Abstract
In this study, shade-induced conversion from a young pale/yellow leaf phenotype to a green leaf phenotype was studied using metabolic and transcriptomic profiling and the albino cultivar ‘Yu-Jin-Xiang’ (‘YJX’) of Camellia sinensis for a better understanding of mechanisms underlying the phenotype shift and the altered catechin and theanine production. Shaded leaf greening resulted from an increase in leaf chlorophyll and carotenoid abundance and chloroplast development. A total of 1,196 differentially expressed genes (DEGs) were identified between the ‘YJX’ pale and shaded green leaves, and these DEGs affected ‘chloroplast organization’ and ‘response to high light’ besides many other biological processes and pathways. Metabolic flux redirection and transcriptomic reprogramming were found in flavonoid and carotenoid pathways of the ‘YJX’ pale leaves and shaded green leaves to different extents compared to the green cultivar ‘Shu-Cha-Zao’. Enhanced production of the antioxidant quercetin rather than catechin biosynthesis was correlated positively with the enhanced transcription of FLAVONOL SYNTHASE and FLAVANONE/FLAVONOL HYDROXYLASES leading to quercetin accumulation and negatively correlated to suppressed LEUCOANTHOCYANIDIN REDUCTASE, ANTHOCYANIDIN REDUCTASE and SYNTHASE leading to catechin biosynthesis. The altered levels of quercetin and catechins in ‘YJX’ will impact on its tea flavor and health benefits.
Similar content being viewed by others
Introduction
A group of albino Camellia sinensis cultivars, such as ‘Yu-Jin-Xiang’ (‘YJX’) and ‘Zhonghuang 2′, produce pale or yellow young leaves in early spring and turn green at later growing stages1,2,3,4. These cultivars are highly desired by the tea industry because young pale (virescent) tea leaves can be used to produce a high quality green tea with an enhanced savory (“umami”) taste and reduced stringency, while mature green leaves can maintain plant growth and regular productivity in successive years. Compared with the mature leaves of an albino cultivar and those of common green cultivars, young albino or yellow leaves usually contain enhanced levels of the non-protein amino acid theanine and reduced levels of catechins, a group of flavan-3-ols found in tea at high levels1,2,4. These metabolites are crucial tea flavor determinants. Catechins and their derivatives are mainly responsible for the bitter/astringent taste and deepen the color of tea infusions5. Theanine, a non-protein amino acid, contributes to the ‘umami’ taste and counteracts the astringent and bitter taste in tea infusions6.
Extensive studies on the biosynthesis of these specialized metabolites have revealed that catehcins are derived from the flavonoid pathway by the action of the biosynthetic genes dihydroflavonol 4-reductase (DFR)7, anthocyanidin reductase (ANR), leucoanthocyanidin reductase (LAR)8, and galloylated by the additional action of epicatechin:1-O-galloyl-β-D-glucose O-galloyltransferase (ECGT)9. Furthermore, the flavonoid pathway in plants is strictly regulated by transcription factors in a triprotein MYB-bHLH-WDR complex in response to different internal and external signals10. However, regulatory mechanisms underlying production of catechins in tea are still poorly understood. Biosynthesis of theanine is reportedly catalyzed by theanine synthase (TS) acting on two substrates (glutamate and ethylamine)11 to form theanine. Three additional enzymes, arginine decarboxylase (ADC), alanine decarboxylase (AIDA) or S-adenosylmethionine decarboxylase (SAMDC), might catalyze tea ethylamine production12,13, but this is not proven decisively. Moreover, in vitro enzyme assays showed bacterial γ-glutamyltranspeptidase (GGT) was able to synthesize theanine directly in an in vitro assay14. Hence, a thorough dissection of differentially expressed tea genes involved in these related pathways and an analysis of the correlation between gene expression and metabolite contents between pale and green leaves should enhance our understanding of the mechanisms underlying specialized tea metabolite biosynthesis and regulation in these two types of tea leaves.
Epigenetic regulation, important for gene transcription and phenotype reconstruction in response to developmental and environmental stimuli15,16, is likely involved (but not proven) in the leaf colour phenotype conversion observed in albino tea cultivars. In Arabidopsis, histone deacetylase HDA15 associated with PHYTOCHROME INTERACTING FACTOR3 regulates chlorophyll biosynthesis17. Moreover, biosynthesis of certain Arabidopsis leaf pigments (lutein and some other carotenoids) are epigenetically regulated by histone lysine methyltransferase SDG818. In apple, methylation within the promoter of the MdMYB10 gene, which regulates many flavonoid pathway genes upstream to anthocyanin and catechin biosynthesis, was supposedly responsible for the anthocyanin-deficient yellow-skin phenotype19. Several genes are also core elements controlling genome-wide epigenetic modification, including DNAMETHYLTRANSFERASE1 (MET1)15 and the methyl-CpG binding protein gene MBD120. Thus, possible involvement of epigenetic regulation in tea leaf color alteration needs to learn.
Direct genetic mutations (as opposite to epigenetic regulation) of thousands of nuclear-encoded chloroplast proteins and more than a hundred plastidial genes could lead to aberrant chloroplasts and their malfunction21,22. To date, characterization of Arabidopsis chloroplast proteins using transposon- or T-DNA-tagged lines revealed that a pale or variegated leaf color can result from as many as 72 defective proteins22. Defective genes such as E3 UBIQUITIN-PROTEIN LIGASE 1 (E3 RING1) in the ubiquitination degradation pathway23 and CLPR2, CLPR4. and CLPP3 in the caseinolytic protease (Clp) system can lead to pale green, yellow, and variegated leaf phenotypes24. Moreover, mutations in 1-DEOXY-D-XYLULOSE 5-PHOSPHATE SYNTHASE (DXS) in the MEP pathway25,26 or in GUN1, a pentatricopeptide repeat-containing protein gene (PPR) functioning in the retrograde signaling pathway27,28, can also lead to albino phenotypes. Hence, determining global transcription changes with an emphasis on those genes related to pale leaf phenotypes of other species should help us to elucidate the mechanisms leading to the alteration in leaf color and specialized metabolite profiles found in tea.
In the current study, we compared transcriptomic and metabolic differences between ‘YJX’ pale green young leaves and their natural greening phenotype under shade treatment, with an emphasis on the changes in characteristic metabolites (catechin and theanine), defective chloroplasts and epigenetic regulation. Our data revealed defective chloroplast development, enhanced antioxidant biosynthesis (especially the redirection of catechins to the flavonol quercetin) and a role for SAMDC on theanine biosynthesis in the ‘YJX’ pale leaves.
