Abstract
Akirin2, a novel nuclear factor, plays an important role in myogenesis. To investigate the role of Akirin2 in proliferation and differentiation of porcine skeletal muscle satellite cells, Akirin2 overexpression and Akirin2 silence technologies were employed. Our results showed that overexpression of Akirin2 markedly enhanced the proliferation and differentiation of porcine skeletal muscle satellite cells, whereas silencing of Akirin2 got the opposite results. Furthermore, our results showed that Akirin2 affected proliferation and differentiation of porcine skeletal muscle satellite cells through extracellular-signal regulated kinase-1/2 (ERK1/2) and NFATc1 signaling pathways. These results indicate that Akirin2 can effectively promote skeletal muscle satellite cells proliferation and differentiation, acting through ERK1/2- and NFATc1-dependent mechanisms.
Similar content being viewed by others
Introduction
Skeletal muscle is mainly composed of postmitotic multinucleated muscle fibers (myofibers). Myogenesis is a multistep process, which involves skeletal muscle satellite cell activation, proliferation, differentiation and formation of multinucleated myofibers1,2. The entire process is regulated by large number of extracellular factors and some distinct signaling pathways, resulting in the subsequent activation of major transcription factors in regulating gene expression3,4.
The extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, a part of the mitogen-activated protein kinase (MAPK) pathway, is involved in regulating many facets of cellular processes such as cell proliferation, differentiation and death5. Previous studies showed that ERK1/2 is required for myoblast proliferation and differentiation6,7,8,9. The activated ERK1/2 pathway enhances the skeletal muscle cell proliferation, but negatively regulates myoblast differentiation10,11. There is some evidence that nuclear factor of activated T cells c1 (NFATc1) cooperates with ERK1/2 signaling in the induction of cell proliferation, apoptosis and differentiation12,13,14.
The nuclear factor Akirin2, a member of the Akirin family, plays a fundamental role in myogenesis15. It may be considered as a potential functional candidate gene of meat quality16,17. The ERK1/2 signaling pathway has been shown to be regulated by Akirin2 in tumor cell lines18,19. However, whether and how Akirin2 affects skeletal muscle cell proliferation and differentiation remains unclear.
In our previous studies, we cloned porcine Akirin2 and examined its effects on expressions of IL-6 and myosin heavy chain (MHC) isoform17,20. In the present study, Akirin2 overexpression and Akirin2 silence were employed to study the effects and the underlying mechanism of Akirin2 on the proliferation and differentiation of porcine skeletal muscle satellite cells.
Results
Expression of Akirin2 in proliferating and differentiating porcine skeletal muscle satellite cells
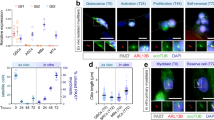
Akirin2 expression in proliferating and differentiating porcine skeletal muscle satellite cells was assessed by real-time quantitative PCR. As shown in Fig. 1, Akirin2 mRNA was was upregulated during porcine skeletal muscle satellite cells proliferation. We also found that Akirin2 mRNA was upregulated during porcine skeletal muscle satellite cells differentiation, which was similar to myogenin mRNA (Fig. 2).
RNA was extracted from the proliferating porcine skeletal muscle satellite cells on the days 1, 2, 3, and 4. Akirin2 mRNA expression was analyzed by real-time quantitative PCR. The amount of Akirin2 mRNA was normalized to the amount of GAPDH mRNA. Data were presented as means ± SE (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001 as compared with the control group (1 d).
RNA was extracted from the differentiating porcine skeletal muscle satellite cells on the days 2, 4, and 6. Akirin2 (A) and myogenin (B) mRNA expression was analyzed by real-time quantitative PCR. The amount of Akirin2 and myogenin mRNA was normalized to the amount of GAPDH mRNA. Data were presented as means ± SE (n = 3). ***P < 0.001 as compared with the control group (2 d).
