Abstract
In young rats, ischemic preconditioning (IPC), which consists of 4 cycles of ischemia and reperfusion alleviated kidney injury caused by 40-min ischemia. However,old rats lost their ability to protect the ischemic kidney by IPC. A similar aged phenotype was demonstrated in 6-month-old OXYS rats having signs of premature aging. In the kidney of old and OXYS rats, the levels of acetylated nuclear proteins were higher than in young rats, however, unlike in young rats, acetylation levels in old and OXYS rats were further increased after IPC. In contrast to Wistar rats, age-matched OXYS demonstrated no increase in lysosome abundance and LC3 content in the kidney after ischemia/reperfusion. The kidney LC3 levels were also lower in OXYS, even under basal conditions, and mitochondrial PINK1 and ubiquitin levels were higher, suggesting impaired mitophagy. The kidney mitochondria from old rats contained a population with diminished membrane potential and this fraction was expanded by IPC. Apparently, oxidative changes with aging result in the appearance of malfunctioning renal mitochondria due to a low efficiency of autophagy. Elevated protein acetylation might be a hallmark of aging which is associated with a decreased autophagy, accumulation of dysfunctional mitochondria, and loss of protection against ischemia by IPC.
Similar content being viewed by others
Introduction
Aging affects both the kidney’s morphology and functioning and it is associated with the increased sensitivity of the kidney to the damaging factors leading to a more severe course of acute kidney injury (AKI) and increased risk of transition from AKI to chronic kidney disease.
Among the molecular mechanisms of aging, an important role is assigned to the processes of acetylation1,2. It is assumed to be a component of the beneficial mechanism of caloric restriction which can increase lifespan through the system associated with the expression of Sirt deacetylases3,4,5. Another target of caloric restriction is mTOR, which controls the processes of autophagy6,7. The decrease in autophagy is an important attribute of the aging cell8. Finally, mitochondria have been implicated in a number of processes, that are crucial in aging9,10,11,12. Distinct functional and morphological changes appear to accumulate in the mitochondria of the aged organ11,12. Currently, these three elements: mitochondria, levels of protein acetylation and autophagy have become critical targets in studies of protection against age-related deleterious changes in organs such as the kidney.
Aging is accompanied by ischemia-associated pathologies and important protective anti-ischemic measures are ischemic preconditioning (IPC) and post conditioning13,14,15 including both direct and remote16, shown for a large number of organs and tissues17,18,19,20, including the kidney21,22. The studies of signaling pathways involved in IPC21,23,24 showed that mitochondria are an essential requisite for implementation of protective mechanisms and dysfunction of these organelles may be related with age-dependent changes in the efficiency of IPC25.
In a contradictory manner, while the majority of patients with AKI are elderly, the plethora of experimental data on this pathology was obtained on young animals. This may be an explanation why most clinical trials of drugs fail although their efficacy has been proven in animal studies. At the same time, the use of old animals is coupled with objective difficulties, and therefore, an important challenge is to find models that examine the phenomenon of aging in vivo. One such model is accelerated-senescence OXYS rats. This strain was established by selection and inbreeding of Wistar rats sensitive to the cataractogenic effects of a galactose-rich diet. Thereafter, in rats of this strain, cataract, a typical disease of old age, developed spontaneously at an early age. OXYS rats demonstrate the signs of premature aging manifested in the development of diseases common to elder age, such as early retinopathy similar to age-related macular degeneration in humans, osteoporosis, arterial hypertension, hypertrophic cardiomyopathy, accelerated thymus involution, sarcopenia and accelerated brain senescence with the features inherent for Alzheimer’s disease26,27,28,29,30,31. It can be assumed that the accelerated aging also affects kidney, making these rats a unique model for studying the effects of ageing on renal diseases.
Presently it is unknown whether the protective effect of IPC is sustained in the kidney during advanced ageing. The goal of this study is to analyze possible changes in natural ischemic tolerance mechanisms in the aged kidney with special attention to protein acetylation, lysosomes and components of mitochondrial autophagy.
Results
Renal hemodynamics after ischemia and IPC
To study the alterations of renal vasculature in response to ischemia/reperfusion (I/R) we measured intrarenal blood flow velocity with Doppler ultrasound. With I/R, the renal blood flow in young rats reached only 80% of the pre-ischemic level after 10 min of reperfusion (Fig. 1). IPC (4 cycles of 15 seconds of ischemia and 15 seconds of reperfusion each) prior to I/R had a beneficial effect on blood flow restoration (Fig. 1).