Results
Leaf color variation and chloroplast ultrastructure observation
Visual and dynamic changes in leaf color of ‘YJX’ were observed over several growing seasons. In early spring (April in Hefei, China), newly generated young leaves of ‘YJX’ were pale green (PL) and then turned much paler (yellowed) and even contained scorched (dried out) edges as leaf development proceeded. By late spring (June), when shoot apical buds formed, the pale leaves recovered naturally to become green (Fig. 1A). A similar leaf color change occurred during the autumn growing season (data not shown), but all leaves of ‘YJX’ were green before the next year’s spring growing season started. Interestingly, pale or yellow leaves of ‘YJX’ recovered their green color under reduced light irradiation after shading for four days in spring (Fig. 1B), which were used for further analyses in this study. Transmission electronic microscopy (TEM) further revealed that non-shaded PL contained fewer and under-developed chloroplasts per transectioned cell (1.59/cell transection) than shade-induced green leaves (SL) (3.21/cell transection) of ‘YJX’ (Fig. 1C–F). SL chloroplasts maintained more visually clear thylakoid membrane systems with more intensively stained chloroplast components (grana stacks) and starch granules compared to PL chloroplasts, (1.20 and 0.61 granules per transectioned chloroplast, respectively) (Fig. 1D,F). These SL chloroplasts were generally comparable in chloroplast ultrastructure such as thylakoid membrane structure and inner component staining intensity to those in the leaves collected at the same developmental stage in ‘Shu-Cha-Zao’ (Fig. 1G,H), a common green leaf cultivar which is widely grown for regular green tea manufacturing in China. Our observations suggested that pale green leaves in ‘YJX’ had aberrant chloroplast development, but that reduced light intensity was able to recover (at least partially) normal leaf chloroplast development and leaf color.
(A) Leaf color alteration over spring growing season of tea plants. (B) Four-day shaded ‘YJX’ pale leaves and ‘SCZ’ leaves. (C) and (D) chloroplasts in pale leaves of ‘YJX’ sampled on April 25th. (E) and (F) chloroplasts in the shaded green leaves of ‘YJX’ sampled on April 25th. (G) and (H) chloroplasts respectively in the non-shaded and shaded leaves of ‘SCZ’ sampled on April 25th. ‘YJX’, ‘Yu-Jin-Xiang’; ‘SCZ’, ‘Shu-Cha-Zao’; Ch, chloroplast; CW, cell wall; Th, thylakoid; Mi, mitochondria; Gr, grana; SG, starch granule; Vc, vacuole. Bars = 20 μm.
Metabolic alteration in leaf pigments and specialized tea metabolites
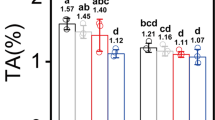
Consistent with the alterations in leaf color and chloroplast ultrastructure in the ‘YJX’ SL and PL, levels of chlorophyll-a (Chla) and -b (Chlb), their sum (Chla + b), and ratio (Chla/b) in PL were dramatically lower than their corresponding values in SL of ‘YJX’ and even lower in leaves of ‘SCZ’ (p < 0.05) at the same developmental stage (Fig. 2A–C). In addition, in ‘YJX’ a significant reduction occurred (P < 0.05) in total carotenoids (11.1%), β-carotene (29.3%), cryptoxanthin (50.0%) and lutein (22.5%). However, a remarkable increase (P < 0.05) in zeaxanthin (69.1%) was found in PL compared to SL, while violaxanthin was at the same level in PL and SL (Fig. 2C–F). Moreover, the abundance of tested carotenoids in the shaded and non-shaded ‘YJX’ leaves were lower than their corresponding counterparts in ‘SCZ’ except for zeaxanthin, whose abundance was conversely higher in ‘YJX’ than in ‘SCZ’ (P < 0.05) (Fig. 2D).
Duncan’s new multiple range test was performed. Columns labeled with the same letter had no significant difference among the four tested leaf measurements, P > 0.05. (A) Chlorophylls. (B) Ratio of chlorophyll a/b. (C–F) Abundances of total carotenoids, β-carotene, zeaxanthin, violaxanthin, cryptoxanthin, and lutein, respectively. (G–I) Abundances of total catechins, EGC, EGCG, EC, and ECG, respectively. (J–L) Abundances of quercetin, kaempferol, caffeine, and theanine, respectively. PL-YJX, pale leaves of ‘YJX’; SL-YJX, shaded green leaves of ‘YJX’; NS-SCZ, non-shaded leaves of ‘SCZ’; S-SCZ, shaded leaves of ‘SCZ’; Chla, chlorophyll a; Chlb, chlorophyll b; EGCG, (−)-epigallocatechin gallate; EGC, (−)-epigallocatechin; EC, (−)-epicatechin; ECG, (−)-epicatechingallate. FW, fresh weight.
Notably, significant changes were found in the abundance of specialized metabolites (flavonols, catechins, and theanine, but not caffeine) in ‘YJX’ compared to their counterparts in ‘SCZ’ (Fig. 2G–L). The levels of total catechins, four dominant catechines epigallocatechin 3-gallate (EGCG), epigallocatechin (EGC), epicatechin 3-gallate (ECG) and epicatechin (EC) in both shaded and non-shaded leaves of ‘YJX’ were all remarkably lower than those in ‘SCZ’ (P < 0.05) (Fig. 2G–I; Supplementary Fig. S1). A four-day shading treatment did not significantly affect the abundance of the tested catechins in ‘YJX’, except for ECG, which was reduced (P < 0.05). However, in ‘SCZ’, shading resulted in a significant increase in galloylated catechins EGCG and ECG (P < 0.05) (Supplementary Fig. S1) and a significant decrease in non-galloylated catechins EC and EGC (P < 0.05) (Fig. 2G–I). Interestingly, high level of quercetin, a product branched off from the metabolic flux towards catechin biosynthesis (Supplementary Fig. S1), was found in PL of ‘YJX’, followed by a significantly lower level in SL, and both were dramatically higher in the two types of ‘YJX’ leaves than in leaves of ‘SCZ’ (P < 0.05) (Fig. 2J). The abundance of kaempferol (Supplementary Fig. S1) in PL was also higher than that in SL (P < 0.05). Similarly, shading led to a significant reduction in kaempferol in ‘SCZ’ leaves (Fig. 2J). No difference was found in caffeine abundance among the different test leaves (Fig. 2K). However, a higher level of theanine was present in the non-shaded leaves of ‘YJX’ than in ‘SCZ’ (P < 0.05), whereas shade elevated theanine to a higher level in ‘SCZ’ leaves than in ‘YJX’ leaves (Fig. 2L).
