parameters of Sr2Si5N8 tended to decrease with the doping of Ce3+ ions, resulting in the shift of XRD peaks to high diffraction angles20.
Comparison of crystal structures, morphology and luminescent properties between Ce3+-doped Sr2Si5N8 synthesized via the CVD and solid-state reaction processes
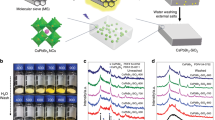
Figure 4(a) displays the comparative XRD patterns for Sr1.94Ce0.06Si5N8 phosphors synthesized via methods A and B at 1600 °C. It was shown that both methods produced pure Sr1.94Ce0.06Si5N8 compound without any impurity. However, the diffraction peak intensity of phosphors prepared via method A is higher than that of phosphors prepared via method B. Figure 4(b,c) shows the scanning electron micrographs of Sr1.94Ce0.06Si5N8 phosphors synthesized via both methods. The phosphors prepared via the method A exhibited a size distribution in the range of 8–10 μm, as shown in Fig. 4(b). In contrast, the particle size of phosphors prepared via method B ranged from 0.5 μm to 5 μm, as shown in Fig. 4(c). These results indicated that phosphors prepared via method A exhibited larger particle size and smaller size distribution than those prepared via the method B.
Figure 5(a,b) shows the particle size distribution of Sr1.94Ce0.06Si5N8 phosphors analyzed using the laser diffraction particle size analyzer. The average particle size of phosphors prepared via method A was 21.7 μm with a standard deviation of 8.7 μm. However, the average particle size of phosphors synthesized via method B was measured to be 9.7 μm with a standard deviation of 9.1 μm. Both the results of SEM and particle size analysis indicated that the CVD process was beneficial to prepare phosphors with large particle sizes and small size distribution.
The reaction mechanism of the formation for Sr2Si5N8 phosphors via methods A and B are proposed in Fig. 6(a,b), respectively. In method A, Sr metal and the mixtures of Si3N4 and CeO2 are placed separately in two crucibles. During the heating process, Sr metal first reacts with N2 gas to form Sr3N221. Then Sr3N2 melts to be liquid and produces Sr3N2 vapor at temperatures above the melting point of Sr3N2 (m.p. = 1030 °C). Sr3N2 vapor then flows with the carrier gas to react with the mixtures in the other crucible to form Sr2−xCexSi5N8. Owing to the high uniformity of gas-solid mixtures for Sr3N2(v), Si3N4(s), and CeO2(s), the reaction occurs homogeneously. Therefore, the formed Sr2−xCexSi5N8 particles exhibited small size distribution. Furthermore, the high mobility of gas may enhance the diffusion process and the corresponding reaction rates, thereby resulting in the formation of large particles22,23. On the other hand, in method B, the mixtures of Sr3N2, Si3N4 and CeO2 are placed in the same crucible. When the temperature is increased over 1030 °C, Sr3N2 melts and flows to the bottom of the mixtures for Si3N4 and CeO2 powders. As a result, the reaction on the top of powders may be incomplete. The incomplete reaction will result in low crystallinity and weak XRD peak intensity24,25. In addition, the liquid-solid contact between Sr3N2(l) and the mixtures of Si3N4(s) and CeO2(s) is heterogeneous and the reaction is inhomogeneous. Therefore, the formed Sr2Si5N8 particles show non-uniform size distribution.
Figure 7(a) presents the PL emission spectra of Sr1.94Ce0.06Si5N8 phosphors synthesized via methods A and B at 1600 °C. Under the blue excitation at 432 nm, Sr1.94Ce0.06Si5N8 prepared via both methods exhibited a broad emission band centered at approximately 550 nm due to the 5d-4f transition of Ce3+ ions26. The emission intensity of phosphors prepared via method A was approximately 40% higher than that prepared via method B. The enhanced emission properties of phosphors synthesized via method A than that synthesized via method B can be attributed to the high crystallinity of the prepared phosphors, as shown in Fig. 4(a)27. Moreover, it is known that small particles usually possess more surface defects than large particles and these surface defects may decrease the photoluminescence intensity of phosphors28,29,30. Therefore, the small particle size of the phosphors synthesized via method B (as shown in Fig. 4(c)) may also be the reason for the relative low photoluminescence intensity. Figure 7(b) displays the excitation spectra of Sr1.94Ce0.06Si5N8 phosphors synthesized via both methods at 1600 °C. The excitation spectra of phosphors prepared via both methods monitored at 550 nm were similar, and both spectra included two broad excitation bands at 230–350 nm and 350–500 nm, respectively. The peak at 285 nm can be attributed to the host lattice excitation, while the broad excitation band from 350 to 500 nm is due to the complex splitting of the 5d1 excited state (4f-5d transition) for Ce3+ ions31,32.