IPC affords protection of only young animals’ ischemic kidney
Kidney I/R resulted in the development of AKI in young (4–6-month-old) rats (Fig. 2A). The impairment of excretory renal function was evidenced by more than 6-fold increase in blood urea (BUN) 48 hours after I/R. IPC prior to I/R alleviated the severity of renal failure in young animals (Fig. 2A). Although the severity of renal failure after I/R in 20–23-month-old rats was about the same as in young rats, IPC of the kidneys of old animals was not protecting (Fig. 2A). Of note, BUN of intact 20–23-month-old rats was the same as in young rats (Fig. 2A).
(A) Young (4–6 month) and old (20–23 month) outbred rats. Number of animals: “young control” n = 11, “young I/R” n = 21, “young IPC+I/R” n = 10, “old control” n = 6, “old I/R” n = 6, “old IPC+I/R” n = 9. (B) Young (6-month) Wistar and OXYS rats. Number of animals: “Wistar control” n = 3, “Wistar I/R” n = 8, “Wistar IPC+I/R” n = 6, “OXYS control” n = 3, “OXYS I/R” n = 7, “OXYS IPC+I/R” n = 7. *p < 0.05 compared to control, **p < 0.05 compared to I/R.
In young rats, the total antioxidant capacity (TAC) of cytosolic fractions of the kidney exposed to IPC was increased by 33% versus that in unexposed animals (from 45.82 ± 0.75 to 61.14 ± 3.66 mmol of trolox-equivalents/mg protein). Unlike in young rats, in old ones TAC decreased after IPC by 43% versus unexposed old rats (from 57.91 ± 14.66 to 33.00 ± 8.20).
The effect of IPC on AKI in OXYS
We have not found the signs of impairment of renal function in 6-month-old OXYS. No significant differences in renal morphology in OXYS rats compared to Wistar were found (Supplementary Figure S1). Kidney I/R in these rats resulted in a profound renal insufficiency with 12-times BUN increase (Fig. 2B). In addition, IPC of 6-month-old OXYS didn’t afford nephroprotection against I/R (Fig. 2B). This response to IPC was quite different from the effect of IPC in young, but similar to the response of old outbred rats, which also demonstrated insensitivity to renal IPC (Fig. 2A).
To exclude the possibility that the response to IPC is due to the difference in the strains of the rats, we studied the kidney functioning in 6-month Wistar rats (which were the origin for raising OXYS) 48 hours after I/R without and with IPC (Fig. 2B). In these age-matched Wistar, BUN was normal and close to that in outbred animals. On the day 2 after I/R, BUN in these animals was increased to 6 times versus control level. IPC of 6-month Wistar as in young outbred rats had a remarkable nephroprotective effect, resulting in reducing BUN (Fig. 2B). Thus, we found no differences in the occurrence of ischemia-induced AKI in young outbred and Wistar rats.
In addition, in intact OXYS, renal TAC was slightly higher than in age-matched Wistar rats (54.68 ± 10.16 and 43.31 ± 4.23, respectively) and after IPC, it decreased in OXYS kidneys to 28.10 ± 2.18 mmoles of trolox-equivalents/mg protein.
Proteins acetylation in young and old animals
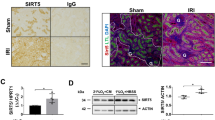
We found that in renal cortex, acetylated lysines are predominantly localized in the nuclei of tubular cells (Fig. 3A). Only 10% of the tubules from young rats have over half of nucleus with apparent fluorescent signal. Such acetylation-positive tubules were twice as abundant in kidneys harvested 40 minutes after I/R compared to baseline (Fig. 3A,B). If before I/R the kidney was subjected to IPC, the levels of acetylated nuclear proteins were significantly lower that after I/R alone (Fig. 3A,B).
Kidneys of young (A,B), old (C,D) and young OXYS (E,F) rats were harvested at baseline, 40 minutes after I/R and I/R with IPC. Graphs in (B,D,F) illustrate the portion of tubules with acetylated nuclei. (A,C,E): Confocal microscopy of kidney cortex sections stained with acetyl-Lys antibodies (green fluorescence) and TRITC-phalloidin (red fluorescence). Scale bars indicate 100 μm. Number of slices: “young control” n = 45, “young I/R” n = 45, “young IPC+I/R” n = 45, “old control” n = 60, “old I/R” n = 60, “old IPC+I/R” n = 45, “OXYS control” n = 45, “OXYS I/R” n = 60, “OXYS IPC+I/R” n = 60. *p < 0.05 compared to control, **p < 0.05 compared to I/R.
The levels of acetylation in the old kidney cortex revealed a significant difference from those of young ones (Fig. 3C). The percentage of acetylation-positive tubules was two times more than in young animals (Fig. 3D vs 3B). I/R of the old kidney led only to a slightly higher number of acetylated tubules (Fig. 3C,D) while priming the kidney with IPC resulted in a remarkably greater number of these (Fig. 3C,D).