Akirin2 promotes proliferation of porcine skeletal muscle satellite cells
Porcine skeletal muscle satellite cells were subjected to cell proliferation analysis after transfection of pcDNA3.1(+)-pAkirin2 plasmid or Akirin2 siRNA for 24 h. The results showed that overexpression of Akirin2 could promote the mRNA (Fig. 3A) and protein expressions of Akirin2 (Fig. 3B) and the cell proliferation (Fig. 4A and B), whereas silencing of Akirin2 inhibited the expression of Akirin2 (Fig. 3C and D) and the cell proliferation (Fig. 4C and D). Taken together, these findings show that Akirin2 functions in promoting the proliferation of porcine skeletal muscle satellite cells.
Approximately 60% confluent porcine skeletal muscle satellite cells were transfected with 0.5 μg of pcDNA3.1(+)-pAkirin2 or 50 nM of Akirin2-siRNA and cultured in proliferation medium for 24 h. (A,C) The amount of Akirin2 mRNA against GAPDH mRNA was measured by real-time quantitative PCR. (B,D) The Akirin2 protein expression was measured by Western blotting. Equal loading was monitored with anti-GAPDH antibody. Data were presented as means ± SE (n = 3). **P < 0.01 and ***P < 0.001 as compared with the control group.
Approximately 60% confluent porcine skeletal muscle satellite cells were transfected with 0.5 μg of pcDNA3.1(+)-pAkirin2 or 50 nM of Akirin2-siRNA and cultured in proliferation medium for 24 h. (A,C) Cell proliferation was evaluated by EdU proliferation assay. The percentage of EdU-positive cells was quantified. Results were presented as mean ± SE (n = 6). (B,D) Western blot analysis of extracts from porcine skeletal muscle satellite cells by using anti-phospho-Histone H3 antibody. Results were presented as means ± SE (n = 3). *P < 0.05 and ***P < 0.001 as compared with the control group.
Akirin2 promotes differentiation of porcine skeletal muscle satellite cells
To investigate the role of Akirin2 in differentiation of porcine skeletal muscle satellite cells, we performed overexpression or silencing of Akirin2 in differentiating cells and measured the protein level of myogenic marker MHC. As shown in Fig. 5, overexpression of Akirin2 significantly increased, whereas silencing of Akirin2 significantly decreased, the protein level of MHC, suggesting that Akirin2 functions in promoting the differentiation of porcine skeletal muscle satellite cells.
(A) Overexpression of Akirin2 promoted porcine skeletal muscle satellite cells differentiation. Approximately 80% confluent porcine skeletal muscle satellite cells were transfected with 1 μg of pcDNA3.1(+)-pAkirin2 and cultured in differentiation medium for 72 h. Total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-Akirin2, MHC and GAPDH antibodies. (B) Akirin2 silencing inhibited porcine skeletal muscle satellite cells differentiation. Approximately 80% confluent porcine skeletal muscle satellite cells were transfected with 50 nM of Akirin2-siRNA and cultured in differentiation medium for 72 h. Total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-Akirin2, MHC and GAPDH antibodies. Results were presented as means ± SE (n = 3). ***P < 0.001 as compared with the control group.
Involvement of ERK1/2 signaling pathway in Akirin2-induced proliferation and differentiation of porcine skeletal muscle satellite cells
To investigate whether Akirin2 affects the ERK1/2 signaling pathway, we probed for phospho-ERK1/2 levels in lysates from Akirin2-overexpressed porcine skeletal muscle satellite cells. The results indicated that overexpression of Akirin2 activated ERK1/2 in proliferating cells (Fig. 6). A similar result was also observed in differentiating cells (data not shown). To determine whether Akirin2 affects proliferation and differentiation of porcine skeletal muscle satellite cells through the ERK1/2 signaling pathway, porcine skeletal muscle satellite cells were treated with specific ERK inhibitor PD98059 and Akirin2 overexpression. The results indicated that inhibition of ERK1/2 signaling pathway significantly eliminated the proliferation (Figs 4A,B and 7) and differentiation (Figs 5A and 8) promotion by Akirin2 overexpression.
