Abstract
What did coral reef ecosystems look like before human impacts became pervasive? Early efforts to reconstruct baselines resulted in the controversial suggestion that pristine coral reefs have inverted trophic pyramids, with disproportionally large top predator biomass. The validity of the coral reef inverted trophic pyramid has been questioned, but until now, was not resolved empirically. We use data from an eight-year tag-recapture program with spatially explicit, capture-recapture models to re-examine the population size and density of a key top predator at Palmyra atoll, the same location that inspired the idea of inverted trophic biomass pyramids in coral reef ecosystems. Given that animal movement is suspected to have significantly biased early biomass estimates of highly mobile top predators, we focused our reassessment on the most mobile and most abundant predator at Palmyra, the grey reef shark (Carcharhinus amblyrhynchos). We estimated a density of 21.3 (95% CI 17.8, 24.7) grey reef sharks/km2, which is an order of magnitude lower than the estimates that suggested an inverted trophic pyramid. Our results indicate that the trophic structure of an unexploited reef fish community is not inverted, and that even healthy top predator populations may be considerably smaller, and more precarious, than previously thought.
Similar content being viewed by others
Introduction
Wildlife management and conservation is fundamentally concerned with assessing the status of wild populations today and forecasting their trends into the future. Two bounding points of reference can play critical roles for different decisions – historical abundances prior to human impacts and population extinction. On land, human impacts have been ubiquitous and enduring for centuries1,2,3,4, and habitats are so altered that recovering species to historical levels is generally unfeasible and rarely even imagined. Instead, terrestrial conservation biologists are disproportionately concerned with the other end of the abundance spectrum – reducing risks of extinction5. By contrast, extinctions in the sea are still comparatively rare6,7, but thousands of species are harvested from wild populations, often at rates that are currently unsustainable8,9. Setting harvest targets that maintain vibrant populations of targeted species and the broader structure of the ecosystem in which they thrive requires a benchmark understanding of marine ecosystems10,11,12. Reconstructing what a pristine ocean looked like is therefore critical to set appropriate management targets for fished species and to recover threatened species10,13.
Unsurprisingly, a great deal of effort has gone into estimating historical baselines of ocean abundance. Most estimates have come from indirect measures, such as reconstructing fisheries landings trajectories to hindcast pre-exploitation abundances (e.g. refs 12, 14, 15, 16) or using patterns of genetic diversity to infer historical population sizes17,18. However, humans began aggressively removing species from the ocean well before we began studying them, making it generally impossible to establish an accurate historical baseline of population abundance for most marine species solely using data from a post-industrial fishing world19. A third method of estimating historical population baselines hinges upon the fact that – unlike on land – there are still places in the ocean that are relatively pristine. Efforts to reconstruct baseline information about marine populations and ecosystem dynamics have focused on the remote central Pacific Ocean20,21,22,23, where the Pacific Remote Islands are protected under U.S. jurisdiction and provide a potential window into the historical ocean. Early studies in one such isolated system, Palmyra atoll, determined that the grey reef shark (Carcharhinus amblyrhynchos), the most abundant predator in terms of biomass24, had a density that ranged from ~200 sharks/km2 (towed-diver survey25 and SCUBA belt transects24) to over 1000 sharks/km2 (SCUBA belt-transect survey25 calculated from shark biomass reported in ref. 23). The latter estimate provided support for the surprising and controversial idea that historical marine food webs may have had an inverted trophic pyramid with larger biomass at the top of the food web than at trophic levels below21,22,23.
If this vision of past ocean ecosystems was correct, and if it applied to other ocean geographies and habitats, the implications for ocean management and the need for ocean restoration would be profound. Not surprisingly, this rather radical scientific revision to our understanding of trophic structure and biomass organization in coral reef ecosystems has also evoked considerable scientific skepticism. Theoretical examinations of biomass distribution and energy flow in ecosystems26 questioned the potential validity of the empirical findings. Such conceptual challenges raised the specter of empirical errors, especially with respect to sampling highly mobile but spatially patchy predators with classical approaches25,27. One path to resolution is to solve the empirical sampling challenges and see if better estimates from pristine habitats in the ocean support a different view of unexploited ocean food webs.