The response of kidney cortex acetylation levels to I/R and IPC in 6-month-old OXYS was qualitatively similar to that observed in 23-month-old outbred rats (Fig. 3E). In the kidneys of intact OXYS rats, the percentage of highly acetylated tubules was much higher than in the kidneys of young outbred rats (Fig. 3F) and was not higher in kidneys subjected to I/R. However, after IPC, a significantly greater percentage of highly acetylated tubules was observed (Fig. 3E,F).
Response of autophagy-lysosomal machinery to I/R in young and OXYS rats’ kidneys
Differences between OXYS and young outbred rats were also evident in the response of autophagy-lysosomal machinery to kidney I/R. In renal cortex slices from intact Wistar rats, we observed uniform Lysotracker staining in the tubules (Fig. 4A). In kidneys harvested 24 hours after I/R, the average intensity of Lysotracker fluorescence in the slices of renal cortex was about twofold higher than in intact rat (Fig. 4A,B). In renal cortex slices from rats exposed to renal IPC before I/R, the average Lysotracker fluorescence intensity was similar to that in intact rats (Fig. 4A,B). The lysosomal staining of renal cortex slices from intact OXYS rats revealed a similar average Lysotracker fluorescence intensity compared to intact Wistars (Fig. 4A,B). However, unlike in age-matched Wistar rats, lysosome abundance 24 hours after I/R or after I/R with IPC was not greater than in controls (Fig. 4A,B).
(A) Confocal microscopy after Lysotracker Green staining of vital renal cortex slices. The scale bar indicates 100 μm. (B) Quantification of Lysotracker Green fluorescence intensity. Number of slices: “Wistar control” n = 75, “Wistar I/R” n = 75, “Wistar IPC+I/R” n = 75, “OXYS control” n = 45, “OXYS I/R” n = 45, “OXYS IPC+I/R” n = 30. (C) Level of autophagy-associated LC3-I protein in kidneys harvested at baseline, 48 hours after I/R and after I/R with IPC, as estimated by Western blotting. Number of animals: “Wistar control” n = 3, “Wistar I/R” n = 4, “Wistar IPC+I/R” n = 3 *p < 0.05 compared to control, **p < 0.05 compared to I/R.
To verify that the changes in lysosome abundance after I/R are due mostly to autophagy-lysosomal machinery, we evaluated the content of the autophagosomal marker LC3 in kidney homogenates of Wistar rats. The response of LC3-I content to I/R and IPC-I/R was similar to that observed in Lysotracker staining (Fig. 4C).
Impaired autophagy and mitophagy in old rats’ kidneys
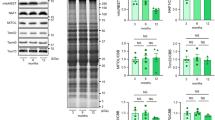
A study of the basal level of autophagy in the kidney revealed the same levels of Beclin-1 but considerably lower LC3-I content in intact OXYS rats compared to Wistar rats (Fig. 5A,B). This proves that already at the age of 6-months, OXYS show the decreased levels of autophagy that are typical for aged tissue8. In accordance with these data, the contents of ubiquitinated proteins and PTEN-induced putative kinase1 (PINK1) in kidney mitochondria of OXYS rats were significantly higher (Fig. 5C,D) than in age-matched Wistar rats, suggesting impaired mitophagy in OXYS. Since intact mitochondria are shown to be essential for the function of protective mechanisms involved in IPC13,14,15 we studied the function of mitochondria isolated from kidneys primed and unprimed with IPC. IPC in young animals did not cause any noticeable effect in the functioning of mitochondria, as assessed by their membrane potential values (Fig. 5E). Mitochondria isolated from the kidney of old rats differed from mitochondria of young rats by exhibiting a remarkably higher fraction with low transmembrane potential (Fig. 5F) and this fraction was even higher with IPC (Fig. 5G).
(A) Level of Beclin-1 in total kidney homogenates from Wistar and OXYS rats (B). Levels of LC3-I protein in total kidney homogenates from Wistar and OXYS rats. (C) Level of ubiqitinated proteins in isolated kidney mitochondria from Wistar and OXYS rats (D) Levels of PINK-1 in isolated kidney mitochondria of Wistar and OXYS rats. Number of animals: “Wistar” n = 3, “OXYS” n = 3. (E–G) Mitochondrial transmembrane potential in kidney mitochondria. Flow cytometry of isolated kidney mitochondria loaded with TMRE. (E) Young rats’ mitochondria in control (blue histogram) and 60 minutes after IPC (red histogram). (F) Transmembrane potential in kidney mitochondria from intact young (blue histogram) and old (red histogram) rats. A fraction of mitochondria with low transmembrane potential is seen as a left shoulder on each histogram. (G) Old rats’ mitochondria in control (blue histogram) and 60 minutes after IPC (red histogram). Note the increased fraction of de-energized mitochondria in old rats after IPC versus control rats. Number of animals n = 3 in all groups.