Approximately 60% confluent porcine skeletal muscle satellite cells were transfected with 0.5 μg of pcDNA3.1(+)-pAkirin2 and cultured in proliferation medium for 24 h. Total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 and ERK1/2 antibodies. Results were presented as mean ± SE (n = 3). ***P < 0.001 as compared with the control group.
(A) Porcine skeletal muscle satellite cells were cultured in proliferation medium. PD98059 (50 μM) or DMSO was added 1 h before transfection with 0.5 μg of pcDNA3.1(+)-pAkirin2 in approximately 60% confluent porcine skeletal muscle satellite cells. After 24 h, cell proliferation was evaluated by EdU proliferation assay. The percentage of EdU-positive cells was quantified. Results were presented as mean ± SE (n = 6). (B) Lysates from porcine skeletal muscle satellite cells treated as described in (A) were immunoblotted with anti-phospho-Histone H3 antibody. Equal loading was monitored with anti-GAPDH antibody. Results were presented as mean ± SE (n = 3). Values with different letters are significantly different (P < 0.05).
PD98059 or DMSO was added 1 h before transfection with 1 μg of pcDNA3.1(+)-pAkirin2 in approximately 80% confluent porcine skeletal muscle satellite cells. On day 3 of differentiation, total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-MHC and GAPDH antibodies. Results were presented as mean ± SE (n = 3). Values with different letters are significantly different (P < 0.05).
Involvement of NFATc1 signaling pathway in Akirin2-induced proliferation and differentiation of porcine skeletal muscle satellite cells
To investigate whether Akirin2 affects the NFATc1 signaling pathway, we probed for NFATc1 levels in lysates from Akirin2-overexpressed porcine skeletal muscle satellite cells. The results showed that overexpression of Akirin2 increased the protein expression of NFATc1 in proliferating cells (Fig. 9). A similar result was also observed in differentiating cells (data not shown). To verify whether NFATc1 signaling pathway is involved in Akirin2-induced proliferation and differentiation promotion of porcine skeletal muscle satellite cells, the cells were treated with the inhibitor CsA and Akirin2 overexpression. The results showed that the inhibitor CsA significantly eliminated the proliferation (Figs 4A,B and 10) and differentiation (Figs 5A and 11) promotion by Akirin2 overexpression.
Total cell lysates prepared as described in Fig. 6 were subjected to SDS-PAGE and immunoblotted with anti-NFATc1 and GAPDH antibodies. Results were presented as mean ± SE (n = 3). ***P < 0.001 as compared with the control group.
(A) Approximately 60% confluent porcine skeletal muscle satellite cells, pretreated with 5 μM CsA for 1 h or left untreated, were transfected with 0.5 μg of pcDNA3.1(+)-pAkirin2. After 24 h, cell proliferation was evaluated by EdU proliferation assay. The percentage of EdU-positive cells was quantified. Data were presented as mean ± SE (n = 6). (B) Lysates from porcine skeletal muscle satellite cells treated as described in (A) were immunoblotted with anti-phospho-Histone H3 and NFATc1 antibodies. Equal loading was monitored with anti-GAPDH antibody. Results were presented as mean ± SE (n = 3). Values with different letters are significantly different (P < 0.05).
CsA or DMSO was added 1 h before transfection with 1 μg of pcDNA3.1(+)-pAkirin2 in approximately 80% confluent porcine skeletal muscle satellite cells. On day 3 of differentiation, total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-NFATc1, MHC and GAPDH antibodies. Data were presented as mean ± SE (n = 3). Values with different letters are significantly different (P < 0.05).