Transcriptomic alteration for various biological processes and pathways
Transcriptomic analysis was conducted for ‘YJX’ non-shaded pale leaves and shaded green leaves based on a reference transcriptomic dataset established using “deep” sequencing of a mixed RNA from the tested leaf samples. In the reference dataset, a 18,1161 transcripts and 82,134 unigenes were assembled having N50 values 1,905 bp and 1,061 bp, respectively (Table 1; Supplementary Table S1; Supplementary Fig. S2). Further, a total of 37,753 unigenes were annotated using six publicly available databases (Table 1; Supplementary Table S2), while 1,196 differentially expressed genes (DEGs) were identified between the two types of leaf samples based on reads per kilobase per million (RPKM) and a fold change ratio limit of >2 (PL/SL) and P-value < 0.0529 (FDR Bonferroni-corrected). Gene Ontology (GO) analysis according to Alexa et al.30 revealed that the identified DEG dataset included significant differences in certain biological processes, molecular functions and cellular components (Supplementary Fig. S3), which were over-represented out of 355, 102, and 64, for the above three different categories respectively identified (data not shown). Enrichment analysis of the GO terms revealed that biological processes ‘chloroplast organization’, ‘response to high light’, and ‘establishment of protein localization to organelle’ were significantly affected (Fig. 3A) in addition to many other processes (Supplementary Fig. S3 and Table S5). KEGG analysis revealed the involvement of the DEGs in many biosynthetic pathways, particularly in protein processing, plant-pathogen interaction and amino acid biosynthesis, as well as theanine biosynthesis and flavonoid biosynthesis among other genes, each representing 5% of annotated genes (Fig. 3B).
(A) Enriched gene ontology biological processes. (B) Affected metabolic pathways revealed by KEGG analysis. Theanine biosynthesis was constructed according to Kanehisa et al.64.
Further analysis revealed that 44 DEGs were related to chloroplast activities, among which 13 were up-regulated and 31 were down-regulated in expression, and some gene products were localized in chloroplasts (Table 2). The 44 DEGs involved in diverse biological activities, and included the LIGHT HARVESTING COMPLEX A-B BINDING PROTEIN 7 (LHCB7) in photosynthesis, ENDOGLUCANASE 8 in carbohydrate metabolism, TRANSLOCON OF THE INNER ENVELOPE OF CHLOROPLASTS 32 (TIC32) in membrane transport, THYMIDINE KINASE in nucleotide metabolism, and the ATP-dependent CLP PROTEASE PROTEOLYTIC SUBUNIT 5 (CLPP5) involved in chloroplast protein catabolism. SUCROSE SYNTHASE 6, transporters and stress defensive genes were largely up-regulated in PL compared to SL, while others were down-regulated (Table 2). In addition, significant alteration was also found in transcript levels of five ubiquitous regulator-ubiquitin proteasome system genes (UPS), seven PPRs, two retrotransposon genes and one terpenoid pathway gene (Supplementary Table S3).
To determine potential epigenetic involvement in the alteration of leaf color and specialized metabolites, we identified 38 DEGs (out of all those identified) related to DNA methylation and histone methylation and acetylation in the ‘YJX’ transcriptome data. Interestingly, all 38 DEGs were lower in expression in PL compared to SL according to their RPKM ratios (Table 3). These included DNA METHYLTRANSFERASE 1 (MET1) (PL/SL = 0.29), HISTONE-LYSINE N-METHYLTRANSFERASE ASHR 3 (PL/SL = 0.21), HISTONE-LYSINE N-METHYLTRANSFERASE ATXR 6 (PL/SL = 0.24) and METHYL-CPG-BINDING DOMAIN-CONTAINING PROTEIN 9 (MBD9) (PL/SL = 0.43) (Table 3).
DEG transcript level validation using quantitative real-time PCR
Quantitative real-time PCR (qPCR) analysis was applied for validation of transcriptomic data and also for differentiation of gene expression under shade and non-shade conditions used to test the two cultivars ‘YJX’ and ‘SCZ’. QPCR results confirmed that ‘YJX’ non-shaded pale leaves had a significantly higher transcript level of the E3 RING1 gene, but significantly lower levels of ClpP5 and LHCB7 (Fig. 4A) compared to shaded green YJX’ leaves. Similarly, the expression of E3 RING1 in ‘SCZ’ was significantly lower in non-shaded leaves compared to shaded leaves. ClpP5 had very low expression in ‘SCZ’ in both non-shaded and shaded leaves compared to their counterparts in ‘YJX’ (Fig. 4A). Moreover, LHCB7 expression was significantly reduced due to shading in ‘SCZ’, which was opposite to what we found in ‘YJX’ (Fig. 4A).
(A) Transcript levels of DEGs related to chloroplast activities relative to E3 RING1 in SL-YJX. ClpP5, Clp protease proteolytic subunit 5; E3 RING1, E3 ubiquitin-protein ligase RING 1; LHCB7, Light harvesting chlorophyll a-b binding protein 7. (B) and (C) Transcript levels of DEGs related to photo-protection relative to APX3 in PL-YJX and VDE-2 in SL-YJX. APX3, ascorbate peroxidase3; GolS2, galactinol synthases 2; GSTF2, phi class of glutathione transferases 2; VDE, violaxanthin de-epoxidase; ZEP, zeaxanthin epoxidase. (D) Transcript levels of DEGs related to epigenetics relative to MBD9 in NS-SCZ. TKRP125, 125 kDa kinesin-related protein; MBD9, Methyl-CpG-binding domain-containing protein 9. The columns labelled with the same letter for the same gene had no significant difference by Duncan’s new multiple range test, P > 0.05. ND, not detectable.
