Abstract
In C. elegans, the transcription factor skinhead-1 (SKN-1), the ortholog of human NF-E2-related factor 2 (Nrf-2), plays important roles in oxidative stress defense and aging processes. It has been documented that the activity of SKN-1 is regulated by its phosphorylation modification. However, whether other posttranslational modifications of SKN-1 affect its function remains unclear to date. Here we report, for the first time, that SKN-1 is O-GlcNAcylated at Ser470 and Thr493 by O-GlcNActransferase OGT-1. By generating the double mutations of Ser470/Thr493 in the wild type and skn-1(zu67) worms, respectively, we found that disruption of O-GlcNAc modification on SKN-1 repressed the accumulation of SKN-1 in the intestinal nuclei, and decreased the activities of SKN-1 in modulating lifespan and oxidative stress resistance. Moreover, under oxidative stress, SKN-1 was highly O-GlcNAcylated, resulting in the decrease of GSK-3-mediated phosphorylation at Ser483 adjacent to the O-GlcNAcylated residues (Ser470 and Thr493). These data suggest that O-GlcNAcylation of SKN-1 is crucial for regulating lifespan and oxidative stress resistance via the crosstalk with its phosphorylation in C. elegans. These findings have important implications for studying the functions of O-GlcNAcylation on Nrf-2 in human aging-related diseases.
Similar content being viewed by others
Introduction
Oxidative stress is a major factor of influencing aging, and is involved in a number of human diseases, such as diabetes, cancer and neurodegenerative diseases1,2. In mammalian cells, the transcription factor NF-E2-related factor 2 (Nrf2) induces the expression of phase II detoxification genes, including NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), thioredoxin reductase 1 (TXNRD1), the modifier subunit (GCLM) and catalytic subunit (GCLC) of glutamate-cysteine ligase, to defense the oxidative stress3, whereas in C. elegans, skinhead-1 (SKN-1), the ortholog of Nrf-2, is required for both oxidative stress resistance and anti-aging through its accumulation in the intestinal nuclei to promote the detoxification target genes4. It has been proven that the subcellular location and activity of SKN-1 are regulated by its phosphorylation modification via a few signaling pathways5,6,7. For instance, the phosphorylation of SKN-1 by p38/mitogen-activated protein kinase (MAPK) signaling is required for SKN-1 translocation into nuclei5. Moreover, glycogen synthase kinase-3 (GSK-3) and insulin/IGF-1-like signaling (IIS) phosphorylate SKN-1 at specific serine and threonine sites, which prevents SKN-1 from accumulating in the intestinal nuclei and suppresses the phase II detoxification gene expressions6,7. However, it remains unknown whether the function of SKN-1 is regulated by other posttranslational modifications to date.
O-GlcNAcylation is a conserved posttranslational modification found in all metazoans, including worms, insects, plants and human8. Uridine diphosphate Nacetyl glucosamine (UDP-GlcNAc), the final product of the nutrient-sensing hexosamine signaling pathway, is the donor substrate of the O-GlcNActransferase (OGT)9,10,11. In the O-GlcNAc cycling, OGT catalyzes the addition of O-linked GlcNAc molecule from UDP-GlcNAc to serine and threonine residues of the target proteins, while O-GlcNAcase (OGA) is responsible for the removal of the O-GlcNAc modification12,13. In C. elegans, OGT-1 and OGA-1 are the only homologs of human OGT and OGA, respectively14. Abnormal O-GlcNAc cycling has a profound impact on diabetes, cancer and age-related neurodegenerative diseases15,16,17. Thus far, a large number of nuclear and cytoplasmic proteins have been known to be O-GlcNAcylated in eukaryote cells. Over the years, accumulating evidence has revealed the important roles of O-GlcNAcylation in many aspects of cellular processes, including signal transduction, transcriptional regulation, protein stability, and cell cycle control13,18,19,20,21,22. As both phosphorylation and O-GlcNAcylation occur at serine and threonine residues, protein O-GlcNAcylation has an extensive crosstalk with the protein phosphorylation during these cellular processes14,23,24.
C. elegans is a classical model for studying the regulation of aging and defense against oxidative, UV and other stresses. It has been reported that the loss of function on ogt-1 or oga-1 altered the lifespan and UV stress susceptibility phenotypes, suggesting O-GlcNAcylation plays key roles on modulating C. elegans aging and stress resistance19,25. However, proteins that are directly modified by O-GlcNAcylation to regulate the lifespan and stress resistance have not been characterized so far. Evidence has shown that both SKN-1 and OGT-1 are expressed in the intestine of worms. Furthermore, previous studies have found that the functions of some mammalian transcription factors, such as FOXO proteins, are regulated by the O-GlcNAc modification on these proteins26. Therefore, whether SKN-1 is a substrate of OGT-1 and undergoes O-GlcNAc modification need to unveil.
In this study, we discovered that SKN-1 was directly O-GlcNAcylated by OGT-1 in C. elegans. The loss of O-GlcNAc modification on SKN-1 via the site-mutations inhibited the intestinal nuclear accumulation of SKN-1 and its activities in anti-aging and oxidative stress defense. Additionally, while the oxidative stress elevated the O-GlcNAcylation levels of SKN-1, the GSK-3-induced phosphorylation of SKN-1 at a nearby serine residue was decreased. Together, our data identified SKN-1 as a novel target of O-GlcNAcylation, which is involved in modulation of oxidative stress resistance and anti-aging in C. elegans.