Photoluminescence characteristics of Ce3+-doped Sr2Si5N8 host
It was reported that Ce3+ ions in different host materials show two characteristics emission bands due to the spin-orbit splitting of the ground state (2F5/2 and 2F7/2) with an energy difference of approximately 2000 cm−1 33. To further investigate the broad emission band of Ce3+ doped Sr2Si5N8, the emission curve was fitted to be four well-separated Gaussian components peaking at approximately 493 nm, 530 nm, 562 nm and 626 nm, as shown in Fig. 8(a). The energy difference between the sub-bands 493 nm and 530 nm was 1416 cm−1, and that between the sub-bands 562 nm and 626 nm was 1819 cm−1. These two values are close to the energy difference of the two ground states 2F5/2 and 2F7/2. Therefore, it can be concluded that Ce3+ ions are located at two different Sr2+ sites and eventually two kinds of luminescent centers are formed. S. Miao et al. suggested that the local environment surrounding Ce3+ ions in the host lattice can affect the positions of the emission band for Ce3+ ions and the positions can be estimated via an empirical relation given as follows34:
(a) Deconvoluted emission spectra of Sr1.94Ce0.06Si5N8, (b) emission spectra of Sr2−xCexSi5N8 (x = 0.02–0.10) phosphors synthesized via the CVD process at 1600 °C, and variation of the (c) emission intensity, peak wavelength, and (d) Stokes shift with the concentration of Ce3+ ions in Sr2−xCexSi5N8.

where E (cm−1) represents the real position of the d-band edge, Q represents the position in energy for the d-band edge of Ce3+ free ions, r is the radius of the host cation, and Ea is the electron affinity of the atoms. The valence of Ce3+ ions and the number of anions in the immediate shell around the ions are denoted by V and n, respectively. In the present case, Q, V, r and Ea are constants. Therefore, the positions of the emission band only depend on the number of anions in the immediate shell around three crystallographically independent cation sites in Sr2Si5N8matrix including Si4+, 8 coordinated Sr2+ and 10 coordinated Sr2+ sites. As per the estimation done in the literature, these three conditions were calculated based on Eq. (1), respectively35. Compared with the calculated values obtained from Si4+ sites (1200–1250 nm), it can be concluded that the Sr2+ sites can act as the favorable cation sites for Ce3+ ion. According to Eq. (1), Ce3+ ions locating at the 8-coordinate Sr2+ sites tend to exhibit longer emission wavelength (560–630 nm) than those occupying the 10-coordinate Sr2+ sites (489–535 nm). Therefore, it is considered that Ce3+ ions with the emissions at 493 nm and 530 nm occupies the 10-coordinate Sr2+ sites, while the other Ce3+ ions showing emissions at 562 nm and 626 nm are related to the 8-coordinate Sr2+ sites.
Figure 8(b) shows the emission spectra of Sr2−xCexSi5N8 (x = 0.02–0.10) phosphors calcined via method A at 1600 °C. The relationship between x and the relative emission intensity is shown in Fig. 8(c). Increasing the doping amount of Ce3+ ions to x = 0.06 led to an increase in the emission intensity of Sr2−xCexSi5N8. However, a further increase in the concentration of Ce3+ ions reduced the emission intensity due to the self concentration quenching phenomena36. The self-concentration quenching effects between two Ce3+ ions may be owing to the non-radiative energy transfer between two Ce3+ ions. In general, the non-radiative energy transfer between two identical ions may take place via exchange interaction, radiation reabsorption, or an electric multipolar interaction. With an increase in the concentration of identical ions, the distance between two ions reduces and the energy transfer starts at a critical distance (Rc)37. Rc can be calculated from the structural parameters including cell volume (V), the number of cations in the unit cell (N), and the critical concentration of Ce3+ (C) in the host via the following formula38:

From the appropriate V, N and C values (363.53 Å3, 2 and 0.06, respectively), Rc in Sr2−xCexSi5N8 was calculated to be 17.95 Å. According to G. Blasse, Rc for the general exchange interaction is estimated to be around 5 Ǻ38. Therefore, the exchange interaction can be neglected in the energy transfer within Sr2−xCexSi5N8 phosphors. In the present case, radiation reabsorption and multipolar interaction may be the main mechanism for concentration quenching. On the other hand, when x was increased from 0.02 to 0.10, the emission peak position for phosphors shifted from 535 nm to 556 nm. Such resultant shift may be due to the re-absorption of high energy emission and the increased Stokes shift owing to the change of crystal field strength39. Figure 8(d) shows the Ce3+ concentration dependent Stokes shift estimated from the energy difference between the last excitation band at low energy and the first emission band at high energy14. It can be seen clearly that the Stokes shift increased with increasing Ce3+ concentrations and hence supported the shifting of emission peak position.