Discussion
The phenomenon of IPC was introduced in 1986 in a model of myocardial infarction17, where brief periods of I/R afforded protection of the tissue from damage caused by subsequent prolonged ischemia. Some elements of kidney IPC have been documented in 1990 and 199432,33. Nowadays, to prevent I/R-induced renal failure, various protocols of IPC are used generally containing several (1–6) episodes of I/R, each lasting 4–11 min34,35,36,37,38.
In this work, we explored the effects of IPC afforded by brief (15 seconds) intermittent episodes of I/R on processes occurring in old and young kidney which are experiencing ischemia-induced AKI. Priming of the kidney prior to an extensive ischemia alleviated damaging effect of I/R on the organ as was evidenced by a drop of BUN.
High autophagic activity in cells is often associated with tissue damage. The levels of lysosomal abundance in the kidney exposed to I/R and the key marker of autophagy LC3-I in the ischemic kidney were significantly increased. Priming the kidney with IPC resulted in relative normalization of these indicators of autophagy activation.
The increase in LC3-I demonstrates the activation of autophagic signaling pathways. Potent inducers of this process are ROS39 and ischemia (possibly as a source of ROS40). A key role of mitochondria in the pathogenesis of ischemia-related pathologies41 and specifically in AKI42 is well recognized. I/R causes mitochondrial dysfunction provoking excessive production of ROS by mitochondrial enzymes41. ROS may promote induction of the permeability-transition-pore in mitochondria (mPTP)43 through the mechanism of the ROS-induced-ROS-release44 which serves as a deadly signal for apoptotic execution of the cell. It is accepted that the ultimate goal of protective signaling pathways in the IPC cascade is the prevention of mPTP-opening42,45. Apparently, alleviation of the ischemic kidney injury by IPC associated with lower autophagy is the evidence for normalization of mitochondrial functioning and accompanying ROS-production.
We have demonstrated that renal protection against ischemic injury may be reached even when the duration of ischemic episodes is reduced from the conventional few minutes to 15 seconds. Apparently, within such a short period of ischemia, a trigger activating the protective signaling cascade is strong enough to induce ischemic tolerance and, therefore, the use of longer (5–10 minutes) ischemic episodes for mPTP-inhibition seems redundant. The most convincing evidence for affording ischemic tolerance as a result of IPC is an increase in total antioxidant capacity of renal tissue. Indirect evidence lies in the fact that to reduce the severity of renal failure after I/R by postconditioning, a few cycles of I/R episodes lasting 10–30 sec46 can be used. However, the ability to protect the organ from I/R-induced injury by an ultra-short preconditioning protocol has never been used.
The findings of the efficiency of such an ultra-short IPC can have a practical value. Such short protocol obviously, has no direct damaging effect on the tissue, and makes it possible to use IPC just before surgery on the organ eliciting the high risk of developing AKI.
Although experimental IPC models have been shown to evoke potent anti-ischemic defence47, IPC-induced protection is diminished in old animals, affording no protection in ischemic heart, brain and liver25,48,49,50,51,52. Renal protection by IPC in aged animals has not been tested yet. In this study, we, for the first time, demonstrate that in old animals the protective effect of IPC on the renal function vanishes and the level of kidney failure in exposed aged unprimed animals to I/R is as high as in primed aged animals. Since the cardioprotective effect of IPC could be restored in old animals performing treadmill exercises with normalization of mitochondrial functioning, it gives an additional argument to consider mitochondria as a key player in lost preconditioning effects with aging53.
To get insight into a mechanism for the age-dependent loss of a protective mechanism of IPC, we tested a hypothesis that some deleterious alterations of proteins participating in protective pathways may occur. Acetylation, an important post-translational modification of proteins, possibly plays a key role in the mechanism of aging. Thus, the activation of the deacetylase Sir2 was shown to increase the lifespan of yeast54 and worms55 and the knockout of Sirt1 (a Sir2 homolog in mammals) results in a decrease in lifespan of mice and abolishes the positive effect of caloric restriction on lifespan56. Inhibition of acetyltransferase, catalyzing acetylation, increased the lifespan of yeast57 and Drosophila58, while the increase in cytosolic acetyl-CoA which acetylates proteins in a non-catalytical way59 accelerated cellular senescence58. These data indicate that a high level of proteome acetylation is probably the cause of those negative processes that are associated with aging. In this study, we confirmed that in renal tissue of old rats the level of nuclear protein acetylation is higher, and this phenomenon is associated with the loss of the protective effect of IPC.