Results
The intestinal nuclear accumulation of SKN-1 and the expression of SKN-1 target genes are regulated by OGT-1/OGA-1
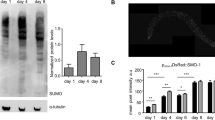
The accumulation of SKN-1 in the intestinal nuclei is the core step to stimulate the activities of this transcription factor in C. elegans. In order to know whether there is the relationship between O-GlcNAc cycling and the activities of SKN-1, we first analyzed the changes of SKN-1’s accumulation in the intestinal nuclei in ogt-1 and oga-1 mutants. The functions of oga-1 and ogt-1 were first proven depleted in C. elegans, by measuring the global O-GlcNAcylation level in these two mutants (Supplementary Fig. S1). The levels of SKN-1B/C::GFP accumulation in the intestinal nuclei were assigned as “high”, “medium” and “low” according to a well-acknowledged approach as described in the method4,6 (Fig. 1a). Results showed that the level of SKN-1B/C::GFP accumulation in the intestinal nuclei was increased in oga-1(ok1207), but not ogt-1(ok1474) (Fig. 1b). Since accumulating evidence has shown that the intestinal nuclear accumulation of SKN-1 can be triggered by oxidative stress4,5,6, we treated N2 with 5 mM t-butyl hydrogen peroxide (tBHP) according to the previous publications4,6,7. Addition of tBHP increased reactive oxygen species (ROS) level in the whole worms by 2-fold as shown by changes in 2, 7-dichlorodihydrofluorescein fluorescence (Supplementary Fig. S2). Moreover, it can be stated 5 mM tBHP treatment significantly increased the intestinal nuclear accumulation of SKN-1B/C::GFP in N2 (Fig. 1c), while only slightly increased in the oga-1 mutants (Fig. 1d). However, when the ogt-1 mutants were treated with tBHP, a lower percentage of the SKN-1B/C::GFP positive intestinal nuclei was observed, compared with N2 (Fig. 1d). At last, by the RT-qPCR analysis, we found the mRNA expression levels of SKN-1 target genes, such as gcs-1, gst-4 and gst-7, were up-regulated in the oga-1 mutants, which were depleted by skn-1 RNAi (Fig. 1e and Supplementary Fig. S3). These results suggest that O-GlcNActransferase OGT-1 and O-GlcNAcase OGA-1 in C. elegans play important roles on regulating the accumulation of SKN-1 in the intestinal nuclei and SKN-1’s target gene expressions.
(a) The patterns of SKN-1B/C::GFP accumulation in the intestinal nuclei were assessed as “high”, “medium” and “low”. Arrows indicated the GFP-tagged SKN-1 proteins accumulated in the intestinal nuclei in wild-type (N2). (b) The intestinal nuclear accumulation of SKN-1B/C::GFP was promoted by loss of oga-1, but not ogt-1. (c) Oxidative stress increased the intestinal nuclear accumulation of SKN-1B/C::GFP in N2. t-butyl hydrogen peroxide (tBHP) was used as a inducer of oxidative stress. (d) The tBHP-induced increase of SKN-1B/C::GFP accumulation in the intestinal nuclei was up-regulated by loss of oga-1, but down-regulated by disruption of ogt-1. (e) Depletion of oga-1 increased the target gene expressions of SKN-1. The mRNA levels of SKN-1 target genes were measured by RT-qPCR in wild type (N2) and oga-1(ok1207) worms treated with control dsRNA, and oga-1(ok1207) worms treated with skn-1 dsRNA. act-1 was used as an internal reference. All of the representative data were from at least three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significance
SKN-1 interacts with and is O-GlcNAcylated by OGT-1
Next, we examined whether OGT-1 could interact with and O-GlcNAcylate SKN-1. GST pull-down assays were performed by utilizing the whole-worm extracts from N2 expressing SKN-1(B/C)::GFP. The results showed that SKN-1 was able to bind to GST-OGT-1, but not GST (Fig. 2a).To confirm their physiological interaction in vivo, we performed immunoprecipitation (IP) with anti-GFP antibody in SKN-1(B/C)::GFP worms and found that OGT-1 interact with SKN-1 in the worm extracts (Fig. 2b).
(a) GST-OGT-1 bound to SKN-1 proteins expressed in the wild type worms. GST pull-down assay was performed by IP with GST-OGT-1 protein from the whole extracts of the wild-type (N2) expressing SKN-1(B/C)::GFP, and followed by immunoblotting with anti-GFP antibody. The arrow on the right side indicated the band of GST-OGT-1 purifed from E. coli BL21. GST protein was as the negative control. (b) OGT-1 interacted with SKN-1 in vivo. co-IP was operated by utilizing the whole extracts of SKN-1B/C::GFP worms with anti-GFP antibody, followed by immunoblotting with anti-OGT antibody. IgG was as the negative control. (c) SKN-1 was O-GlcNAcylated by OGT-1. In O-GlcNAcylation assay, the O-GlcNAcylated SKN-1 was purified and detected with the anti-O-GlcNAc antibody upon the co-expression of HIS-OGT-1 and GST-SKN-1 in Escherichia coli BL21. (d) SKN-1 was O-GlcNAcylated in C.elegans. SKN-1 proteins were immunoprecipitated with anti-GFP antibody from the whole extracts of SKN-1B/C::GFP worms. The O-GlcNAcylation of SKN-1was detected by immunoblotting with anti-O-GlcNAc antibody. 100 mM free GlcNAc was pre-incubated with anti-O-GlcNAc antibody during immunoblotting to confirm the specific signal of the O-GlcNAcylated SKN-1. IgG was as the negative control. (e) OGT-1/OGA-1 regulated the O-GlcNAcylation cycle of SKN-1. SKN-1 proteins were immunoprecipitated with anti-GFP antibody by using the whole extracts from the oga-1(ok1207), ogt-1(ok1474) and wild-type (N2) expressing SKN-1B/C::GFP, respectively. Then the O-GlcNAcylation level of SKN-1 was measured by immunoblotting with anti-O-GlcNAc antibody. IB means immunoblotting.