The expression levels of ASCORBATE PEROXIDASE 3 (APX3), GALACTINOL SYNTHASE 2 (GolS2), GLUTATHIONE TRANSFERASE 2 (GSTF2), VIOLAXANTHIN DE-EPOXIDASE (VDE) and ZEAXANTHIN EPOXIDASE (ZEP), related to the biological process ‘response to high light’ in GO analysis were also examined by qPCR. Transcript levels of all these unigenes were higher in PL than in SL in ‘YJX’ (P < 0.05) (Fig. 4B,C). However, the expression of these genes (P < 0.05) in ‘SCZ’ shaded and non-shaded leaves were not significantly changed, except for GSTF2 which had a lower expression level in shaded leaves than in shaded ones (Fig. 4B). VDE and ZEP had higher expression in PL than in SL (Fig. 4C), a finding which correlated well with the difference in violaxanthin and zeaxanthin (Fig. 2D–F). Notably, VDE-2 and ZEP-2 were expressed at very low levels in ‘SCZ’ in both shaded and non-shaded leaves (Fig. 4C). These data suggested that the photo-protection system had a higher expression level in PL of ‘YJX’ than in SL and ‘SCZ’. Moreover, the transcript levels of the 125 KDA KINESIN-RELATED PROTEIN (TKRP125) and MBD9 genes were significantly lower in the non-shaded leaves than in the shaded leaves in both cultivars (P < 0.05). As well, all qPCR data were highly consistent with the transcriptomic results.
Differentially expressed genes in the flavonoid pathway
Flavonoid-derived phenolic compounds, especially the dihydroxyflavonol quercetin, are specialized compounds not only functioning as antioxidants in photo-protection to quench reactive oxygen species31, but also determining tea flavor and having health. Unigenes annotated as CHALCONE SYNTHASE (CHS) -2 and -3 were found within our transcriptomic data out of the three CHS isoforms previously reported in tea32,33. Sequences common to both CHS-2 and -3 were used for qPCR primer design (Supplementary Table S3) to quantify these two isoforms together. A full length FLAVONOL SYNTHASE (FLS) cDNA was found to be identical to the posted sequence (GenBank EF205150.1). In addition to the functionally characterized LAR1 in tea (Genbank GU992401)8, LAR2, which was previously reported to have a lower expression than LAR133, was found. For FLAVANONE 3′-HYDROXYLASE (F3′H), CHALCONE ISOMERASE (CHI), and ANTHOCYANIDIN SYNTHASE (ANS), quantification of their transcripts was performed only using annotated unigenes with the longest sequences, since these three genes had not been previously characterized so far and the longer sequences would be functionally more reliable than short assembled sequences present in the transcriptome data.
The transcript levels of CHS, CHI, F3H, and FLAVONOID 3′,5′-HYDROXYLASE (F3′5′H), all functioning in the upstream pathway, were significantly higher (P < 0.05) in PL than in SL of ‘YJX’, while DIHYDROFLAVONOL 4-REDUCTASE (DFR) maintained the same level in both leaf types and FLAVONOL SYNTHASE (FLS) was expressed at a lower level in PL than in SL of ‘YJX’ (Fig. 5). In ‘SCZ’, the expression of several of these genes was almost at the same level under shading and non-shading conditions, except for CHI, DFR, and FLS (P < 0.05) (Fig. 5). Significantly higher expression of CHI and FLS and lower expression of DFR were found in the non-shaded leaves compared to the shaded leaves in ‘SCZ’ (Fig. 5F,G). Moreover, high expression of FLS and low expression of DFR were noted in ‘YJX’ compared to ‘SCZ’. However, the expression of downstream pathway genes LAR1 and -2, ANS and ANTHOCYANIDIN REDUCTASE (ANR) -1 and -2 were lower (P < 0.05) in PL than in SL in ‘YJX’, and their expression showed the same pattern in ‘SCZ’ (Fig. 5H–J). These results suggested that transcription changes in flavonoid pathway genes favored enhanced biosynthesis of quercetin rather than catechins in ‘YJX’.
Duncan’s new multiple range test was performed and the levels labeled with the same letter for the same gene exhibited no significant difference among the tested samples, P > 0.05. CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol 4-reductase; FLS, flavonol synthase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase; ANS, anthocyanidin synthase.
Differentially expressed genes in the theanine biosynthetic pathway
Theanine was enriched in PL of ‘YJX’ compared with common green tea cultivars1,4. For a better understanding of theanine production in ‘YJX’ and ‘SCZ’ under shade conditions, expression of genes supposedly related to theanine biosynthesis was examined except for ARGININE DECARBOXYLASE (ADC) and ALANINE DECARBOXYLASE (AIDA) since no annotated unigenes were found in our transcriptome data. For transcript quantification, all unigenes annotated as ALANINE AMINOTRANSFERASE (ALT) and GGT were quantified using qPCR. GLUTAMATE DEHYDROGENASE (GDH) -1 and SAMDC were also chosen for qPCR since our transcriptomic data indicated that they were differentially expressed between PL and SL (data not shown). For other related genes, the unigenes with the longest sequences were quantified.
The expression levels of GLUTAMINE SYNTHETASE (GS), GLUTAMATE SYNTHASE (GOGAT), GLUTAMATE DEHYDROGENASE (GDH-2), and NAD-GLUTAMATE DEHYDROGENASE (NAD-GDH), all related to the bioconversion between glutamate and glutamine, were enhanced in the non-shaded PL compared with the shaded SL of ‘YJX’ (Fig. 6). The expression of ALANINE AMINOTRANSFERASE (ALT), responsible for the biosynthesis of the precursor L-alanine, was unchanged between PL and SL. In contrast, the expression of SAMDC, responsible for the biosynthesis of the immediate precursor ethylamine, was lower in PL than in SL. TS-1, TS-2, and GGT-1, GGT-2, catalyzing theanine production, were expressed higher in PL than in SL of ‘YJX’. Interestingly, transcription levels of all the tested genes in ‘SCZ’ were higher in shaded leaves than in non-shaded ones and consistent with the contents of theanine in ‘SCZ’.
Duncan’s new multiple range test was performed and the levels labeled with the same letter for the same gene exhibited no significant difference among the tested samples, P > 0.05. Transcript level of GOGAT gene was set as 1. GS, glutamine synthetase; GOGAT, glutamate synthase; GDH, glutamate dehydrogenase; NAD-GDH: NAD-dependent glutamate dehydrogenase; TS, theanine synthetase; ALT, alanine aminotransferase; SAMDC: S-adenosylmethionine decarboxylase; AIDA, alanine decarboxylase; ADC, arginine decaboxylase; GGT, γ-glutamyltranspeptidase.
Discussion
In this study, phenotypic alteration, transcriptomic reprogramming and metabolic flux redirection were studied using the conversion of a young pale/yellow leaf phenotype into a green leaf phenotype in the albino tea (Camellia sinensis) cultivar ‘YJX’ under shaded conditions. By comparing this conversion with changes that occurred in leaves of the common green tea cultivar ‘SCZ’ under the same shade conditions, dynamic differences in ‘YJX’ were elucidated.