The most common site for acetylation of proteins is the amino-group of lysine and it also is the main site of ubiquitination. Therefore, these two processes may be in a competitive interaction. It can make acetylation as an alternative to the mechanism of disposal of impaired proteins through ubiquitination. Indeed, acetylation prevented the proteasomal degradation of proteins60. Thus, excessive acetylation and low activity/expression of deacetylases in the cell resulting in increased levels of defective proteins would neutralize the efficiency of the cellular system of quality control. This may explain the loss of the protective action of the IPC in aged animals, as ubiquitination of proteins including mitochondrial plays a key role in segregation and destruction of dysfunctional cell components, including organelles. The mechanism of mitochondrial quality control is based on the principle of homeostasis of the mitochondrial transmembrane potential. When it drops as a result of impairment in oxidative phosphorylation (which could be also age-dependent), it cannot drive PINK1 into the mitochondria where it is normally cleaved by the presenilins-associated rhomboid-like protein (PARL). As a result, the PINK1 remains on the outer membrane of dysfunctional mitochondrion where it facilitates the ubiquitination by Parkin61 which is a starting point for disposal of impaired mitochondrion through autophagy/mitophagy.
Our data shows that in OXYS rats demonstrating signs of premature aging, the levels of protein ubiqutination and the content of PINK1 in the kidney mitochondria were elevated demonstrating an accumulation of impaired mitochondria. This suggests that although the OXYS kidney accumulates more low-functional mitochondria, the process of their elimination through mitophagy is retarded. Indeed, in the entire population of mitochondria isolated from the kidney of old rat, there was an essential portion of mitochondria with low membrane potential. While in young rats the IPC of the kidney does not cause changes in the membrane potential of their mitochondria, in old animals the IPC causes an increase of low-potential mitochondrial population, i.e., instead of IPC beneficial effect, we observed a deleterious effect of IPC in aged animals. These changes were associated with higher level of protein acetylation.
Thus, a high level of acetylated proteins in old animals is associated with accumulation in the kidney of partially de-energized mitochondria which are more susceptible to oxidative stress41. The level of protein acetylation in young OXYS was very high, even higher than in the 23-month-old outbred rats. IPC leads to an increased ischemic tolerance apparently by lowering the production of oxidants and increasing the antioxidative redox buffering capacity. In the renal tissue of young animals, excessive ROS are quickly scavenged by a redox buffer. As a result, the IPC-induced ROS-burst has enough time to ignite the protective signaling pathway but not enough to activate the damaging cascade. Our data that IPC improves the total antioxidative capacity support this suggestion. On the contrary, in old rats IPC causes an essential drop of ROS-buffering capacity. Under these conditions, an IPC-induced ROS-burst develops a profound oxidative stress which is high enough to damage tissue rather than activate protective signaling. Possibly, oxidative stress is either a cause or a result of enhanced levels of acetylation leading to limited healing ability due to a low autophagic activity in old animals.
Materials and Methods
Animals
Male outbred rats of age 4–6 and 20–23 months and 6-month-old Wistar and OXYS rats were used. The animal protocols were evaluated and approved by the animal ethics committee of Belozersky Institute of Physico-Chemical Biology (Protocol 2/13 from April 8, 2013). They were in accordance with the Federation of Laboratory Animal Science Associations (FELASA) guidelines.
I/R and IPC protocols
The animals anesthetized with chloralhydrate (300 mg/kg, i.p.) were subjected to 40-minute warm ischemia of the left kidney as described21,62. The renal vascular bundle was occluded with a microvascular clip for 40 minutes. Circulation was restored by removing the clip. The lack of blood flow during ischemia (Fig. 1) and renal hemodynamics during reperfusion were assessed by 25-MHz ultrasound Doppler transducer (Doppler Minimax, Russia). Nephrectomy of the right kidney was executed simultaneously with ischemia of the left one. During operation, the body temperature of the rat was maintained at 37 ± 0.5 °C.
IPC was performed by clamping renal arteries with a microvascular clip and consisted of 4 cycles with 15-second ischemia and 15-second reperfusion each, immediately before the clamping of renal pedicle for 40 minutes. Blood samples were taken 48 hours after ischemia to determine BUN using AU480 Chemistry System (Beckman Coulter, USA).
Immunofluorescence
Kidney slices were fixed for 24 hours in 4% formaldehyde with PBS at 4 °C, and permeabilized in 0.02% Triton X-100 for 60 minute at 20 °C. Sections were incubated overnight with 1:200 anti-acetyl-Lys Ab (CellSignaling, USA) and after three rinses in PBS were incubated 1 hour with secondary antibodies diluted 1:200 (FITC-conjugated anti-rabbit IgG; Jackson ImmunoResearch Laboratories, USA). For actin staining, kidney slices were incubated with 1:100 Phalloidin-TRITC (Invitrogen, USA).