To further investigate whether SKN-1 could be directly O-GlcNAcylated by OGT-1, we performed the O-GlcNAcylation assay according to the method reported previously27. The constructs expressing HIS-tagged OGT-1 and GST-tagged SKN-1 were co-transfected into Escherichia coli BL21. Then, the GST-SKN-1 was purified with glutathione Sepharose 4B beads and examined by immunoblotting with anti-O-GlcNAc antibody (RL2). As expected, SKN-1 exhibited a strong O-GlcNAc modification signal (Fig. 2c). To prove whether SKN-1 could also be O-GlcNAcylated in vivo, IP was performed with anti-GFP antibody using whole extracts of SKN-1(B/C)::GFP worms. The specific signal of O-GlcNAcylated SKN-1 was observed in these worms, which was further confirmed via the GlcNAc competition experiment by pre-incubation of anti-O-GlcNAc antibody with free GlcNAc during immunoblotting (Fig. 2d).
Importantly, extracts of oga-1 and ogt-1 mutant worms expressing SKN-1(B/C)::GFP were then used in the IP assays. While a higher level of O-GlcNAc modification of SKN-1 was observed in oga-1 mutants, a lower level was detected in ogt-1 mutants, compared to N2 (Fig. 2e). Taken together, these data suggest that SKN-1 was O-GlcNAcylated by OGT-1 in C. elegans.
SKN-1 is O-GlcNAcylated preferentially at Ser470 and Thr493 by OGT-1
To identify the specific serine and threonine residues of SKN-1 modified by OGT-1, we next purified GST-tagged SKN-1 from E. coli BL21, in which GST-tagged SKN-1 plasmids and HIS-tagged OGT-1 plasmids were co-transformed. Then, the mass spectrometric (MS) analysis was conducted according to the previous study27. The results revealed that the sites of Thr445, Ser446, Ser449, Ser470 and Thr493 of SKN-1 were O-GlcNAcylated (Fig. 3a–c and Supplementary Fig. S4a,b). Then, each of these O-GlcNAc modification sites was individually mutated into alanine, and co-expressed with HIS-tagged OGT-1 in BL21. By using the O-GlcNAcylation assay, we found that the O-GlcNAcylation level of SKN-1 was decreased significantly with the single point mutations of Ser470 and Thr493 (Fig. 3d). These data indicate that the Ser470 and Thr493 of SKN-1 are the major O-GlcNAcylation sites catalyzed by OGT-1.
(a,b) The O-GlcNAcylation sites of SKN-1 were analyzed by mass spectrometry. The residues of O-GlcNAcylation sites Ser470 and Thr493 were identified from the fragmentation of the SKN-1 peptides HSFSDCTTDSSSTCSR and YTSESSTGTHESR by mass spectrometry. (c) The O-GlcNAcylated residues were shown in the animo acid sequnce of the analyzed SKN-1 peptides. the O-GlcNAcylated residues of SKN-1 were indicated in red. (d) Ser470 and Thr493 were the major O-GlcNAcylation sites catalyzed by OGT-1. Each serine or threonine of the idetified O-GlcNAcylation sites on SKN-1(321–623aa) peptide was mutated into alanine. GST-OGT-1 and SKN-1 peptides (wild type or mutant) were co-expressed in BL21. The O-GlcNAcylation level were then measured by using anti-O-GlcNAc antibody. IB means immunoblotting.
Ser470/Thr493 O-GlcNAcylation of SKN-1 regualtes its functions in lifespan and oxidative stress resistance
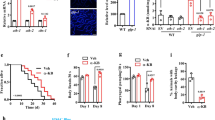
To investigate whether O-GlcNAc modification at Ser470 and Thr493 is crucial for the functions of SKN-1 in C. elegans, the SKN-1B/C S470A/T493A::GFP transgenic worms with the loss of O-GlcNAcylation on SKN-1 were generated on the wild type background. Treatment with tBHP increased the intestinal nuclear accumulation of SKN-1 in SKN-1B/C::GFP worms, but not in SKN-1B/C S470A/T493A::GFP worms (Fig. 4a,c). Furthermore, the increase in the level of SKN-1 in intestinal nuclei, which was induced by loss of oga-1, was significantly repressed by the expression of SKN-1B/C S470A/T493A::GFP in oga-1 mutants (Fig. 4b,c).
(a) The double mutations of Ser470/Thr493 down-regulated the intestinal nuclear accumulation of SKN-1 promoted by tBHP treatment. The intestinal nuclear accumulation of SKN-1 was scored in the wild type worms expressing SKN-1B/C::GFP or SKN-1B/C S470A/T493A::GFP, with or without tBHP treatments. (b) Loss of O-GlcNAcylation at Ser470/Thr493 inhibited the increase in the intestinal nuclear accumulation of SKN-1 induced by disruption of oga-1. The intestinal nuclear accumulation of SKN-1 was scored in oga-1(ok1207) worms expressing SKN-1B/C::GFP or SKN-1B/C S470A/T493A::GFP under normal condition. (c) The presentative images to show the intestinal nuclear accumulation of SKN-1 described A and B. The accumulated SKN-1 in the intestinal nuclei was observed by confocal microscopy (arrows). (d,e) The loss of Ser470/Thr493 O-GlcNAcylation depressed the function of SKN-1 on longevity. The lifespan was assayed in the wild type worms and skn-1(zu67) worms harboring rol-6;SKN-1B/C::GFP and rol-6;SKN-1B/C S470A/T493A transgenes, respectively. (f,g) The depletion of Ser470/Thr493 O-GlcNAcylation on SKN-1 resulted in its decrease of oxidative stress defense. The wild type and skn-1(zu67) worms expressing rol-6;SKN-1B/C::GFP and rol-6;SKN-1B/C S470A/T493A transgenes were treated with 9.125 mM tBHP in NGM plates as oxidative stress, and following the survival analysis respectively. All the representative data were from at least three independent experiments. ***p < 0.001.