Interaction effects between ERK1/2 and NFATc1 signaling pathways in porcine skeletal muscle satellite cells
Porcine skeletal muscle satellite cells were treated with inhibitors PD98059 or CsA for 1 h before transfection with pcDNA3.1(+)-Akirin2 plasmid for 24 h in proliferation medium and for 72 h in differentiation medium, respectively. The results of Western blot indicated that PD98059 and CsA inhibited NFATc1 and phosphorylation of ERK1/2, respectively, in both proliferation and differentiation medium (Fig. 12).
(A,B) PD98059 or DMSO was added 1 h before the transfection of pcDNA3.1(+)-pAkirin2. After cell proliferation (A) treatment with Akirin2 for 24 h or differentiation (B) treatment with Akirin2 for 72 h, total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-NFATc1 and GAPDH antibodies. (C,D) CsA or DMSO was added 1 h before the transfection of pcDNA3.1(+)-pAkirin2. After cell proliferation (C) treatment with Akirin2 for 24 h or differentiation (D) treatment with Akirin2 for 72 h, total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-phosphorylation of ERK1/2 and ERK1/2 antibodies. Results were presented as mean ± SE (n = 3). Values with different letters are significantly different (P < 0.05).
Discussion
The process of myogenesis is controlled by several myogenic regulatory factors (MyoG, MyoD, Myf5, Myf6, and so on) which further regulate the expression of many muscle specific genes21. This process is also guided by various environmental cues3,4. There is some evidence that many peptidic factors are able to regulate skeletal muscle cells proliferation or differentiation through distinct signaling pathways9,22. Akirin2 has been identified as a potential regulator of myogenesis15. Here, we addressed the questions whether Akirin2 might participate in regulation of skeletal muscle satellite cells proliferation and differentiation. The results of our work demonstrated that Akirin2 promotes proliferation and differentiation of porcine skeletal muscle satellite cells.
Phospho-Histone H3 protein was used as a mitotic cell cycle biomarker for cell proliferation23. MHC is the late differentiation markers of myoblasts24,25. To study the effects of Akirin2 on the proliferation and differentiation of porcine skeletal muscle satellite cells, we used two approaches: over-expression and RNA interference with Akirin2. Our results showed that overexpression of Akirin2 significantly increased the abundance of phospho-Histone-H3, a S/G2 and G2/M phase marker protein. Furthermore, overexpression of Akirin2 significantly increased the protein expression levels of MHC. Moreover, silencing of Akirin2 decreased the proliferation and differentiation of porcine skeletal muscle satellite cells. Our in vitro study revealed that Akirin2 plays an important role in proliferation and differentiation of porcine skeletal muscle satellite cells. However, Sun et al. reported that Akirin2 could promote the proliferation but not the differentiation of duck myoblasts26. Those findings suggest that the function of Akirin2 in skeletal myogenesis exists the cells or species difference.
There is a lot of evidence to indicate that ERK1/2 pathway is involved in regulating the proliferation of muscle cells27,28,29. To gain insight into the mechanisms by which Akirin2 stimulates the proliferation and differentiation of porcine skeletal muscle satellite cells, we evaluated the signaling events. We found that Akirin2 increased the phosphorylation level of ERK1/2 in proliferating porcine skeletal muscle satellite cells. To test the functional role of ERK1/2 activation induced by Akirin2 in porcine skeletal muscle satellite cells proliferation, we next explored the effects of ERK1/2 inhibitor (PD98059) on Akirin2-induced proliferation promotion of porcine skeletal muscle satellite cells. Inhibition of the ERK1/2 pathway by PD98059 decreased Akirin2-induced proliferation promotion of porcine skeletal muscle satellite cells. These results suggest that the ERK1/2 pathway mediates the stimulatory effects of Akirin2 on the proliferation of porcine skeletal muscle satellite cells.