To this end, we set out to re-estimate shark abundance and density at the same unfished coral reef – Palmyra atoll – that inspired the idea of inverted trophic biomass pyramids in coral reef ecosystems. Palmyra atoll is a U.S. National Wildlife Refuge in the central Pacific that was established in 2001 and is one of the few remaining unfished coral reef ecosystems on the planet. Animal movement is suspected to have significantly biased the early biomass estimates at Palmyra, with the reported bias exaggerated for top predators, which tend to be highly mobile27. We therefore focused our reassessment on the most mobile and most abundant predator at Palmyra24, the grey reef shark, and used survey techniques and analytical methods that can be applied over large spatial areas while accounting explicitly for movement on and off of study areas by target species as well as heterogeneous probability of detection28,29,30. With data from an eight-year, spatially explicit tag-recapture field program, we used spatial capture-recapture (SCR) models to estimate population abundance, density, and individual movement using precise capture-recapture locations and predictions based on standard detection models (Supplementary Methods). We conducted a Bayesian analysis of a SCR model to produce the first spatially and temporally explicit island-wide baseline population abundance and density estimates for a dominant reef shark at an unexploited coral reef. Our refined density estimate allows us to reconstruct top predator biomass and consider the consequences for the trophic biomass structure of an unfished foodweb. Most importantly, we then discuss the management implications of inflated baseline abundance and density estimates for shark populations.
Results
Our tag-recapture field program began in October 2006 and concluded in October 2014. During that time, we captured 1356 individual grey reef sharks (887 female, 469 male, 1 unrecorded) over 88 total days of fishing (Figs 1 and 2; Supplementary Fig. 1). Of these, 389 individuals were recaptured during unique sampling periods (Fig. 2). We examined various parameterizations of our SCR model that included effects of distance from activity center, fishing effort, sex, and size of each individual shark, all of which are known to affect capture probability (e.g. Espinoza et al.31; Heupel & Simpfendorfer32). Given that none of the estimated Bayesian credible intervals (CIs) in the model that included all covariates overlapped zero, we report results from the full model only. Grey reef shark adult and sub-adult density was 21.3 sharks/km2 (95% CI 17.8, 24.7), and total population abundance was 8344 individuals (95% CI 6977–9698) (Fig. 3; Supplementary Table 1). The unfished shark population was also stable through time; there were no significant differences in grey reef shark abundance or density through the study period (Fig. 4).
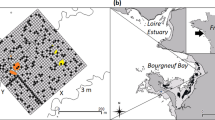
A map of Palmyra Atoll, a U.S. Wildlife Refuge with a ban on extractive fishing, showing forereef/offshore, backreef, and lagoon habitats, all of which were sampled during our 8-year capture-recapture study. Capture and recapture locations are shown for all sampling periods (x). Circles (o) denote the 2 km sampling grid used for the spatial capture-recapture models. Figure was created using R (version 3.1.3 [ www.r-project.org/]). Bathymetry map is from the National Oceanic and Atmospheric Administration’s (NOAA) Coral Reef Ecosystem Division and the Pacific Islands Benthic Habitat Mapping Center data collections 2016 (http://www.soest.hawaii.edu/).
Capture (N = 1356) and recapture (N = 389) locations for C. amblyrhynchos captured over 88 days of handline fishing between 2006–2014. Figure was created using the ggmap61 function in R (version 3.1.3 [ www.r-project.org/]).
Spatially explicit density estimates from capture-recapture data for grey reef shark adults and sub-adults at an unfished coral reef (mean 21.3 sharks/km2; 95% CI 17.8–24.7). Density is color-coded (red = highest and green = lowest). Figure was created using R (version 3.1.3 [ www.r-project.org/]). Habitat information is from NOAA’s NCCOS data collections 2016 (products.coastalscience.noaa.gov).