Next, we analyzed the lifespan and oxidative stress tolerance of N2, in which SKN-1B/C::GFP and SKN-1B/C S470A/T493A::GFP transgenes were expressed, respectively. The results showed that the overexpression of SKN-1B/C::GFP increased the longevity and oxidative stress tolerance of N2 (Fig. 4d,f and Supplementary Table S1,2). While, the lifespan and their resistance to oxidative stress of N2 were not altered by expressing SKN-1B/C S470A/T493A::GFP (Fig. 4d,f and Supplementary Table S1,2).
Furthermore, the SKN-1B/C::GFP and SKN-1B/C S470A/T493A::GFP transgenic worms were then generated on the skn-1(zu67) background. We found that the lifespan of skn-1(zu67) worms were extended by the overexpression of SKN-1B/C::GFP, but not SKN-1B/C S470A/T493A::GFP (Fig. 4e and Supplementary Table S1). In addition, analysis of oxidative stress sensitivity revealed that SKN-1B/C S470A/T493A::GFP did not contribute to the oxidative stress resistance in the skn-1(zu67) worms (Fig. 4g and Supplementary Table S2). Apparently, the O-GlcNAcylation of SKN-1 at Ser470/Thr493 is essential for promoting SKN-1 accumulation in the intestinal nuclei, and activating its function in modulating lifespan and oxidative stress resistance in C. elegans.
Oxidative stress enhances the O-GlcNAcylation of SKN-1, resulting in the decrease of phosphorylation at Ser483 by GSK-3
Since our data showed that the tBHP-induced accumulation of SKN-1 in the intestinal nuclei was suppressed by lacing of O-GlcNAcylation on SKN-1, we next intended to determine whether this modification functions as the response to the oxidative stress. Therefore, the wild type worms harboring SKN-1(B/C)::GFP expression were treated with tBHP, and followed by co-IP experiments. The results showed that the O-GlcNAcylation level of SKN-1 was elevated by this oxidative stress in the wild type worms, but not in the ogt-1 mutants (Fig. 5a). Meanwhile, tBHP treatment increased the binding of OGT-1 to SKN-1 (Fig. 5b). These data suggest O-GlcNAcylation of SKN-1 is a physical response in C. elegans to defense against the oxidative stress.
(a) Oxidative stress enhanced the O-GlcNAcylation level of SKN-1. The immunoprecipitations were performed by using anti-GFP antibody from the whole extracts of the wild type (N2) or ogt-1(ok1474) worms expressing SKN-1B/C::GFP with or without tBHP treatments. The O-GlcNAcylation level of SKN-1 was detected by immunoblotting with anti-O-GlcNAc antibodies. The SKN-1B/C::GFP worms on the wild type (N2) background without tBHP treatment were as the negative control. (b) Oxidative stress elevated the interaction between OGT-1 and SKN-1. The wild type worms expressing SKN-1B/C::GFP were treated with tBHP or not. SKN-1 was then immunoprecipitated from the whole cell extracts with anti-GFP antibody, followed by immunoblotting with anti-OGT antibody. (c) The O-GlcNAcylation of SKN-1 down-regulated GSK-3-mediated phosphorylation in vitro. Recombinant GSK-3 were incubated with wild type SKN-1(321–623aa), or the SKN-1(321–623aa) peptides with single mutation of S470A or T493A, or double mutations of S470A/T493A, respectively. The phosphorylation level and O-GlcNAcylation level of the wild type or mutant SKN-1 proteins were then detected by immunoblotting with the anti-phosphoserine antibody (Pho) and anti-O-GlcNAc antibody. (d) Oxidative stress decreased the phosphorylation level of SKN-1 at Ser483. SKN-1 proteins were immunoprecipitated with anti-GFP antibody by using the whole cell extracts of SKN-1B/C::GFP worms with or without tBHP treatment, followed by immunoblotting with anti-phospho-SKN-1(Ser483) antibody (Ser483 Pho) and anti-O-GlcNAc antibody. (e) The loss of O-GlcNAcylation on SKN-1 at Ser470/Thr493 inhibited its intestinal nuclear accumulation promoted by gsk-3 RNAi. The wild type worms harboring SKN-1B/C S470A/T493A or SKN-1B/C::GFP transgenes were treated with gsk-3 RNAi and control RNAi, respectively. The accumulation of SKN-1 in the intestinal nuclei was then scored in these worms. All of the representative data from at least three independent experiments. ***p < 0.001. (f) The roadmap illustrating that O-GlcNAcylation of SKN-1 regulates its intestinal nuclear accumulation and functions on anti-aging and the oxidative stress resistance in C. elegans.
It has been documented that the crosstalk between O-GlcNAcylation and phosphorylation usually occurs at the residues close to each other28. Thus, we examined the sequence surrounding Ser470 and Thr493, and focused on Ser483, which has been reported to be phosphorylated by GSK-36. (Supplementary Fig. S5). The antibody specifically recognizing the phosphorylated Ser483 of SKN-1 was prepared (Supplementary Fig. S6). By using this antibody, we found that loss of oga-1 increased the O-GlcNAcylation level of SKN-1, while decreased the phosphorylation level of Ser483 (Supplementary Fig. S7). As a substantial elevation of GSK-3 in both ogt-1 and oga-1 mutants has been reported previously29, we then examined whether O-GlcNAcylation of SKN-1 inhibits the phosphorylation of Ser483 directly by GSK-3 kinase assay in vitro. The GST-SKN-1(321–623aa) co-expressed in E. coli BL21 with or without HIS-OGT-1, was purified and used as the substrate of GSK-3. The kinase assays revealed that the phosphorylation level of SKN-1 was down-regulated by the O-GlcNAcylation of SKN-1. In contrast, this phosphorylation level was restored upon the single mutation of T493A, as well as the double mutations of S470A and T493A (Fig. 5c). These data indicate that the O-GlcNAcylation of SKN-1 is able to suppress the GSK-3-mediated phosphorylation on this protein directly.