We have also demonstrated that Akirin2 promotes the myogenic differentiation of porcine skeletal muscle satellite cells. The stimulatory effect was characterized by increasing protein expression level of MHC, a myogenic differentiation-related protein. Previous studies suggested that the effect of MAPK on muscle cells differentiation is controversial. Some studies suggest that activation of the ERK pathway prevents skeletal muscle differentiation29,30,31, and other studies believe that it functions at two stages of skeletal muscle differentiation10,32. However, recent data indicate that ERK1/2 may positively regulate myogenic differentiation6,28,33. In this study, we showed that Akirin2 promotes differentiation of porcine skeletal muscle satellite cells through ERK1/2 signaling pathway.
Calcineurin (CaN) has been reported to be a possible candidate in the signaling of skeletal muscle cellular growth, and plays an important role in regulating cell proliferation34 and differentiation35,36,37. CaN has also been reported to affect the muscle regeneration by association with NFATc1, a downstream target of CaN signaling37. As Akirin2 affects both the proliferation and differentiation of porcine skeletal muscle satellite cells, we hypothesized that Akirin2 affects the proliferation and differentiation of porcine skeletal muscle satellite cells via the NFATc1 signaling pathway. The immunosuppressive drug CsA is a well-known inhibitor of CaN, and thus inhibits NFAT activity by blocking its dephosphorylation38. CsA has also been reported to inhibit myoblast differentiation35,36. Here we demonstrated that CsA inhibited proliferation and differentiation of porcine skeletal muscle satellite cells, and Akirin2 promoted the NFATc1 protein expression in porcine skeletal muscle satellite cells during both proliferation and differentiation stages.
In the present study, we demonstrated that Akirin2 activated both ERK1/2 and NFATc1 signaling pathway in porcine skeletal muscle satellite cells. So we speculated that ERK1/2 and NFATc1 signaling pathways might crosstalk with each other. This speculation was partly supported by the data of the present study. Further study is warranted to verify the speculation more thoroughly.
In summary, the present study demonstrated that Akirin2 plays an important role in proliferation and differentiation of porcine skeletal muscle satellite cells, and further revealed that Akirin2 promotes proliferation and differentiation of porcine skeletal muscle satellite cells through ERK1/2 and NFATc1 signaling pathways. However, it is necessary to further investigation of the role of Akirin2 in skeletal muscle development by in vivo study.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Sichuan Agricultural University. All experimental protocols were approved by the Animal Care Advisory Committee of Sichuan Agricultural University.
Reagents
The calcineurin inhibitor cyclosporin A (CsA) was obtained from Amresco (USA) and resolved in DMSO. The specific ERK inhibitor PD98059 was purchased from Sigma (St. Louis, MO, USA) and resolved in DMSO, then used at 50 μM. Anti-NFATc1 (Cat. No. 8032) antibody was obtained from Cell signaling Technology (Danvers, MA, USA). Anti-phospho-ERK1/2 (Cat. No. sc-16982), phospho-Histone H3 (Cat. No. sc-8656-R), MHC (Cat. No. sc-20641) and GAPDH (Cat. No. sc-20357) antibodies were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-ERK1/2 (Cat. No. 16443–1-AP) antibody was obtained from ProteinTech Biotechnology (Chicago, IL, USA).
Isolation of porcine skeletal muscle satellite cells
Porcine skeletal muscle satellite cells were isolated from 3-day-old male Duroc × Yorkshire × Landrace (DLY) pigs as described previously39 with some modifications. Briefly, skeletal muscles were digested with 0.2% collagenase type II (Sigma) and then filtered successively through 200-mesh and 400-mesh cell sieves. The collected cells were purified by differential adhesion method. The resulting mononuclear cells were cultured in DMEM/F12 (Invitrogen) supplemented with 20% FBS, 100 U/mL penicillin and 100 μg/μL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. The cells were identified by immunofluorescence with anti-Pax7 antibody (PAX7, DSHB, USA) (data not shown).
Cell culture
For stimulation experiments, cells were treated with recombinant plasmid pcDNA3.1(+)-pAkirin2, and PD98059 (ERK1/2 inhibitor) or cyclosporin (CsA, CaN inhibitor) were added 1 h before the treatment. Myogenic differentiation was induced by changing the medium to DMEM/F12 supplemented with 2% horse serum and penicillin/streptomycin.