Lysosome abundance
Kidneys were excised 24 hours after I/R and placed in the incubation medium (Hank’s Balanced Salt Solution for cell cultures added with 10 mM Hepes-NaOH, pH 7.4) to wash out the blood. Then, 10–15 μm thick sections through the cortical zone of the kidney were made. Tissue sections were washed using the incubation medium (all procedures and incubation were done at 25 °C) and stained for 30 minutes with 1 μM Lysotracker Green (Invitrogen, USA).
Confocal microscopy
Kidney slices after Lysotracker or immune staining were imaged with an LSM510 inverted confocal microscope (Carl Zeiss, Jena, Germany). Images were processed using ImageJ software (NIH, Bethesda, MD, USA).
Western blotting
Kidney homogenate samples were loaded onto 15% Tris–glycine polyacrylamide gels (10 μg protein/lane). After electrophoresis, gels were blotted onto PVDF membranes (Amersham Pharmacia Biotech, UK). Membranes were blocked with 5% non-fat milk in PBS/0.1% Tween-20 and subsequently incubated with primary antibodies: anti-LC3 A/B (CellSignaling, USA), anti-Beclin-1 1:1000 (CellSignaling, USA), anti-ubiquitin - polyclonal rabbit 1:1000 (Abcam, USA), anti-B-actin monoclonal mouse 1:1500 (Sigma, USA), anti-PINK1 polyclonal rabbit 1:1000 (Abcam, USA). Membranes were stained with secondary antibodies: anti-mouse IgG or anti-rabbit IgG conjugated with horseradish peroxidase 1:10000 (Jackson ImmunoResearch, USA). Detection was performed by V3 Western Blot Imager (BioRad, USA).
Mitochondria isolation
The rat kidney mitochondria were isolated by differential centrifugation in the medium containing 0.25 M sucrose, 10 mM Tris-HCl, 1 mM EDTA, 0.1% BSA, pH 7.4 and incubated in the same medium without BSA, EDTA, or DTT. Protein concentration was measured by bicinchoninic acid assay (Sigma, USA).
Flow cytometry
Flow cytometry was performed by using a Cytomics FC500 (Beckman Coulter, USA). TMRE-mediated fluorescence reflecting a magnitude of transmembrane potential was measured on the FL2-channel with λex = 488 nm. The incubation medium contained 120 mM KCl, 3 mM HEPES, 1 mM EGTA, 5 mM K2PO4, 100 nM TMRE, 5 mM succinate, and 200 μg mitochondrial protein/ml, pH 7.2–7.4.
Total antioxidative capacity
TAC of kidney homogenates was measured by the hemoglobin-hydrogen peroxide-luminol chemiluminescence. Briefly, 10 μl of the sample were added to 100 μl of reaction medium (0.21 μM hemoglobin, 10 μM luminol in PBS) and the reaction was initiated by 20 μM H2O2. TAC was measured by assessing the luminescence quenching through scavenging the radicals generated by hemoglobin-hydrogen peroxide. The outcomes were expressed in mmoles of Trolox equivalent per mg protein.
Statistics
All groups contained 6–10 animals. Western blots, immunohystochemistry, lysotracker staining and flow cytometry experiments were performed at least in triplicate. Data are presented as mean ± SEM. Comparisons between groups were made using a Student t-test with a P ≤ 0.05 taken to indicate statistical significance.
Additional Information
How to cite this article: Jankauskas, S. S. et al. The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci. Rep. 7, 44430; doi: 10.1038/srep44430 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Famulski, K. S. & Halloran, P. F. Molecular events in kidney ageing. Curr. Opin. Nephrol. Hypertens. 14, 243–248 (2005).
Bandyopadhyay, D. & Medrano, E. E. The emerging role of epigenetics in cellular and organismal aging. Exp. Gerontol. 38, 1299–1307 (2003).
Cohen, H. Y. et al. Calorie Restriction Promotes Mammalian Cell Survival by Inducing the SIRT1 Deacetylase. Science 305, 390–392 (2004).
Nakamura, A., Kawakami, K., Kametani, F., Nakamoto, H. & Goto, S. Biological significance of protein modifications in aging and calorie restriction. Ann. N. Y. Acad. Sci. 1197, 33–39 (2010).
Hebert, A. S. et al. Calorie Restriction and SIRT3 Trigger Global Reprogramming of the Mitochondrial Protein Acetylome. Mol. Cell 49, 186–199 (2013).
Morselli, E. et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy 6, 186–188 (2010).