Phenotypic alterations in leaf color and specialized metabolite abundance occurred with leaf development and light intensity in ‘YJX’. Natural high light shining upon pale leaves in spring led to a more extreme pale phenotype, whereas reduced light intensity through shading resulted in the rapid greening of leaf color of ‘YJX’. Similar light dependent leaf phenotypic changes have been reported previously in maize and Arabidopsis34,35. Our data revealed that the non-shaded pale leaves of ‘YJX’ contained aberrant chloroplasts with undeveloped thylakoid membranes and significantly reduced chlorophylls and total carotenoids, both of which play crucial roles in photosystem assembly and, light-harvesting and the latter in photo-protection in chloroplasts36,37, were found in PL compared to SL of ‘YJX’. Moreover, a dramatic reduction for four catechins and an increase for zeaxanthin occurred in ‘YJX’ compared to ‘SCZ’, as reported previously in some other albino tea cultivars1. In addition, a high level of quercetin was found in PL, followed by that in SL of ‘YJX’, and both were tremendously higher than their counterparts in ‘SCZ’ leaves. This is highly consistent with the finding that plants undergoing severe stress conditions (such as high light stress) preferentially accumulate more effective antioxidants such as the dihydroxy B-ring-substituted flavonoid quercetin36,38 and the carotenoid zeaxanthin (a crucial component of the violaxanthin cycle in plant photo-protection)36,37,39. These results indicated that metabolic flux redirection towards zeaxanthin and quercetin enhancement occurred in PL and SL of ‘YJX’ to varying extents compared to ‘SCZ’.
Interestingly, catechin abundance between non-shaded PL and shaded SL of ‘YJX’ was not significantly different, while in shaded leaves of ‘SCZ’ the galloylated catechins (EGCG and ECG) were higher and non-galloylated catechins (EGC and EC) lower than in non-shaded leaves (P < 0.05). This finding in the green cultivar ‘SCZ’ was consistent with previous reports on the changes in the abundance of galloylated and non-galloylated catechins in shaded and non-shaded tea leaves40,41. However, the data for albino ‘YJX’ differed from a previous report42 in which a significant reduction occurred in catechin levels after 3 weeks of shading compared to non-shaded green tea cultivar42. This difference could result from a change in metabolic flow in albino tea leaves and from differently applied shading periods compared with our study43. Significant difference in the abundance of carotenoids, chlorophylls, quercetin, and theanine were also noted between PL and SL of ‘YJX’ and compared to their counterparts in ‘SCZ’ leaves. These data suggest that regulation of catechins differ from that of the other metabolites we studied. Metabolic flux redirection was also reported for theanine enhancement under shade condition as reported for some other albino tea cultivars1,4. Due to the multiple health benefits and flavor contributions of quercetin5 and theanine6, it would be interesting to find out the possible changes in tea flavor and health functions of the albino ‘YJX’.
Transcriptome reprogramming was observed in the PL of ‘YJX’ based on establishment of a deep sequencing reference transcriptome since tea genome information is still unavailable. A total of 1,196 DEGs were identified between the ‘YJX’ PL and SL, which compared favorably with DEG profiles found in Arabidopsis under high light stress44. For validation of the identified DEGs, qPCR was performed on a small subset of DEGs, including E3 RING1, ClpP5, LHCB7 related to aberrant chloroplasts, APX3, GolS2, GSTF2, VDE and ZEP related to photo-protection, and TKRP125 and MBD9 related to epigenetic regulation. GO enrichment analysis revealed that a wide range of biological processes and metabolic pathways were likely affected by these DEGs, including ‘chloroplast organization’, ‘responses to high light intensity’, ‘flavonoid biosynthesis’ and ‘amino acid biosynthesis’. However, light sensor genes45, including PHYTOCHROME A/B/E, CRYPTOCHROME 1, PHOTOTROPIN 1/2 and UV RESISTENCE LOCUS 8, were found not significantly changed in shaded and non-shaded ‘YJX’ leaves. Moreover, downstream genes in the light transduction pathways proposed by Chory45 were neither affected by the shade treatment, nor found in our transcriptomic data. It is interesting to learn the roles of light transduction pathways in the observed transcriptomic and metabolic changes in this study. Consistent with the aberrant chloroplasts we observed, a total of 44 DEGs related to chloroplast activities were found in PL. Interestingly, an extraordinarily high expression level (>18 fold) of ClpP5, a component of the Clp proteasomal complex in the chloroplast, was found in in ‘YJX’ (SL > PL) compared to the control cultivar ‘SCZ’. Abnormal transcription of ClpP5 in maize34 and Arabidopsis35 also results in a virescent leaf phenotype, which can be restored by reduced light intensity. Further investigations are required to find out whether ClpP5 or other subunits of the Clp complex is responsible for the leaf color phenotypic alteration in ‘YJX’.
Chlorophyll deficiency in albino plants generally results in a host of responses to high light stress46 not only by enhancing antioxidant metabolites (including ascorbate, glutathione47,48, zeaxanthin36,37,39, lutein36, and quercetin36,38) but also reprogramming to activate transcriptional responses to high light intensity. Transcriptomic reprogramming also occurred in ‘YJX’ to the genes related to epigenetic regulation, as expected, since the observed leaf phenotypic conversion in ‘YJX’ was activated by plant development and shading, both of which are processes governed by epigenetic modification15. Our data suggested that the 36 DEGs involved in DNA and histone methylation, including H3K27 trimethylation, likely regulate light-induced gene transcription as seen in many other plant species such as Arabidopsis and tobacco49,50. Thus lower expression of these methylation genes negatively correlated with the higher expression of most tested genes in non-shaded leaves compared to shaded ones both in ‘YJX’ and ‘SCZ’15. More detailed investigations are needed for determining the role of specific epigenetic genes on the ‘YJX’ pale phenotype.