The siRNA and plasmid transfection
A pair of 21-nucleotide siRNA sequences targeting Akirin2 was designed and synthesized by GenePharm (Shanghai, China). The sense strand of the Akirin2 siRNA was 5′-GCUGUACUUCUGAUGCACATT-3′, and the antisense strand was 5′-UGUGCAUCAGAAGUACAGCTT-3′. The sense strand of the negative control siRNA was 5′-UUCUCCGAACGUGUCACGUTT-3′, and the antisense strand was 5′-ACGUGACACGUUCGGAGAATT-3′. The siRNA was dissolved in DEPC-treated water, and the final concentration was 50 nM. The pcDNA3.1(+)-pAkirin2 plasmid was constructed by our lab20. The lipofectamine 2000 (Invitrogen, California, USA) was used to transfect the porcine skeletal muscle satellite cells according to the manufacturer’s instruction.
Cell proliferation analysis
To investigate cell proliferation, markers of two phases of the cell cycle were analyzed by using EdU (5-ethynyl-2′-deoxyuridine) for synthesis phase and phospho-histone H3 for G2-M phase. EdU proliferation assay was performed as described by Chen et al.40 using a Click-iT EdU Alexa Fluor 594 Imaging Kit (Invitrogen). The level of phospho-histone H3 protein was detected by western blot analysis.
RNA extraction and real-time quantitative PCR
Total RNA isolation was performed according to the RNAiso Plus reagent (TaKaRa, Dalian, China) protocol. cDNA was synthesized by using PrimeScript® RT reagent Kit with gDNA Eraser (TaKaRa) according to the manufacture’s instructions. For real-time quantitative PCR analysis, synthesized cDNA and SYBR select Master Mix (Applied Biosystems, Foster, CA, USA) were run on an ABI 7900HT Real-time PCR system. The gene specific primers used are listed in Table 1. The PCR cycling conditions were as following: 45 cycles at 95 °C for 15 s and 60 °C for 30 s. Relative gene expression was determined using the comparative Ct method41 with GAPDH as an endogenous control.
Western blotting
Protein was extracted from porcine skeletal muscle satellite cells using RIPA cell lysis buffer (Pierce, Rockford, IL, USA) supplemented with protease inhibitor cocktail (Sigma). Protein concentrations were assessed by BCA protein assay kit (Pierce). Equal amounts of protein were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blocked in 3% non-fat milk in TBS-0.1% Tween-20 for 1 h and incubated overnight with primary antibody at 4 °C, followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology) for 1 h at 37 °C. The bound antibodies were visualized with a ClarityTM Western ECL Substrate (Bio-Rad, Hercules, CA, USA) using a ChemiDoc XRS Imager System (Bio-Rad). Housekeeping protein GAPDH was used as a control for equal protein loading. The density of the protein bands was determined using Gel-Pro Analyzer 4.2 software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
All data, expressed as mean ± SE, were subjected to one-way ANOVA analysis or Tukey test using SPSS 11.0 software and P < 0.05 was considered significant.
Additional Information
How to cite this article: Chen, X. et al. Akirin2 regulates proliferation and differentiation of porcine skeletal muscle satellite cells via ERK1/2 and NFATc1 signaling pathways. Sci. Rep. 7, 45156; doi: 10.1038/srep45156 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wagers, A. J. & Conboy, I. M. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122, 659–667 (2005).
Buckingham, M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Current Opinion in Genetics & Development 16, 525–532 (2006).
Perry, R. L. S. & Rudnick, M. A. Molecular mechanisms regulating myogenic determination and differentiation. Frontiers in Bioscience 5, D750–D767 (2000).
Ge, Y., Sun, Y. & Chen, J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. Journal of Cell Biology 192, 69–81 (2011).
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews 22, 153–183 (2001).