Ning, Y. C. et al. Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage. Mech. Ageing Dev. 134, 570–579 (2014).
Cuervo, A. M. Autophagy and aging: keeping that old broom working. Trends Genet. 24, 604–612 (2008).
Harman, D. The biologic block: the mitochondria? J. Am. Geriatr. Soc. 20, 145–147 (1972).
Bratic, A. & Larsson, N. The role of mitochondria in aging. J. Clin. Invest. 123, 951–957 (2013).
Skulachev, V. P. Mitochondria, reactive oxygen species and longevity: some lessons from the Barja group. Aging Cell 3, 17–19 (2004).
Zorov, D. B. Mitochondrial damage as a source of diseases and aging: a strategy of how to fight these. Biochim. Biophys. Acta 1275, 10–15 (1996).
Tsang, A., Hausenloy, D. J., Mocanu, M. M. & Yellon, D. M. Postconditioning: A form of ‘modified reperfusion’ protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ. Res. 95, 230–232 (2004).
Szwarc, I. et al. Ischemic postconditioning prevents ischemic acute renal failure. Transplant. Proc. 39, 2554–2556 (2007).
Bon, D. et al. New strategies to optimize kidney recovery and preservation in transplantation. Nat. Rev. Nephrol. 8, 339–347 (2012).
Wever, K. E. et al. Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: the role of adenosine. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 26, 3108–3117 (2011).
Murry, C. E., Jennings, R. B. & Reimer, K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136 (1986).
Schroeder, C. A. et al. Preconditioning with ischemia or adenosine protects skeletal muscle from ischemic tissue reperfusion injury. J. Surg. Res. 63, 29–34 (1996).
Nuzzo, G. et al. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl. 10, S53–S57 (2004).
Kitagawa, K. et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 528, 21–24 (1990).
Plotnikov, E. Y. et al. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 72, 1493–1502 (2007).
Yoon, Y. E. et al. Preconditioning strategies for kidney ischemia reperfusion injury: Implications of the ‘time-window’ in remote ischemic preconditioning. PLoS One 10, 1–11 (2015).
Juhaszova, M. et al. Role of glycogen synthase kinase-3β in cardioprotection. Circ. Res. 104, 1240–1252 (2009).
Hausenloy, D. J., Ong, S. B. & Yellon, D. M. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res. Cardiol. 104, 189–202 (2009).
Juhaszova, M., Rabuel, C., Zorov, D. B., Lakatta, E. G. & Sollott, S. J. Protection in the aged heart: Preventing the heart-break of old age? Cardiovasc. Res. 66, 233–244 (2005).
Markovets, A. M. et al. Alterations of retinal pigment epithelium cause AMD-like retinopathy in senescent-accelerated OXYS rats. Aging (Albany. NY). 3, 44–54 (2011).
Muraleva, N. A., Ofitserov, E. N., Tikhonov, V. P. & Kolosova, N. G. Efficacy of glucosamine alendronate alone & in combination with dihydroquercetin for treatment of osteoporosis in animal model. Indian J. Med. Res. 135, 221–227 (2012).
Bobko, A. A. et al. 19F NMR measurements of NO production in hypertensive ISIAH and OXYS rats. Biochem. Biophys. Res. Commun. 330, 367–370 (2005).
Obukhova, L. A., Skulachev, V. P. & Kolosova, N. G. Mitochondria ‐ targeted antioxidant SkQ1 inhibits age ‐ dependent involution of the thymus in normal and senescence ‐ prone rats. Aging (Albany. NY). 1, 389–401 (2009).
Vays, V. B. et al. Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany. NY). 6, 140–148 (2014).
Stefanova, N. A., Muraleva, N. A., Skulachev, V. P. & Kolosova, N. G. Alzheimer’s disease-like pathology in senescence-accelerated OXYS rats can be partially retarded with mitochondria-targeted antioxidant SkQ1. J. Alzheimer’s Dis. 38, 681–694 (2014).
Zager, R. A., Iwata, M., Burkhart, K. M. & Schimpf, B. A. Post-ischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int. 45, 1760–1768 (1994).
Yoshioka, T. et al. Role of intrinsic antioxidant enzymes in renal oxidant injury. Kidney Int. 38, 282–8 (1990).
Kapitsinou, P. P. & Haase, V. H. Molecular Mechanisms of Ischemic Preconditioning in the Kidney. Am. J. Physiol. - Ren. Physiol. 309, F821–F834 (2015).
Wever, K. E. et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One 7, 1–10 (2012).
Mahfoudh-Boussaid, A. et al. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1α in ischemic kidney: the role of nitric oxide. J. Biomed. Sci. 19, 7 (2012).