Quantum efficiency and thermal stability of Ce3+-doped Sr2Si5N8 host
The integrating sphere was applied for determining the absolute quantum efficiency of the as-prepared phosphors. BaSO4 powders with a reflectivity of 95% in visible light were used as the standard to calculate the spectrum of the excitation source. Figure 9 shows the luminescence spectra of BaSO4 powders and Sr1.94Ce0.06Si5N8 phosphors under the excitation at 460 nm. The external quantum efficiency (EQE) can be calculated using the formula ηo = Nem/Nexc × 100%, where Nem and Nexc are the number of emitted and excited photons, respectively. The internal quantum efficiency (IQE) can be calculated from ηi = Nem/Nabs × 100%, where Nabs is the number of absorbed photons40. The EQE and IQE for Sr1.94Ce0.06Si5N8 phosphors were calculated to be 51% and 71%, respectively. These results indicate that Sr1.94Ce0.06Si5N8 phosphors have potential to be used in 460 nm InGaN-based LED chips.
Figure 10 plots the normalized emission intensity of Sr1.94Ce0.06Si5N8 phosphor as a function of temperature under the blue excitation at 460 nm. The photoluminescence intensity was observed to decrease with the increase in temperature. At 150 °C, the emission intensity remained approximately 73% of that recorded at room temperature. The decrease in emission intensity was fit with Boltzmann sigmoidal function properly with R2 value larger than 0.99 and the fitting were employed in the estimation of TQ1/2 value. TQ1/2 is the temperature at which the phosphor loses half of its emission efficiency. From the fitting data, TQ1/2 was obtained as a high value of 513 K (±9 K). When the temperature was increased, the nonradiative relaxation probability induced by enhanced phonon-electron interaction was also increased41. The activation energy (Ea) for thermal quenching can be obtained using the equation listed below42,43:

where I0 and IT are the luminescence intensities at room and testing temperatures, respectively, C is a constant, and k is the Boltzmann constant (8.617 × 10−5 eV K−1). The inset in Fig. 10 plots ln[(I0/IT) − 1] vs. 1/kT to calculate the activation energy for Sr1.94Ce0.06Si5N8. From Eq. (3), Ea was estimated to be 0.33 eV (±0.04 eV). The high value of activation energy indicates high thermal stability for the present phosphors. Such high thermal stability due to the compact crystal lattice of Sr2Si5N8-based structure is suitable for LED applications.