Gredinger, E., Gerber, A. N., Tamir, Y., Tapscott, S. J. & Bengal, E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. The Journalof Biological Chemistry 273, 10436–10444 (1998).
Jones, N. C., Fedorov, Y. V., Rosenthal, R. S. & Olwin, B. B. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. Journal of Cellular Physiology 186, 104–115 (2001).
Koyama, T. et al. Interaction of Scaffolding Adaptor Protein Gab1 with Tyrosine Phosphatase SHP2 Negatively Regulates IGF-I-dependent Myogenic Differentiation via the ERK1/2 Signaling Pathway. The Journal of Biological Chemistry 283, 24234–24244 (2008).
Yang, H. S. et al. Chemerin regulates proliferation and differentiation of myoblast cells via ERK1/2 and mTOR signaling pathways. Cytokine 60, 646–652 (2012).
Bennett, A. M. & Tonks, N. K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278, 1288–1291 (1997).
Jones, N. C., Fedorov, Y. V., Rosenthal, R. S. & Olwin, B. B. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. Journal of Cellular Physiology 186, 104–115 (2001).
Cicek, M. et al. TGF-β inducible early gene 1 regulates osteoclast differentiation and survival by mediating the NFATc1, AKT, and MEK/ERK Signaling Pathways. PLoS ONE 6, e17522 (2011).
Robbs, B. K., Lucena, P. I. & Viola, J. P. B. The transcription factor NFAT1 induces apoptosis through cooperation with Ras/Raf/MEK/ERK pathway and upregulation of TNF-α expression. Biochimica et Biophysica Acta 1833, 2016–2028 (2013).
Wu, W. W. et al. NFATC1 promotes cell growth and tumorigenesis in ovarian cancer up-regulating c-Myc through ERK1/2/p38 MAPK signal pathway. Tumor Biology 37, 4493–4500 (2016).
Chen, X. L. et al. Role of Akirin in skeletal myogenesis. International Journal of Molecular Sciences 14, 3817–3823 (2013).
Ma, J. S., Xu, G. Y., Wan, L. & Wang, N. L. Molecular cloning, sequence analysis and tissue-specific expression of Akirin2 in Tianfu goat. Gene 554, 9–15 (2015).
Chen, X. L. et al. Effect of porcine Akirin2 on skeletal myosin heavy chain isoform expression. International Journal of Molecular Sciences 16, 3996–4006 (2015).
Komiya, Y. et al. A novel binding factor of 14-3-3β functions as a transcriptional repressor and promotes anchorage-independent growth, tumorigenicity and metastasis. Journal of Biological Chemistry 283, 18753–18764 (2008).
Macqueen, D. J. & Johnston, I. A. Evolution of the multifaceted eukaryotic akirin gene family. BMC Evolutionary Biology 9, 34 (2009).
Chen, X. L., Huang, Z. Q., Jia, G., Wu, X. Q. & Wu, C. M. Molecular cloning, tissue distribution, and functional analysis of porcine Akirin2. Animal Biotechnology 23, 124–131 (2012).
Knight, J. D. & Kothary, R. The myogenic kinome: protein kinases critical to mammalian skeletal myogenesis. Skeletal Muscle 1, 29 (2011).
Florini, J. R., Ewton, D. Z. & Magri, K. A. Hormones, growth factors, and myogenic differentiation. Annual Review of Physiology 53, 201–216 (1991).
Hendzel, M. J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997).
Burattini, S. et al. C2C12 murine myoblasts as a model of skeletal muscle development: morphofunctional characterization. European Journal of Histochemistry 48, 223–233 (2004).
Braun, T. & Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nature Reviews Molecular Cell Biology 12, 349–361 (2011).
Sun, W. Q. et al. Akirin2 could promote the proliferation but not the differentiation of duck myoblasts via the activation of the mTOR/p70S6K signaling pathway. International Journal of Biochemistry & Cell Biology 79, 298–307 (2016).