Since quercetin was enhanced and catechins decreased in ‘YJX’, flavonoid pathway gene expression was also examined with a focus on metabolic flux redirection in ‘YJX’. Catechins are highly abundant in tea leaves, and their biosynthesis and accumulation have been extensively studied in this species7,8,9. In tea plants, LAR and ANR8, F3′5′H51, and ECGT9 are key downstream enzymes catalyzing the biosynthesis of catechins. Nevertheless, functional characterization of genes for catechin biosynthesis is still limited in tea plants8,51. In this study, qPCR results showed that expression of many upstream flavonoid genes such as CHS, CHI, and F3H, were significantly enhanced in PL compared to SL of ‘YJX’, suggesting the possibility of an enhanced metabolic flux within this part of flavonoid pathway. Higher expression levels of F3H and F3′5′H in PL, genes which are responsible for dihydroflavonol and flavonol quercetin biosynthesis52, were also consistent with an enhanced level of quercetin in PL. Lower levels of ANR1 and -2, ANS, and LAR1 and -2 in ‘YJX’ than in ‘SCZ’ also correlated with decreased levels of different catechins in ‘YJX’. Furthermore, high expression of FLS and low expression of DFR in ‘YJX’ compared to ‘SCZ’ was consistent with the difference in flavonol and flavan-3-ols between the two cultivars. This consistency is further supported by the functional characterization of the two genes7,31,53 and FLS expression is also correlated negatively to catechin abundance in tobacco50 and tea3, but positively correlated with quercetin53. Our data suggested that these genes could be ‘turning points’ for the metabolic flux redirection in ‘YJX’.
The enhanced level of theanine in albino leaves or by shade observed in this study and reported earlier1,4,54,55 suggested potential transcription changes for genes related to theanine biosynthesis. Theanine is reportedly synthesized by theanine synthetase from glutamic acid and ethylamine56, which is derived from L-alanine in tea plants11,57 by the action of AIDA, ADC or SAMDC12,13. However, only SAMDC out of these three decarboxylases was mapped in our tea transcriptome data14,15. This higher expression of SAMDC was consistent with the variation of theanine abundance in shaded and non-shaded leaves of the two cultivars. Additionally, all theanine genes tested by qPCR were also expressed at a higher level in shaded ‘SCZ’ leaves than in non-shaded ones, consistent with the change in theanine level between the two types of ‘SCZ’ leaves but not in ‘YJX’. Hence, the underlying biological mechanisms underlying theanine biosynthesis in ‘YJX’ remain unclear.
In summary, alterations in leaf color, leaf pigments, flavonoids, and theanine as well as global transcription specifically related to flavonoid and theanine pathways clearly revealed that metabolic flux redirection and transcriptomic reprogramming occurred in the pale leaves of ‘YJX’ compared with shaded leaves of this cultivar and the green cultivar ‘SCZ’. Consistency between dramatically altered abundance of multiple metabolites and expression of multiple corresponding genes in flavonoid pathway in ‘YJX’ suggested the action of a regulatory mechanism in ‘YJX’ underlying a concerted expression regulation of many genes upon high light stress. The identified differentially expressed genes related to chloroplast activities and epigenetic regulation may lead to a new horizon to elucidate mechanisms underlying over the ‘YJX’ leaf phenotypic conversion. Our study also has a great potential for tea flavour and health benefit improvement.
Methods
Plant material and shading treatment
Six-year old plants of Camellia sinensis cv. ‘Yu-Jin-Xiang’ (‘YJX’) and ‘Shu-Cha-Zao’ (‘SCZ’) were grown in the tea farm of Anhui Agricultural University located at 31°55′42.8″N, 117°12′09.1″E; Hefei City, Anhui, China with identical cultivation management. Shade treatment was started for young pale leaves of ‘YJX’ and ‘SCZ’ leaves at the same developmental stage on April 22, 2015 for four consecutive days using two layers of black shading nets, resulting in light intensity reduced to 18 mol m−2 s−1 (12.5% of natural sunlight intensity). After four days of treatment, shaded and non-shaded leaves of ‘YJX’ and ‘SCZ’ were collected for chemical profiling, electronic microscope observations, or in some cases transcriptomic sequencing. In detail, TEM, chemical and qPCR analyses were performed using the four types of leaves to give direct comparisons. For transcriptomic data analysis, non-shaded pale and shaded re-greening leaves in ‘YJX’ were used since the changes in ‘YJX’ over the leaf color conversion were focused and leaf transcript analysis of normal green cultivars under shade and non-shade conditions had been reported before42,58.
RNA Isolation, RNA-Seq library construction and illumina sequencing
Total RNA was extracted from tea leaves by using the RNAprep pure Plant Kit (TianGen Biotech., Ltd, Beijing, China). RNA quality and quantity were determined using both agarose gels and a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). RNA samples with A260/280 ratio between 1.8 to 2.0, A260/230 ratio between 2.0 to 2.2 and RIN (RNA integrity number)59 more than 8.0, were used for transcriptomic sequencing. Purified RNA samples (one mixture of young, old and shading leaves and three biological replicates each for PL and SL) were sent to Biomarker Technologies Corporation (Beijing, China) for cDNA library construction and sequencing. For each sample, 10 ng RNA was used for cDNA synthesis with oligo (dT) for mRNA enrichment. Enriched mRNA was fragmented and amplified using an Ovation RNA-Seq System V2 kit (Nugen Technologies, San Carlos, USA) following the manufacturer’s protocols. The cDNA was purified and 1 μg amplified cDNA for each sample was used to generate multiplexed RNA-Seq libraries (mean size about 360 bp) and sequenced using the Illumina HiSeqTM 2000 platform (Illumina, Inc., Shanghai, China).
Functional annotation of the unigenes
The unigene sequences of the mixed tea leaf sample aligned using BLASTX against the COG, GO, KEGG, Swissprot, TrEMBL and NR databases (E-value < = 1E-5) to retrieve functional annotations based on sequence similarity. Unigenes that could not be aligned to any of these databases were analyzed by the ESTScan software60. Gene ontology (GO) analysis was conducted using the TopGO package30. The GO terms with p-values <0.05 were considered significantly enriched. The biological processes of enriched GO terms were then visualized using TopGO30.
Differentially expressed genes functional enrichment
All usable reads were normalized into RPKM values (reads per kb per million reads)61. Differential expression of unigenes between two RNAseq samples were calculated based on “base mean” value obtained from the DESeq package. Only unigenes with an PL/SL RPKM ratio >2 and a false discovery rate (FDR) multiple test score of P < 0.0529, were considered to be differentially expressed unigenes.
Chloroplast ultrastructure observation and chlorophyll abundance measurement
For ultrastructural observation, fresh leaves were excised and infiltrated with 4% glutaraldehyde solution using a syringe and then soaked in the solution according to Li et al.4. Glutaraldehyde-infiltrated tea leaves were cut into 2 × 2 mm pieces and further sectioned using a TCS CM1900 freezing microtome (Leica, Germany). The ultrathin section was double lead stained according to Daddow et al.62 and then observed using a HT-7700 transmission electron microscope (TEM) (Hitachi, Japan).