Electroluminescence properties of phosphors-converted white LEDs
Herein, an effective synthesis route has been designed to produce high quality Sr2Si5N8:Ce3+ nitridosilicate phosphors for LEDs. The developed phosphors synthesized via the novel CVD route showed high yellow-emission intensity, adequate quantum efficiency, and very low thermal quenching behaviors. The research outcomes directly indicate the suitability of the present phosphors for possible LED applications. Therefore, the optimum composition derived from the CVD route was finally incorporated in LED packaging to check the suitability of the phosphors for industrial applications. Figure 11 shows the electroluminescence (EL) spectra of LEDs driven by a current of 280 mA. When Sr1.94Ce0.06Si5N8 phosphor was applied to a LED chip, the electroluminescence spectrum presented a blue peak at 460 nm as well as the yellow emission band of Sr1.94Ce0.06Si5N8. The corresponding CIE coordinate was (0.27, 0.37) with a near white CCT of 8167 K and a Ra value of 64. On the other hand, the conventional YAG:Ce3+ phosphors were also packed with blue LEDs having a 460-nm emission. The corresponding CIE coordinate of YAG:Ce3+-coated LEDs was (0.29, 0.36) with a CCE of 7161 K and a Ra value of 69. Both two kinds of LEDs showed low CRI values owing to the lack of red emissions. The high CCT result indicates that Sr1.94Ce0.06Si5N8-coated LED exhibits the color temperature in the cold white region43. For improving the CCT and CRI of LEDs, Sr2Si5N8:Eu2+ phosphors were blended with Sr1.94Ce0.06Si5N8 and coated on another LED chip. The resulting electroluminescence spectrum exhibited a combination of blue, yellow, and red emissions with corresponding CIE coordinates of (0.33, 0.33), a pure white CCT of 5953 K, and Ra of 84 which is close to standard daylight at noon (D65, 6500 K) and can be applied for different commercial appliances44. Table 2 lists the full set of CRI and average CRI (Ra) values. The insets of Fig. 11 present the images of the packaged WLEDs. The light emission with high brightness was seen clearly. As a consequence of the present work, yellow emission Sr2−xCexSi5N8 with high crystallinity and high brightness were successfully synthesized via the CVD process. The potential of the present phosphors for application in WLEDs was demonstrated.
Conclusions
A chemical vapor deposition (CVD) process was newly developed to synthesize Sr2Si5N8: Ce3+ phosphors through the reaction between Sr3N2(v) and the mixtures of Si3N4(s) and CeO2(s). The phosphors prepared via the CVD process had high crystallinity, uniform particle size distribution in the range of 8–10 μm and efficient photoluminescence due to the homogeneous gas-solid reaction. On the other hand, the phosphors prepared via the solid-state reaction process showed low crystallinity, nonuniform size distribution in the range of 0.5–5 μm, relatively low photoluminescence because of the inhomogeneous liquid-solid reaction. As the concentration of Ce3+ ions in Sr2−xCexSi5N8 was increased from x = 0.02 to 0.10, a red shift of the emission peak from 535 nm to 556 nm was observed under blue light excitation. Meanwhile, phosphors exhibited the maximum emission intensity at x = 0.06. The critical distance (Rc) of energy transfer, the external and internal quantum efficiencies were calculated to be 17.95 Å, 51% and 71%, respectively. The activation energy of thermal stability for Sr2Si5N8:Ce3+ was counted to be 0.33 eV. A white LED with a color rendering index of 84 and a color temperature of 5953 K was fabricated via utilizing the mixture of Sr2Si5N8:Ce3+ and Sr2Si5N8:Eu2+ phosphors with a InGaN LED chip (460 nm). This research demonstrated a potential synthesis technique to prepare nitride phosphors for white LEDs.
Materials and Methods
Synthesis of Ce3+-doped Sr2Si5N8 phosphors via the CVD process
In the present work, Sr2−xCexSi5N8 phosphors were prepared via the chemical vapor deposition (CVD) process. Figure 12(a) illustrates the schematic diagram of the CVD process, which is defined as method A. 0.012 mol of strontium metal was put in a molybdenum crucible. The mixtures of analytical-grade Si3N4 (0.005 mol) and CeO2 (0.003x mol, x = 0.02–0.10) powders were put in another molybdenum crucible. Then both molybdenum crucibles were placed in a tubular furnace for heating under a H2/N2 mixed atmosphere. The partial pressures of H2 and N2 were 76 torr H2 and 684 torr, respectively. The annealing temperature was increased to 800 °C and maintained for 1 h to nitridize strontium metal to be strontium nitride. Then the heating temperatures were further increased to 1400–1600 °C and maintained for 8 h to evaporate strontium nitride onto the mixture powders to form Sr2−xCexSi5N8 (x = 0.02–0.10) phosphors through the CVD process.
Synthesis of Ce3+-doped Sr2Si5N8 phosphors via the solid-state reaction process
In order to compare the phosphors prepared via the CVD process with those prepared via the conventional process, Sr1.94Ce0.06Si5N8 phosphors were also synthesized via the conventional solid-state reaction process. Figure 12(b) illustrates the schematic diagram of the solid-state reaction process, which is defined as method B. Analytical-grade Sr3N2 (0.00194 mol), Si3N4 (0.005 mol) and CeO2 (0.00018 mol) powders were thoroughly ground and mixed according to the chemical formula Sr1.94Ce0.06Si5N8 in an argon-filled glove box. Then the mixed powders were placed in a molybdenum crucible and calcined at 1600 °C for 8 h in a reduced atmosphere (76 torr H2 and 684 torr N2) to form Sr1.94Ce0.06Si5N8 phosphors.