To further confirm the crosstalk between O-GlcNAcylation and GSK-3-mediatd phosphorylation on SKN-1 at Ser483 under oxidative stress in C. elegans, the wild-type (N2) expressing SKN-1(B/C)::GFP were treated with tBHP, followed by IP assay. As expected, the phosphorylation level of SKN-1 at Ser483 was decreased in the tBHP-treated worms, which had a higher O-GlcNAcylation level of SKN-1 compared with the untreated worms (Fig. 5d).
Moreover, we knocked down the gsk-3 in SKN-1B/C::GFP and SKN-1B/C S470A/T493A::GFP transgenic worms, respectively. As expected, the intestinal nuclear accumulation of SKN-1 was significantly increased by gsk-3 RNAi in the SKN-1B/C::GFP worms, but rarely raised in the SKN-1B/C S470A/T493A::GFP worms (Fig. 5e). These results show that the oxidative stress increases the O-GlcNAcylation level of SKN-1, leading to the decrease of GSK-3-mediated phosphorylation on Ser483 in C. elegans.
Discussion
SKN-1, the ortholog of mammalian Nrf2, is a crucial transcription factor engaged in modulation of oxidative stress resistance and longevity, whose functional regulation is predominantly achieved by posttranscriptional modification4,5,6,7,30. Unlike Nrf2, of which many types of protein modification have been extensively documented, such as phosphorylation, ubiquitination, acetylation and sumoylation31,32,33,34, SKN-1 is primarily as the substrate of phosphorylation5,6,7,30. In the present study, we identified for the first time that SKN-1 is O-GlcNAcylated at Ser470 and Thr493 by OGT-1 in C. elegans. This modification of SKN-1 plays a crucial role on promoting the activities of SKN-1 in modulating lifespan and the tolerance to oxidative stress.
It is well known that the ortholog of C. elegans OGT-1 in human, named OGT, regulates the global levels of O-GlcNAc modification of cells, and has a large number of substrates35,36,37. To investigate whether Nrf2 could be a substrate of OGT in human cells, we performed O-GlcNAcylation assay and found Nrf2 was transiently O-GlcNAcylated by human OGT in vitro (Supplementary Fig. S8). Therefore, the physiological and pathological roles of Nrf2’s O-GlcNAcylation in human yet to be further explored.
It is noteworthy that loss of oga-1 or ogt-1 did not remarkably affect the SKN-1 expression in the polymodal sensory neurons (ASI neurons) (Supplementary Fig. S9). ASIs are important polymodal sensory neurons, which are regarded as the putative hypothalamus in C. elegans. It has been reported SKN-1 expression in these neurons is important to extend lifespan through dietary restriction (DR)38. This suggests that the O-GlcNAc modification of SKN-1 might regulate C. elegans lifespan independent of DR pathway. We found that loss of ogt-1 decreased the intestinal nuclear accumulation of SKN-1 promoted by tBHP treatment. However, some percentage of SKN-1 was still observed in the intestinal nuclei in ogt-1 mutant with tBHP treatment (Fig. 1d). Preliminary studies also reported that disruption of ogt-1 did not completely eliminate the intestinal nuclear accumulation of SKN-1 induced by sodium azide39. These data imply some factors might exist in C. elegans to counteract the decrease in the accumulation of SKN-1 in the intestinal nuclei triggered by loss of ogt-1.
Mounting evidence has suggested that O-GlcNAc plays a critical role in regulating chromatin structure40. Some O-GlcNAc sites on histones H2A, H2B, and H4 were identified by mass spectrometry, and the changes of histone O-GlcNAcylation were detected during mitosis and with heat shock41,42. Our results showed that the expressions of SKN-1 target genes were up-regulated by the loss of oga-1 (Fig. 1e). Therefore, we further investigated whether the transcriptional expressions of SKN-1 target genes can be promoted by the histone O-GlcNAcylation on their promoters. According to the published data of whole-genome chromatin immunoprecipitation (ChIP)-on-chip and transcriptional profiling on the wild-type, oga-1 and ogt-1 mutant worms19, few positive signals of histone O-GlcNAcylation were detected on the promoters of phase II detoxification genes, which were known as the classical target genes of SKN-1. These data further verify that the activities of SKN-1 on anti-aging and oxidative stress resistance are regulated by the O-GlcNAc modification on this transcription factor itself.
The existing evidence has pointed to the extensive crosstalk between O-GlcNAcylation and phosphorylation that play important roles in various aspects of cellular processes14,21,24,28. Since the sites of O-GlcNAcylation on SKN-1 discovered in our study are close to Ser483, which is reported phosphorylated by GSK-3, we further explored the crosstalk between GSK-3-mediated phosphorylation and O-GlcNAcylation of SKN-1.The GSK-3 kinase assays showed that the phosphorylation level of SKN-1 mediated by GSK-3 was directly inhibited by the O-GlcNAcylation of this protein at Ser470 and Thr493 (Fig. 5c). Moreover, gsk-3 RNAi failed to elevate the intestinal nuclear accumulation of SKN-1 in the SKN-1B/C S470A/T493A::GFP worms, indicating that the O-GlcNAc modification of SKN-1 was required for maintaining the functions of SKN-1 controlled by GSK-3 pathway. Previous studies have reported the phosphorylation of SKN-1catalyzed by other kinases, such as AKT-1 and AKT-2 in the Insulin/IGF-1-like pathway, can also inhibit the accumulation of SKN-1 in the intestinal nuclei and decrease its activities in regulating lifespan and oxidative stress resistance7. Therefore, it is possible that there is the relationship between the O-GlcNAcylation of SKN-1 and its phosphorylation mediated by other kinases.