Lee, H. T. & Emala, C. W. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A 1 and A 3 receptors. Am. J. Physiol. Ren. Physiol. 278, F380–F387 (2000).
Cochrane, J. et al. Ischemic preconditioning attenuates functional, metabolic, and morphologic injury from ischemic acute renal failure in the rat. Ren. Fail. 21, 135–145 (1999).
Kiffin, R., Bandyopadhyay, U. & Cuervo, A. M. Forum Review: Oxidative stress and autophagy. Antioxid. Redox Signal. 8, 152–162 (2006).
Zweier, J. L., Flaherty, J. T. & Weisfeldt, M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84, 1404–1407 (1987).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 94, 909–950 (2014).
Ralto, K. M. & Parikh, S. M. Mitochondria in Acute Kidney Injury. Semin. Nephrol. 36, 8–16 (2016).
Hunter, D. R. & Haworth, R. A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 195, 453–459 (1979).
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L. & Sollott, S. J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 (2000).
Juhaszova, M. et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 113, 1535–1549 (2004).
Liu, X. et al. Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochem. Biophys. Res. Commun. 359, 628–634 (2007).
Silachev, D. N. et al. The Mitochondrion as a Key Regulator of Ischaemic Tolerance and Injury. Hear. Lung Circ. 10, 897–904 (2014).
Bickler, P. E., Fahlman, C. S. & Gray, J. J. Hypoxic preconditioning failure in aging hippocampal neurons: Impaired gene expression and rescue with intracellular calcium chelation. J. Neurosci. Res. 88, 3520–3529 (2010).
He, z. et al. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2, 365–374 (2005).
Ebrahim, Z., Yellon, D. M. & Baxter, G. F. Ischemic preconditioning is lost in aging hypertensive rat heart: Independent effects of aging and longstanding hypertension. Exp. Gerontol. 42, 807–814 (2007).
Adam, T., Sharp, S., Opie, L. H. & Lecour, S. Loss of cardioprotection with ischemic preconditioning in aging hearts: role of sirtuin 1? J. Cardiovasc. Pharmacol. Ther. 18, 46–53 (2013).
Selzner, N., Boehnert, M. & Selzner, M. Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: Basic mechanisms and translational applications. Transplant. Rev. 26, 115–124 (2012).
Wang, W. et al. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid. Med. Cell. Longev. 2014, 1–14 (2014).
Howitz, K., Bitterman, J. & Cohen, H. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Mercken, E. M. et al. SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell 13, 193–196 (2014).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Eisenberg, T. et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme A stimulates autophagy and prolongs lifespan. Cell Metab. 19, 431–444 (2014).
Wagner, G. R. & Payne, R. M. Widespread and enzyme-independent N(epsilone)-acetylation and N(epsilone)-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 288, 29036–29045 (2013).
Hershko, A., Heller, H., Eytan, E., Kaklij, G. & Rose, I. A. R. Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown. Proc. Natl. Acad. Sci. USA 81, 7021–7025 (1984).
Jin, S. M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010).
Plotnikov, E. Y. et al. New-generation Skulachev ions exhibiting nephroprotective and neuroprotective properties. Biochemistry. (Mosc). 75, 145–150 (2010).
Acknowledgements
The study was supported by Russian Science Foundation grant 14-15-00147. The authors are very thankful to Dr. Kenneth W. Fishbein (LCI, NIA) for his valuable critique and help.
Author information
Authors and Affiliations
Contributions
S.S.J. designed experiments, performed animal experiments, analyzed data and wrote the main manuscript text; E.Y.P. designed experiments, performed confocal microscopy experiments, analyzed data, and edited the paper; I.B.P. and L.D.Z. performed Western blotting and TAC assessment; N.V.A. performed immunofluorescence experiments; V.A.P. performed measuring of mitochondrial potential; D.N.S., N.G.K. and D.B.Z. designed experiments, analyzed data and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jankauskas, S., Pevzner, I., Andrianova, N. et al. The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci Rep 7, 44430 (2017). https://doi.org/10.1038/srep44430
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44430
This article is cited by
-
High-fat diet-induced mitochondrial dysfunction is associated with loss of protection from ischemic preconditioning in renal ischemia reperfusion
Pflügers Archiv - European Journal of Physiology (2023)
-
Clinical Characteristics and Predictors of Mortality in Minority Patients Hospitalized with COVID-19 Infection
Journal of Racial and Ethnic Health Disparities (2022)
-
Ischemic Preconditioning of the Kidney
Bulletin of Experimental Biology and Medicine (2021)
-
Autophagy in kidney homeostasis and disease
Nature Reviews Nephrology (2020)
-
Septic acute kidney injury: a review of basic research
Clinical and Experimental Nephrology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.