Characterization of phosphors
The structural analysis of the obtained samples was carried out using X-ray diffractometer (Rigaku, Ultima IV) with a standard CuKα X-ray source. The PDXL program was used to refine the structure. The microstructures of the prepared phosphors were performed using a field emission scanning electron microscope (FE-SEM) (JEOL JSM-7600F) and a field emission transmission microscope (FE-TEM) (Philips Tecnai F30). The particle size distribution of phosphors was carried out using a laser diffraction particle size analyzer (Coulter, LS230). The photoluminescence characteristics of the prepared phosphors were investigated using a fluorescence spectrophotometer (Hitachi, F-4500) with a Xe lamp as the excitation source. The quantum efficiency was measured using a CCE spectrophotometer (BRC112E) with an integrating sphere. The thermal stability of the as-prepared phosphors was measured using a CCE spectrophotometer (BRC112E) and a heater.
Fabrication and characterization of WLEDs
For fabricating white LEDs, the as-prepared phosphors were mixed with commercial Sr2Si5N8:Eu2+ phosphors and dispersed in transparent silicon resin to prepare phosphor mixtures. The mixtures were then coated on 460 nm InGaN-based LED chips to fabricate LED devices. The photoluminescence characteristics of fabricated LEDs were measured using a CCE spectrophotometer (BRC112E). The Commission Internationale de I’Eclairage (CIE) coordinates were converted from the photoluminescence spectra using the color calculator software.
Additional Information
How to cite this article: Yang, C.-Y. et al. Synthesis of Sr2Si5N8:Ce3+ phosphors for white LEDs via efficient chemical vapor deposition. Sci. Rep. 7, 45832; doi: 10.1038/srep45832 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11 July 2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has been fixed in the paper.
11 July 2017
Scientific Reports 7: Article number: 45832; published online: 31 March 2017; updated: 11 July 2017. The original version of this Article contained typographical errors in the title, where: “Synthesis of Sr2Si5N8: Ce3+ phosphors for white LEDs via an efficient chemical deposition”. now reads: “Synthesis of Sr2Si5N8: Ce3+ phosphors for white LEDs via efficient chemical vapor deposition”.
References
Chen, J. et al. Design of a Yellow-Emitting Phosphor with Enhanced Red Emission via Valence State-control for Warm White LEDs Application. Sci. Rep. 6, 31199, doi: 10.1038/srep31199 (2016).
Hsu, C. H., Das, S. & Lu, C. H. Color-Tunable, Single Phased MgY4Si3O13: Ce3+, Mn2+ Phosphors with Efficient Energy Transfer for White-Light-Emitting Diodes. J. Electrochem. Soc. 159, J193–J199 (2012).
Das, S., Yang, C. Y. & Lu, C. H. Structural and Optical Properties of Tunable Warm-White Light-Emitting ZrO2:Dy3+–Eu3+ Nanocrystals. J. Am. Ceram. Soc. 96, 1602–1609 (2013).
Zhu, J. et al. Moisture-induced degradation and its mechanism of (Sr, Ca)AlSiN3:Eu2+, a red-color-converter for solid state lighting. J. Mater. Chem. C 3, 3181–3188 (2015).
Lin, H. C., Yang, C. Y., Das, S. & Lu, C. H. Red-emission improvement of Eu2+–Mn2+ co-doped Sr2Si5N8 phosphors for white light-emitting diodes. Ceram. Inter. 40, 12139–12147 (2014).
Xie, R. J., Hirosaki, N., Li, Y. & Takeda, T. Rare-Earth Activated Nitride Phosphors: Synthesis Luminescence and Applications. Mater. 3, 3777–3793 (2010).
Hsu, C. H. & Lu, C. H. Color-tunable Y2Si4N6C:Ce3+ Carbonitride Phosphors for Ultraviolet Light-Emitting Diodes. J. Am. Ceram. Soc. 94, 1691–1694 (2011).
Tiwary, M. et al. Enhancement of photoluminescence in Er-doped Ag–SiO2nanocomposite thin films: A post annealing study. Vacuum 85, 806–809 (2011).