Fresh leaves (0.1 g, accurate to 0.001 g) were used for chlorophyll abundance quantification according to Feng et al.1. The excised fresh leaves were cut into small pieces. Chlorophyll was extracted overnight using 10 mL (5% acetone: 95% ethanol, v/v) until the leaves became completely white, then the extract was measured using an ultraviolet spectrophotometer (U-5100, Hitachi, Japan) at A645 and A663. The chlorophyll contents were calculated using the following formula according to Feng et al.1:



All experiments were replicated using three independent harvests and light treatments using 20 leaves and 3 tea plants per replicate, and illustrated as ±SD. Duncan’s new multiple range test was performed for significance analysis.
Quercetin and kaempferol, catechin and carotenoid content
Extraction and quantification of catechins and carotenoids were carried out as previously reported1. The extraction of quercetin and kaempferol prior to hydrolysis was carried out following the protocol of Xiong et al.3: tea leaves (ca. 1 g dry weight) were mixed with 40 ml of 60% ethanol and 5 ml HCl (6 M). After refluxing at 95 °C for 2 h, the hydrolyzed solutions were filtered through filter paper, then diluted to 50 ml each using 60% ethanol. Abundances of flavonols, catechins and carotenoid were measured according to previous reports1,3, using HPLC with a C18 Column (4.6 × 250 mm; Phenomenex, Shanghai, China). The compounds were quantified using the standard curves. All compounds were measured using three replicates and shown as means ± SD. Statistical analysis was performed using Duncan’s new multiple range test.
Quantitative real-time PCR
Q-PCR expression assays were performed on a CFX96 platform (Bio-Rad, www.bio-rad.com/), using specific primers (listed in Table S4), and the Top Green qPCR SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. PCR reaction efficiencies for all test genes were over 90% and their transcript levels were calculated using the 2−ΔΔCt method63. All experiments were done using three replicates and shown as means ± SD. Duncan’s new multiple range test was performed for significance analysis.
Additional Information
How to cite this article: Liu, G.-F. et al. Metabolic Flux Redirection and Transcriptomic Reprogramming in the Albino Tea Cultivar ‘Yu-Jin-Xiang’ with an Emphasis on Catechin Production. Sci. Rep. 7, 45062; doi: 10.1038/srep45062 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Feng, L. et al. Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 155, 98–104 (2014).
Wei, K. et al. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 130, 720–724 (2012).
Xiong, L. G. et al. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 71, 132–143 (2013).
Li, Q. et al. Proteomic analysis of young leaves at three developmental stages in an albino tea cultivar. Proteome Sci. 9(1), 44 (2011).
Chaturvedula, P. & Prakash, I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 5, 2110–2124 (2011).
Chu, D., Kobayashi, K., Juneja, L. R. & Yamamoto, T. Chemistry and applications of green tea. (Eds Yamamoto, T., Juneja, L. R., Chu, D. & Kim, M. ) 129–135 (CRC, 1997).
Punyasiri, P. A. N. et al. Flavonoid biosynthesis in the tea plant Camellia sinensis: properties of enzymes of the prominent epicatechin and catechin pathways. Arch. Biochem. Biophys. 431, 22–30 (2004).
Pang, Y. Z. et al. Functional characterization of proanthocyanidin pathway enzymes from tea and their application for metabolic engineering. Plant Physiol. 161, 1103–1116 (2013).
Liu, Y. J. et al. Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 287, 44406–44417 (2012).
Xu, W. J., Dubos, C. & Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 20, 1360–1385 (2015).
Deng, W. W., Ogita, S. & Ashihara, H. Biosynthesis of theanine (γ-ethylamino-L-glutamic acid) in seedlings of Camellia sinensis . Phytochem. Lett. 1, 115–119 (2008).
Shi, C. Y. et al. Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC Genomics 12(1), 131 (2011).
Tai, Y. L. et al. Transcriptomic and phytochemical analysis of the biosynthesis of characteristic constituents in tea (Camellia sinensis) compared with oil tea (Camellia oleifera). BMC Plant Biol. 15(1), 190 (2015).
Suzuki, H., Izuka, S., Miyakawa, N. & Kumagai, H. Enzymatic production of theanine, an “umami” component of tea, from glutamine and ethylamine with bacterial γ-glutamyltranspeptidase. Enzyme Microb. Tech. 31, 884–889 (2002).
Feng, S. H., Jacobsen, S. E. & Reik, W. Epigenetic reprogramming in plant and animal development. Science 330, 622–627 (2010).
Chinnusamy, V. & Zhu, J. K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 12, 133–139 (2009).
Liu, X. C. et al. PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chlorophyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell 25, 1258–1273 (2013).
Cazzonelli, C. I. et al. Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell 21, 39–53 (2009).
El-Sharkawy, I., Liang, D. & Xu, K. N. Transcriptome analysis of an apple (Malus × domestica) yellow fruit somatic mutation identifies a gene network module highly associated with anthocyanin and epigenetic regulation. J. Exp. Bot. 66, 7359–7376 (2015).
Sarraf, S. A. & Stancheva, I. Methyl-CpG binding protein MBD1 couples histone H3 methylation at Lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol. Cell 15, 595–605 (2004).
Pogson, B. J. & Albrecht, V. Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol. 155, 1545–1551 (2011).
Myouga, F. et al. The chloroplast function database II: a comprehensive collection of homozygous mutants and their phenotypic/genotypic traits for nuclear-encoded chloroplast proteins. Plant Cell Physiol. 54(2), e2–e2 (2013).
Ling, Q., Huang, W., Baldwin, A. & Jarvis, P. Chloroplast biogenesis is regulated by direct action of the ubiquitin-proteasome system. Science 338, 655–659 (2012).
Olinares, P. D. B., Kim, J. & van Wijk, K. J. The Clp protease system; a central component of the chloroplast protease network. BBA-Bioenergetics 1807, 999–1011 (2011).
Araki, N., Kusumi, K., Masamoto, K., Niwa, Y. & Iba, K. Temperature-sensitive Arabidopsis mutant defective in 1-deoxy-D-xylulose 5-phosphate synthase within the plastid non-mevalonate pathway of isoprenoid biosynthesis. Physiol. Plantarum 108, 19–24 (2000).
Vranová, E., Coman, D. & Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 64, 665–700 (2013).
Koussevitzky, S. et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 (2007).