Taken together, our findings identify a new posttranslational modification O-GlcNAcylation of SKN-1. This modification, as a response of the oxidative stress, may directly inhibit its phosphorylation mediated by GSK-3, to stimulate the expression of its target genes involved in regulating lifespan and oxidative stress resistance in C. elegans (Fig. 5f). Notably, we find the O-GlcNAcylation level of SKN-1 was increased in response to oxidative stress, indicating that the OGT-1-mediated O-GlcNAcylation of SKN-1 is a dynamic process. The factors that stimulate OGT-1 to interact with and modify SKN-1, thereby activating the downstream target genes, should be unveiled further. Importantly, these data provide the evidence for the conserved function of the O-GlcNAcylation of Cap’n’Colla transcription factors over the evolution.
Methods
Strains
The following strains were used in this work: N2 Bristol (wild-type), oga-1(ok1207); ogt-1(ok1474), LD1(ldls7) were provided by the Caenorhabditis Genetic Center (CGC), oga-1(ok1207) and ogt-1(ok1474) were crossed to LD1(ldls7) to generate oga-1(ok1207);ldls7, ogt-1(ok1474);ldls7 strains. The strains of oga-1(ok1207) and ogt-1(ok1474) from CGC were backcrossed at least three times before used. The transgenic worms: N2 [rol-6], N2 [SKN-1B/C::GFP;rol-6], N2 [SKN-1B/C S470A/T493A::GFP;rol-6], skn-1(zu67) [rol-6], skn-1(zu67)[SKN-1B/C::GFP;rol-6], skn-1(zu67)[SKN-1B/C S470A/T493A::GFP;rol-6], oga-1(ok1207) [rol-6], oga-1(ok1207) [SKN-1B/C::GFP;rol-6], oga-1(ok1207) [SKN-1B/C S470A/T493A::GFP;rol-6] were made in our lab.
Transgenesis
The over expression plasmid SKN-1B/C::GFP was a generous gift from Prof. T. Keith Blackwell (Research Division, Joslin Diabetes Center). We created the mutant constructs of SKN-1B/C S470A/T493A::GFP by using the protocol of QuikChange (Stratagene). The transgenic strains were generated by injecting plasmid into the germlines of young adult animals. The constructs SKN-1B/C::GFP and SKN-1B/C S470A/T493A::GFP were respectively injected into wild-type (N2), oga-1(ok1207) and skn-1(zu67) at 10 ng/μl, along with the rol-6 marker (pRF4) at 50 ng/μl.
Lifespan analysis
Prior to experiments, all animals were maintained at the permissive temperature and grown for at least two generations in the presence of food to assure health. Lifespan analyses were conducted at 20 °C. Synchronized L1 worms were fed with OP50, grown to young adult, and then 30 worms were transferred to a new plate. Animals were tapped every day and scored as dead when they did not respond to the platinum wire pick. All of the life span assays were repeated at least three times. Survival plots, p values (Log-Rank), and proportional hazards were determined by using GraphPad Prism 5 software.
Oxidative stress resistance assay
To examine the accumulation of SKN-1B/C::GFP in the intestinal nuclei and SKN-1 target gene expressions by RT-qPCR under oxidative stress, young adults were exposed to 5 mM t-butyl hydrogen peroxide tBHP (Sigma) for 90 minutes. To assess the viability, day 1 later adults were transferred to NGM (nematode growth media) plates containing tBHP at 9.125 mM. Animals were incubated at 20 °C and periodically scored for survival.
Cellular localization of SKN-1B/C::GFP
The animals were incubated for 24 h at 20 °C and allowed to lay eggs. Their surviving progeny were grown to L4 larvae and young adults, and then the accumulation of SKN-1B/C::GFP in the intestinal nuclei was scored according to the protocol in the previous study4,5. Briefly, “High” indicates that a strong SKN-1B/C::GFP signal is present in all of the intestinal nuclei. “Medium” indicates that nuclear SKN-1B/C::GFP is present at high levels anteriorly, posteriorly, or anteriorly and posteriorly, but barely detectable midway through the intestine, or that a weak SKN-1B/C::GFP signal was observed in all intestinal nuclei. “Low” indicates that SKN-1B/C::GFP is barely detectable in the intestinal nuclei.
RNA isolation and quantitative PCR
To prepare worm sample, animals were picked and placed to clean plates to minimize contamination. Then approximately 200 animals suspended in 50 μl M9 buffer. Total RNAs from worms and cells were prepared by using Trizol reagent kit (TaKaRa) according to the manufacturer’s instructions. The cDNA was generated with oligo(dT) primers (Promega) by using the Reverse Transcription System (Promega). The Quantitative Real Time PCR was carried out using the SYBR Green Real time PCR Master Mix (TOYOBO) and operating on a Light Cycler 480 Real-Time PCR system Roche and then normalized to act-1 for worms.
Plasmids, reagents and antibodies
The cDNAs for skn-1, skn-1(321–623aa) and ogt-1 of C. elegans were amplified by PCR and cloned into pGEX-6P-1 and PET-28a vectors, respectively. All of the point mutations of SKN-1 were generated by site-directed mutagenesis according to the QuikChange (Stratagene) protocal. The antibodies of anti-GFP, anti-GST (Tianjin Sungene Biotech), anti-β-actin (Sigma), anti-O-GlcNAc RL2 (Abcam), anti-OGT (sigma) and anti- phosphoserine (Millipore) were purchased from the commercial channels. The anti-OGT and anti-GFP antibodies were first analyzed and make sure of their specific binding to the OGT-1 and GFP in C. elegans (Supplementary Fig. S10a,b). Rabbit polyclonal antibodies against phospho-SKN-1(Ser483) were generated by using synthetic peptides, TTDSSSTCS(#)RLSSESPRYTSE. # means the phosphorylated residue Ser483.