Faraji, N. et al. Visible-Light Driven Nanoscale Photoconductivity of Grain Boundaries in Self-Supported ZnO Nano- and Microstructured Platelets. Adv. Electron. Mater. 2, 1600138 (2016).
Jin, X. et al. A Novel Concept for Self-Reporting Materials: Stress Sensitive Photoluminescence in ZnO Tetrapod Filled Elastomers. Adv. Mater. 25, 1342–1347 (2013).
ten Kate, O. M., Zhang, Z., Dorenbos, P., Hintzen, H. T. & ven der Kolk, E. 4f and 5d energy levels of the divalent and trivalent lanthanide ions in M2Si5N8 (M = Ca, Sr, Ba). J Solid State Chem. 197, 209–217 (2013).
Van den Eeckhout, K., Smet, P. F. & Poelman, D. Luminescent afterglow behavior in the M2Si5N8:Eu family (M = Ca, Sr, Ba). Mater. 4, 980–990 (2011).
Li, Y. Q. et al. Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J. Alloys Compd. 417, 273–279 (2006).
Li, Y. Q., de With, G. & Hintzen, H. T. Luminescence properties of Ce3+-activated alkaline earth silicon nitride M2Si5N8 (M = Ca, Sr, Ba) materials. J. Lumin. 116, 107–116 (2006).
Duan, C. J., Otten, W. M., Delsing, A. C. A. & Hintzen, H. T. Preparation and photoluminescence properties of Mn2+-activated M2Si5N8 (M = Ca, Sr, Ba) phosphors. J. Solid State Chem. 181, 751–757 (2008).
Xie, R. J., Hirosaki, N., Suehiro, T., Xu, F. F. & Mitomo, M. A Simple, Efficient Synthetic Route to Sr2Si5N8:Eu2+-Based Red Phosphors for White Light-Emitting Diodes. Chem. Mater. 18, 5578–5583 (2006).
Piao, X., Machida, K., Horikawa, T. & Yun, B. Acetate reduction synthesis of Sr2Si5N8:Eu2+ phosphor and its luminescence properties. J. Lumin. 130, 8–12 (2010).
Suehiro, T., Xie, R. J. & Hirosaki, N. Facile Synthesis of (Sr, Ca)2Si5N8:Eu2+-Based Red-Emitting Phosphor for Solid-State Lighting. Ind. Eng. Chem. Res. 52, 7453–7456 (2013).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Miao, S. et al. Effect of Al/Si substitution on the structure andluminescence properties of CaSrSiO4:Ce3+ phosphors: analysis based on the polyhedral distortion. J. Mater. Chem. C 3, 4616–4622 (2015).
Chandrasekharaiah, M. S. & Margrave, J. L. The Kinetics of Oxidation and Nitridation of Lithium, Calcium, Strontium, and Barium. J. Electrochem. Soc. 108, 1008–1012 (1961).
Dill, K. A. & Bromberg, S. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology, 309–310 (Garland Science, 2003).
Champion, D., Meste, M. L. & Simatos, D. Towards an improved understanding of glass transition and relaxations in foods: molecular mobility in the glass transition range. Trends Food Sci. Technol. 11, 41–55 (2000).
Lupan, O. et al. Rapid switching and ultra-responsive nanosensors based on individual shell–core Ga2O3/GaN:Ox@SnO2 nanobelt with nanocrystalline shell in mixed phases. Sens. Actuators B 221, 544–555 (2015).
Lupan, O. et al. Enhanced ethanol vapour sensing performances of copper oxide nanocrystals with mixed phases. Sens. Actuators B 224, 434–448 (2016).
Hsu, C. H. & Lu, C. H. Microwave-hydrothermally synthesized (Sr1−x-yCexTby)Si2O2−δN2+μ phosphors: efficient energy transfer, structural refinement and photoluminescence properties. J. Mater. Chem. 21, 2932–2939 (2011).
Chung, C. Y., Hsu, C. H. & Lu, C. H. Preparation and Mechanism of Nest-Like YBO3:Tb3+ Phosphors Synthesized Via the Microemulsion-Mediated Hydrothermal Process. J. Am. Ceram. Soc. 94, 2884–2889 (2011).
Stryganyuk, G. et al. Luminescence of Ce3+doped LaPO4 nanophosphors upon Ce3+ 4f–5d and band-to-band excitation. J. Lumin. 128, 355–360 (2008).