Chi, W., Sun, X. & Zhang, L. Intracellular signaling from plastid to nucleus. Annu. Rev. Plant Biol. 64, 559–582 (2013).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Alexa, A., Rahnenführer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006).
Agati, G., Azzarello, E., Pollastri, S. & Tattini, M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196, 67–76 (2012).
Takeuchi, A., Matsumoto, S. & Hayatsu, M. Chalcone synthase from Camellia sinensis: isolation of the cDNAs and the organ-specific and sugar-responsive expression of the genes. Plant Cell Physiol. 35, 1011–1018 (1994).
Jiang, X. L. et al. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. Plos One 8, e62315 (2013).
Xing, A. Q. et al. A pair of homoeolog ClpP5 genes underlies a virescent yellow-like mutant and its modifier in maize. Plant J. 79, 192–205 (2014).
Kim, J. et al. Subunits of the plastid ClpPR protease complex have differential contributions to embryogenesis, plastid biogenesis, and plant development in Arabidopsis. Plant Cell 21, 1669–1692 (2009).
Brunetti, C., Guidi, L., Sebastiani, F. & Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 119, 54–62 (2015).
Cazzonelli, C. I. & Pogson, B. J. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 15, 1360–1385 (2010).
Agati, G. & Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 186, 786–793 (2010).
Pinnola, A. et al. Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens . Plant Cell 25, 3519–3534 (2013).
Lee, L. S. et al. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 61, 332–338 (2013).
Saijo, R. Pathway of gallic acid biosynthesis and its esterification with catechins in young tea shoots. Agric. Biol. Chem. 47, 455–460 (1983).
Wang, Y. S. et al. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 141, 7–16 (2012).
Wade, N. L., Tan, S. C. & Kavanagh, E. E. White light prevents increased catechin synthesis by ultraviolet irradiation in banana fruits. J. Hortic. Sci. 68, 637–644 (1993).
Kleine, T., Kindgren, P., Benedict, C., Hendrickson, L. & Strand, Å. Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol. 144, 1391–1406 (2007).
Chory, J. Light signal transduction: an infinite spectrum of possibilities. Plant J. 61(6), 982–991 (2010).
Wu, Q. J., Chen, Z. D., Sun, W. J., Deng, T. T. & Chen, M. J. De novo sequencing of the leaf transcriptome reveals complex light-responsive regulatory networks in Camellia sinensis cv. Baijiguan . Front. Plant Sci. 7, 332 (2016).
Li, Z., Wakao, S., Fischer, B. B. & Niyogi, K. K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 (2009).
Foyer, C. H. & Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100 (2011).
Jang, I. C., Chung, P. J., Hemmes, H., Jung, C. & Chua, N. H. Rapid and reversible light-mediated chromatin modifications of Arabidopsis phytochrome A locus. Plant Cell 23, 459–470 (2011).
Miguel, C. & Marum, L. An epigenetic view of plant cells cultured in vitro: somaclonal variation and beyond. J. Exp. Bot. 62, 3713–3725 (2011).
Wang, Y. S. et al. Functional analysis of flavonoid 3′,5′-hydroxylase from tea plant (Camellia sinensis): critical role in the accumulation of catechins. BMC Plant Biol. 14(1), 347 (2014).
Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126, 485–493 (2001).
Mahajan, M., Ahuja, P. S. & Yadav, S. K. Post-transcriptional silencing of flavonol synthase mRNA in tobacco leads to fruits with arrested seed set. Plos One 6(12), e28315 (2011).
Deng, W. W. et al. Effect of shade treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Growth Regul. 71, 295–299 (2013).
Zhang, Q. F., Shi, Y. Z., Ma, L. F., Yi, X. Y. & Ruan, J. Y. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. Plos One 9(11), e112572 (2014).
Ashihara, H. Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-L-ethylamide) in plants: a comprehensive review. Nat. Prod. Commun. 10(5), 803–810 (2015).
Takeo, T. L-Alanine as a precursor of ethylamine in Camellia sinensis . Phytochemistry 13, 1401–1406 (1974).
Li, N. N. et al. Effects of sunlight on gene expression and chemical composition of light-sensitive albino tea plant. Plant Growth Regul. 78, 253–262 (2016).
Andreas, S. et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 7(1), 3 (2006).
Iseli, C., Jongeneel, C. V. & Bucher, P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 99, 138–148 (1999).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Daddow, L. Y. M. A double lead stain method for enhancing contrast of ultrathin sections in electron microscopy: a modified multiple staining technique. J. Microsc. 129, 147–153 (1983).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Kanehisa, M., Goto, S., Kawashima, S., Okuno, Y. & Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 32, 277–280 (2004).
Acknowledgements
The authors thank master R.D. and bachelor H.Z. assistance in the arrangement of the transcriptome data. This study was financially supported by the National Natural Science Foundation of China [#31070614 and 31370687] and the Chinese Research Fund for the Doctoral Program of Higher Education [20123418110002].
Author information
Authors and Affiliations
Contributions
G.-F.L. performed majority of the experiments and conducted the data analysis and manuscript draft preparation; Z.-X.H. assisted with HPLC analysis; Z.-X.H. and L.F. helped with the preparation of RNA samples for transcriptome data analysis. M.-J.G., and M.Y.G., revised the manuscript; Z.-L.Z., L.-P.G., T.X., and X.-C.W. were involved in the parts in flavonoid and theanine pathways, respectively; S.W. conceived and designed the study, analyzed the data, and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, GF., Han, ZX., Feng, L. et al. Metabolic Flux Redirection and Transcriptomic Reprogramming in the Albino Tea Cultivar ‘Yu-Jin-Xiang’ with an Emphasis on Catechin Production. Sci Rep 7, 45062 (2017). https://doi.org/10.1038/srep45062
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45062
This article is cited by
-
Metabolite profiling in albino tea mutant Camellia sinensis ‘Fuyun 6’ using LC–ESI–MS/MS
Trees (2022)
-
Light control of catechin accumulation is mediated by photosynthetic capacity in tea plant (Camellia sinensis)
BMC Plant Biology (2021)
-
Review: the effect of light on the key pigment compounds of photosensitive etiolated tea plant
Botanical Studies (2021)
-
Significantly increased amino acid accumulation in a novel albino branch of the tea plant (Camellia sinensis)
Planta (2019)
-
Metabolite profiling and transcriptomic analyses reveal an essential role of UVR8-mediated signal transduction pathway in regulating flavonoid biosynthesis in tea plants (Camellia sinensis) in response to shading
BMC Plant Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.