Yu, T. et al. Leptin promotes proliferation and inhibits differentiation in porcine skeletal myoblasts. Bioscience, Biotechnology, and Biochemistry 72, 13–21 (2008).
Lee, J., Tachibana, H., Morinaga, Y., Fujimura, Y. & Yamada, K. Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids. Life Sciences 84, 415–420 (2009).
Otani, M., Furukawa, S., Wakisaka, S. & Maeda, T. A novel adipokine C1q/TNF-related protein 3 is expressed in developing skeletal muscle and controls myoblast proliferation and differentiation. Molecular and Cellular Biochemistry 409, 271–282 (2015).
Coolican, S. A., Samuel, D. S., Ewton, D. Z., McWade, F. J. & Florini, J. R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. Journal of Biological Chemistry 272, 6653–6662 (1997).
Khurana, A. & Dey, C. S. Subtype specific roles of mitogen activated protein kinases in L6E9 skeletal muscle cell differentiation. Molecular and Cellular Biochemistry 238, 27–39 (2002).
Wu, Z. et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Molecular and Cellular Biology 20, 3951–396 (2000).
Li, J. & Johnson, S. E. ERK2 is required for efficient terminal differentiation of skeletal myoblasts. Biochemical and Biophysical Research Communications 345, 1425–1433 (2006).
Wang, S. et al. Calcineruin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Digestive Diseases and Sciences 57, 3184–3188 (2012).
Abbott, K. L., Friday, B. B., Thaloor, D., Murphy, T. J. & Pavlath, G. K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Molecular Biology of the Cell 9, 2905–2916 (1998).
Friday, B. B., Horsley, V. & Pavlath, G. K. Calcineurin activity is required for the initiation of skeletal muscle differentiation. Journal of Cell Biology 149, 657–666 (2000).
Sakuma, K. et al. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathologica 105, 271–280 (2003).
Park, J., Yaseen, N. R., Hogan, P. G., Rao, A. & Sharma, S. Phosphorylation of the transcription factor NFATp inhibits its DNA binding activity in cyclosporin A-treated human B and T cells, Journal of Biological Chemistry 270, 20653–20659 (1995).
Yang, T. et al. MicroRNA-27a promotes porcine myoblast proliferation by downregulating myostatin expression. Animal 8, 1867–1872 (2014).
Chen, X. L. et al. Expression and purification of porcine Akirin2 in Escherichia coli . Turkish Journal of Biology 38, 339–345 (2014).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31472108) and the Specific Research Supporting Program for Discipline Construction in Sichuan Agricultural University.
Author information
Authors and Affiliations
Contributions
X.C. and Z.H. conceived and designed the experiments. X.C. wrote the manuscript. Y.L. carried out the research. Z.H., X.C., G.J., G.L. and H.Z. contributed reagents/materials/analysis tools. All authors contributed to data interpretation, revised the manuscript critically for important intellectual content, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, X., Luo, Y., Huang, Z. et al. Akirin2 regulates proliferation and differentiation of porcine skeletal muscle satellite cells via ERK1/2 and NFATc1 signaling pathways. Sci Rep 7, 45156 (2017). https://doi.org/10.1038/srep45156
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45156
This article is cited by
-
TLR4 activation inhibits the proliferation and osteogenic differentiation of skeletal muscle stem cells by downregulating LGI1
Journal of Physiology and Biochemistry (2022)
-
Decoding the role of inflammation-related microRNAs in cancer cachexia: a study using HPV16-transgenic mice and in silico approaches
Journal of Physiology and Biochemistry (2022)
-
Contingent intramuscular boosting of P2XR7 axis improves motor function in transgenic ALS mice
Cellular and Molecular Life Sciences (2022)
-
2-D08 treatment regulates C2C12 myoblast proliferation and differentiation via the Erk1/2 and proteasome signaling pathways
Journal of Muscle Research and Cell Motility (2021)
-
Akirin proteins in development and disease: critical roles and mechanisms of action
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.