In vitro pull-down assays
The proteins of GST and GST-OGT-1 were expressed in BL21(DE3) bacteria and purified with glutathione Sepharose 4B beads (for GST fusion proteins, GE Healthcare), respectively. GST-fusion protein was incubated with the whole worm lysates of LD1(ldls7) and glutathione Sepharose 4B beads. After extensive washes, bound proteins were analyzed by western blotting with an anti-GFP antibody. Twenty percent of proteins used for the pull down reaction were shown as input.
Immunoprecipitation (IP) and western blotting
To prepare C. elegans proteins, synchronized young adult worms were grown on a 9.5 cm plates at 20 °C, and washed off from the plates with M9 buffer. The worms were lysed by sonication in lysis buffer (50mMTris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, phosphatase inhibitors, and protease inhibitors) and then immunoprecipitated with anti-GFP antibody, followed by western blotting. For IP assay, total protein lysates were incubated overnight with corresponding antibodies with gentle shacking at 4 °C., followed by addition of 40 μl of Pure Proteome protein A/G Mix Magnetic Beads (Millipore) for another 3 h. The beads were resuspended in 60 μl of 2 × loading buffer and boiled for 10 min. Then the supernatant was subjected to SDS-PAGE, transferred to polyvinylidene fluoride membrane (Millipore) and visualized by using appropriate primary antibodies coupled with HRP-conjugated secondary antibodies by ECL reagent (GE Healthcare).
O-GlcNAcylation assay and mass spectrometry (MS) analysis
For O-GlcNAcylation assay, the pGEX-6p-1plamids cloned with the wild type skn-1 cDNA or each point mutants of skn-1 cDNA, and the vectors pET28a(+) cloned with C. elegans ogt-1 cDNA, were co-transfected into E. coli BL21(DE3) competent cells and selected on LB-agar plates containing 50 mg/l kanamycin and 100 mg/l ampicillin (Amresco). One colony was picked, and the protein expression was induced at 16 °C for 20 h with 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) after the absorbance at 600 nm reached 0.8. GST-tagged protein was purified using glutathione Sepharose 4B beads (GE Healthcare) and concentrated in a buffer containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40 and 10% glycerol. Purified proteins that were co-expressed with or without OGT-1 were subjected to western blotting analysis by using anti-O-GlcNAc antibody (abcam).
For mass spectrometry (MS) analysis, the Coomassie blue stained band of purified GST-SKN-1 co-expressed with HIS-OGT in BL21 (DE3) bacteria. was excised for LC-MS analysis performed in the company of Shanghai Applied Protein Technology.
In vitro kinase assay
The peptides were incubated with GST-GSK-3 in buffer containing 20 mM Tris (pH 7.5), 5 mM DTT, 20 mM MgCl2, cold 200 μM ATP at 30 °C for 1 hour. The reaction was terminated by addition of SDS-Loading buffer and analyzed by western blotting.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). For the RT-qPCR assays, p values were determined by Student’s t-test. For the lifespan and oxidative stress resistance assays, p values were determined by log-rank test. For the nuclear localization of SKN-1B/C::GFP, a chi2 test was used, and the differences were considered as significance at P < 0.05.
Additional Information
How to cite this article: Li, H. et al. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci. Rep. 7, 43601; doi: 10.1038/srep43601 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247, doi: 10.1038/35041687 (2000).
Lithgow, G. J. & Walker, G. A. Stress resistance as a determinate of C. elegans lifespan. Mechanisms of ageing and development 123, 765–771 (2002).
McMahon, M. et al. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer research 61, 3299–3307 (2001).
An, J. H. & Blackwell, T. K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes & development 17, 1882–1893, doi: 10.1101/gad.1107803 (2003).
Inoue, H. et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes & development 19, 2278–2283, doi: 10.1101/gad.1324805 (2005).
An, J. H. et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proceedings of the National Academy of Sciences of the United States of America 102, 16275–16280, doi: 10.1073/pnas.0508105102 (2005).
Tullet, J. M. et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038, doi: 10.1016/j.cell.2008.01.030 (2008).
Olszewski, N. E., West, C. M., Sassi, S. O. & Hartweck, L. M. O-GlcNAc protein modification in plants: Evolution and function. Biochimica et biophysica acta 1800, 49–56, doi: 10.1016/j.bbagen.2009.11.016 (2010).
Dorfman, A. et al. The biosynthesis of hyaluronic acid by group A Streptococcus. IV. Role of glucosone as an intermediate in the synthesis of glucosamine. The Journal of biological chemistry 216, 549–552 (1955).
Ghosh, S., Blumenthal, H. J., Davidson, E. & Roseman, S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. The Journal of biological chemistry 235, 1265–1273 (1960).
Marshall, S., Bacote, V. & Traxinger, R. R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. The Journal of biological chemistry 266, 4706–4712 (1991).
Dong, D. L. & Hart, G. W. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. The Journal of biological chemistry 269, 19321–19330 (1994).
Wells, L., Vosseller, K. & Hart, G. W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291, 2376–2378 (2001).
Wang, Z., Gucek, M. & Hart, G. W. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proceedings of the National Academy of Sciences of the United States of America 105, 13793–13798, doi: 10.1073/pnas.0806216105 (2008).
Dias, W. B. & Hart, G. W. O-GlcNAc modification in diabetes and Alzheimer’s disease. Molecular bioSystems 3, 766–772, doi: 10.1039/b704905f (2007).
Yang, X. et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969, doi: 10.1038/nature06668 (2008).
Caldwell, S. A. et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29, 2831–2842, doi: 10.1038/onc.2010.41 (2010).