Su, L. T. et al. Photoluminescence phenomena of Ce3+-doped Y3Al5O12 nanophosphors. J. Appl. Phys. 102, 083541 (2007).
Prashantha, S. C., Lakshminarasappa, B. N. & Nagabhushana, B. M. Photoluminescence and thermoluminescence studies of Mg2SiO4:Eu3+ nano phosphor. J. Alloys Compd. 509, 10185–10189 (2011).
Li, H. L., Xie, R. J., Hirosaki, N. & Yajima, Y. Synthesis and Photoluminescence Properties of Sr2Si5N8:Eu2+ Red Phosphor by a Gas-Reduction and Nitridation Method. J. Electrochem. Soc. 155, J378–J381 (2008).
Im, W. B. et al. A yellow-emitting Ce3+ phosphor, La1−xCexSr2AlO5, for white light-emitting diodes. Appl. Phys. Lett. 93, 091905 (2008).
Blasse, G. & Grabmaier, B. C. Luminescent Materials Ch. 3, 45–46 (Springer-Verlag, 2012).
Van Uitert, L. G. An empirical relation fitting the position in energy of the lower d-band edge for Eu2+ OR Ce3+ in various compounds. J. Lumin. 29, 1–9 (1984).
Liu, C., Xia, Z., Lian, Z., Zhou, J. & Yan, Q. Structure and luminescence properties of greenemitting NaBaScSi2O7:Eu2+ phosphors for near-UVpumped light emitting diodes. J. Mater. Chem. C 1, 7139–7147 (2013).
Yang, C. Y., Das, S. & Lu, C. H. Tunable photoluminescence properties and energy transfer in oxyapatite-based Ca2Tb8(SiO4)6:Eu3+ phosphors for UV-LEDs. J. Lumin. 168, 199–206 (2015).
Zhong, J. et al. Synthesis, structure and luminescence properties of new blue-green-emitting garnet-type Ca3Zr2SiGa2O12:Ce3+ phosphor for near-UV pumped white-LEDs. RSC Adv. 6, 2155–2161 (2016).
Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 62, 207–211 (1986).
Schlieper, T., Milius, W. & Schnick, W. Nitrido-silicate. II [1]. Hochtemperatur-Synthesen und Kristallstrukturen von Sr2Si5N8 und Ba2Si5N8 . Z. Anorg. Allg. Chem. 621, 1380–1384 (1995).
Nakajima, T., Isobe, M., Uzawa, Y. & Tsuchiya, T. Rare earth-free high color rendering white light-emitting diodes using CsVO3 with highest quantum efficiency for vanadate phosphors. J. Mater. Chem. C 3, 10748–10754 (2015).
Janulevicius, M., Grigorjevaite, J., Merkininkaite, G., Sakirzanovas, S. & Katelnikovas, A. Luminescence and luminescence quenching of Eu2Mo4O15 . J. Lumin. 179, 35–39 (2016).
Das, S., Yang, C. Y., Lin, H. C. & Lu, C. H. Structural and luminescence properties of tunable white-emitting Sr0.5Ca0.5Al2O4:Eu2+, Dy3+ for UV excited white-LEDs. RSC Adv. 4, 64956–64966 (2014).
Som, S. et al. The energy transfer phenomena and colour tunability in Y2O2S:Eu3+/Dy3+ micro-fibers for white emission in solid state lighting applications. Dalton Trans. 43, 9860–9871 (2014).
Guo, N. et al. White-light emission from a single-emitting-component Ca9Gd(PO4)7:Eu2+, Mn2+ phosphor with tunable luminescent properties for near-UV light-emitting diodes. J. Mater. Chem. 20, 9061–9067 (2010).
Acknowledgements
The authors would like to thank the Ministry of Science and Technology, Taiwan, the Republic of China, for partial financially supporting this research.
Author information
Authors and Affiliations
Contributions
C.-H.L. conceived, designed and directed the project. C.-Y.Y. synthesized and characterized the present samples. C.-Y.Y., S.S. and S.D. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, CY., Som, S., Das, S. et al. Synthesis of Sr2Si5N8:Ce3+ phosphors for white LEDs via efficient chemical vapor deposition. Sci Rep 7, 45832 (2017). https://doi.org/10.1038/srep45832
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45832
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.