Shark movement was assessed using both the SCR model and multi-year passive acoustic monitoring data available for a subset of the population that was acoustically tagged in 2010–2012 and tracked through August 2015 (N = 37 sharks). Mean activity space estimates from the SCR model and the mean bivariate normal kernel utilization distribution (KUD) estimated from acoustic monitoring were nearly identical at the 99% level (25.2–28.4 km2 (95% CI 20.5–31.0 km2) and 28.8 km2 (95% CI 19.8–37.8 km2), respectively). However, there was a significant amount of individual heterogeneity in 99% KUDs estimated from the acoustic data (minimum <1 km2, maximum 117.4 km2) (Supplementary Table 2). Female sharks also had smaller activity spaces than males (25.2 and 28.4 km2 maximum 99% activity space KUD, female and male respectively), indicated by a sharper decline in the detection function at increasing distances from activity centers (Supplementary Table 1). Gelman-Rubin convergence diagnostics reported  values < 1.1 for all σ, λ, and β parameters (Supplementary Table 1).
values < 1.1 for all σ, λ, and β parameters (Supplementary Table 1).
Discussion
We estimate that grey reef shark density at a near-pristine coral reef is 21.3 sharks/km2, which translates to a total population size of 8344 sharks (Supplementary Table 1). These results suggest that unexploited predator density and abundance are substantially lower than was previously reported from the same location (i.e. ~200–1000 sharks/km223,24,25). The latter estimate led to the controversial suggestion that pristine coral reefs have inverted trophic pyramids, with disproportionally large top predator biomass. If all species in the system were similarly over-counted, then our finding would not challenge the idea of the inverted trophic biomass pyramid in pristine coral reef ecosystems, because all trophic levels would scale equally. However, it is unlikely that all trophic groups were over-counted equivalently. Ward-Paige et al.27 used simulation models to demonstrate a direct correlation between animal mobility and over-counting bias in non-instantaneous diver-based underwater surveys: animals that moved faster were over-counted, while slow-moving, sedentary animals were more likely to be accurately assessed.
For this reason, we assessed the validity of the inverted trophic pyramid by focusing on the grey reef shark – the species with the highest reported biomass that also happens to be the most mobile predator at Palmyra. Through this lens, our results provide clear evidence that the concept of an inverted trophic biomass pyramid inaccurately represents the overall biomass structure of coral reef ecosystems as a result of overestimated top predator densities likely due to noted sampling biases25,26,27. If all top predators (i.e. sharks) at Palmyra were similarly overestimated by traditional underwater diver surveys due to the mobility bias, our estimate would reduce total top predator biomass by nearly 56% compared to the numbers used as evidence of an inverted biomass pyramid at Palmyra23. This substantial biomass reduction reshapes the previously proposed trophic structure into a top-heavy, but not inverted pyramid (Fig. 5), and aligns the biomass profile with known size-based constraints on trophic pyramids26. Although it was recently shown that coral reef systems can support inverted trophic biomass pyramids over extremely limited spatial and temporal scales (a single pass during a spawning aggregation)33, our results indicate that the inverted pyramid does not accurately describe the entire coral reef ecosystem, as was previously suggested. Our findings are consistent with predator-heavy biomass structures reported for well-protected Mediterranean reefs34,35 and kelp forest ecosystems36, and can likely be explained by energetic subsidies as mobile consumers move across multiple habitats. Palmyra’s top predators are known to consume pelagic as well as nearshore resources, thereby creating important linkages across habitats and expanding the resource base of the nearshore coral reef ecosystem37. Ultimately, predator mobility biases both the numerator and the denominator in density estimates. Movement inflates the number of sharks counted by traditional survey methods (sensu Ward-Paige et al.27), while at the same time predator mobility obfuscates the spatial scale of the coral reef foodweb as mobile consumers move between and use resources from multiple habitats (sensu Trebilco et al.36). By accounting for mobility directly and estimating density over space occupied by individuals (via 99% activity space KUDs), our reassessment of shark density and abundance effectively corrects both forms of bias. For species with such complicated behaviors as grey reef sharks, an accurate representation of the coral reef ecosystem and its spatial variability is required to set appropriate management and conservation targets.