Hart, G. W., Housley, M. P. & Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022, doi: 10.1038/nature05815 (2007).
Love, D. C. et al. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proceedings of the National Academy of Sciences of the United States of America 107, 7413–7418, doi: 10.1073/pnas.0911857107 (2010).
Gambetta, M. C., Oktaba, K. & Muller, J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 325, 93–96, doi: 10.1126/science.1169727 (2009).
Kamemura, K., Hayes, B. K., Comer, F. I. & Hart, G. W. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. The Journal of biological chemistry 277, 19229–19235, doi: 10.1074/jbc.M201729200 (2002).
Yang, W. H. et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nature cell biology 8, 1074–1083, doi: 10.1038/ncb1470 (2006).
Wang, Z., Pandey, A. & Hart, G. W. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Molecular & cellular proteomics: MCP 6, 1365–1379, doi: 10.1074/mcp.M600453-MCP200 (2007).
Wang, Z. et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Science signaling 3, ra2, doi: 10.1126/scisignal.2000526 (2010).
Rahman, M. M. et al. Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans. Aging 2, 678–690 (2010).
Housley, M. P. et al. O-GlcNAc regulates FoxO activation in response to glucose. The Journal of biological chemistry 283, 16283–16292, doi: 10.1074/jbc.M802240200 (2008).
Guo, B. et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nature cell biology 16, 1215–1226, doi: 10.1038/ncb3066 (2014).
Butkinaree, C., Park, K. & Hart, G. W. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochimica et biophysica acta 1800, 96–106, doi: 10.1016/j.bbagen.2009.07.018 (2010).
Forsythe, M. E. et al. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proceedings of the National Academy of Sciences of the United States of America 103, 11952–11957, doi: 10.1073/pnas.0601931103 (2006).
Kell, A., Ventura, N., Kahn, N. & Johnson, T. E. Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free radical biology & medicine 43, 1560–1566, doi: 10.1016/j.freeradbiomed.2007.08.025 (2007).
Bloom, D. A. & Jaiswal, A. K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. The Journal of biological chemistry 278, 44675–44682, doi: 10.1074/jbc.M307633200 (2003).
Cullinan, S. B., Gordan, J. D., Jin, J., Harper, J. W. & Diehl, J. A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Molecular and cellular biology 24, 8477–8486, doi: 10.1128/MCB.24.19.8477-8486.2004 (2004).
Sun, Z., Chin, Y. E. & Zhang, D. D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Molecular and cellular biology 29, 2658–2672, doi: 10.1128/MCB.01639-08 (2009).
Malloy, M. T. et al. Trafficking of the transcription factor Nrf2 to promyelocytic leukemia-nuclear bodies: implications for degradation of NRF2 in the nucleus. The Journal of biological chemistry 288, 14569–14583, doi: 10.1074/jbc.M112.437392 (2013).
Wells, L., Whelan, S. A. & Hart, G. W. O-GlcNAc: a regulatory post-translational modification. Biochemical and biophysical research communications 302, 435–441 (2003).
Vosseller, K., Sakabe, K., Wells, L. & Hart, G. W. Diverse regulation of protein function by O-GlcNAc: a nuclear and cytoplasmic carbohydrate post-translational modification. Current opinion in chemical biology 6, 851–857 (2002).
Nandi, A. et al. Global identification of O-GlcNAc-modified proteins. Analytical chemistry 78, 452–458, doi: 10.1021/ac051207j (2006).
Bishop, N. A. & Guarente, L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549, doi: 10.1038/nature05904 (2007).
Hanover, J. A. et al. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proceedings of the National Academy of Sciences of the United States of America 102, 11266–11271, doi: 10.1073/pnas.0408771102 (2005).
Hanover, J. A. Epigenetics gets sweeter: O-GlcNAc joins the “histone code”. Chemistry & biology 17, 1272–1274, doi: 10.1016/j.chembiol.2010.12.001 (2010).
Sakabe, K. & Hart, G. W. O-GlcNAc transferase regulates mitotic chromatin dynamics. The Journal of biological chemistry 285, 34460–34468, doi: 10.1074/jbc.M110.158170 (2010).
Sakabe, K., Wang, Z. & Hart, G. W. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proceedings of the National Academy of Sciences of the United States of America 107, 19915–19920, doi: 10.1073/pnas.1009023107 (2010).
Acknowledgements
We thank Prof. T. Keith Blackwell (Research Division, Joslin Diabetes Center) for providing the SKN-1B/C::GFP plasmids, and Prof. Long Miao, Dr. Yanmei Zhao and Dr. Xiangming Wang (Institute of Biophysics, Chinese Academy Sciences) for helping generating the transgenic worms. We thank Prof. Xueqing Ba for the advice in revising the manuscript (The Institute of Genetics and Cytology, Northeast Normal University). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by the grants from the National Natural Science Foundation of China (Grant numbers 31000575, 31570718, 31571478, 31571317 and 31371294), and The Jilin Provincial Science & Technology Department (Grant Number 20150101069JC).
Author information
Authors and Affiliations
Contributions
Hongyuan Li, Jun Lu and Xiaoxue Li conceived and designed the experiments. Hongyuan Li, Xin Liu and Dan Wang performed the experiments. Tingting Zhao, Zhongwei Li and Cong Lin prepared the compounds used in this study and figures. Hongyuan Li, Liangping Su and Yu Zhang analyzed the data. Hongyuan Li, Xiaoxue Li and Baiqu Huang wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, H., Liu, X., Wang, D. et al. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci Rep 7, 43601 (2017). https://doi.org/10.1038/srep43601
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43601
This article is cited by
-
Effects of excess sugars and lipids on the growth and development of Caenorhabditis elegans
Genes & Nutrition (2020)
-
O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle
Journal of Bioenergetics and Biomembranes (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.