The consequences of a 56% reduction to total top predator biomass (area shown in red) based on our density estimates as compared to the numbers used to motivate the claim of an inverted biomass pyramid (original estimate modified from data presented in Sandin et al.23).
We also found no significant trend in grey reef shark population density or abundance through time (Fig. 4), indicating that the population is temporally stable. Grey reef sharks have a reported intrinsic rebound potential of 0.05 yr−1 (r2M), which equates to a doubling time of 12.8 years38. The observed population stability may therefore indicate true population recovery from potential fishing pressure prior to the establishment of the marine refuge in 2001, validating the use of our population estimate as representative of a historical baseline.
An additional important observation to emerge from our spatially extensive sampling design was the discovery of density hotspots on the eastern and western forereefs, with shark densities up to an order of magnitude lower on the north and south forereefs, on the backreefs, and within the lagoons (Fig. 3). This spatial organization would likely bias spatially limited population surveys or surveys conducted with non-random sampling, further highlighting the necessity of comprehensive spatial sampling to establish historical baselines accurately. Interestingly, while shark density is variable across space, grey reef sharks displayed strong residency, consistent with previous findings for relatively isolated populations39,40,41. Activity space estimates from our spatial capture-recapture model were similar to mean 99% KUD estimates from grey reef shark passive acoustic monitoring (25.2–28.4 km2 and 28.8 km2, respectively; Supplementary Table 2). However, 95% utilization distributions estimated using a Brownian bridge model were substantially smaller at 4.4 ± 1.3 km2 and revealed that sharks used core areas that were highly stable over several years (Papastamatiou, unpublished data). We also found that male sharks had slightly larger activity spaces than females (Supplementary Table 1), similarly consistent with published results31,32; however, all of our acoustically monitored sharks made occasional excursions all or part of the way around Palmyra’s forereefs and/or into backreef and lagoon habitat (J. Caselle & Y. Papastamatiou, unpublished data). A similar observation was made by Heupel and Simpfendorfer42, who showed that grey reef sharks maintained discrete home ranges, had consistency in space use through time and a high degree of overlap between 50% and 95% KUDs, but also undertook larger scale movements occasionally. The drivers of these infrequent excursions are currently unknown and merit further investigation, but island-wide movement has important implications for the spatial management of reef sharks and could explain why small marine reserves may be insufficient to maintain and recover reef shark populations31,43,44.
From a conservation perspective, our lower than expected reef shark density and abundance estimates are both troubling and encouraging. If healthy coral reef ecosystems tend to support far fewer sharks than previously thought, then recovering reef shark populations may not be an insurmountable goal. Decline rates for reef shark species (e.g. over 90% reported in Graham et al.45 and Robbins et al.43) may have been greatly overestimated for some species of reef shark, if they were based on equally overstated baseline population numbers. This could be good news for sharks and shark conservation. On the other hand, if stable shark populations are indeed smaller than expected as our study has shown, they are consequently more precarious when faced with a given level of harvest. Even with our low intensity, single hook, hand-line fishing, we estimate that we interacted with 16–22% of all grey reef sharks at Palmyra; a short visit by a commercial longline vessel could reset population trajectories for this species in a matter of days. In fact, it has long been reported that fisheries are able to expediently devastate reef shark populations45, and over the last decade global shark landings have declined due to population reductions caused by overfishing and poor management46,47. These fishing-induced population declines may be the result of inflated harvest quotas and insufficient protection status resulting from an overestimated baseline, as has been previously speculated27. It is not uncommon for findings from fisheries independent underwater visual census to be translated into management targets for shark populations (e.g. Robbins et al.43), which could be devastating for these species. To be broadly applicable, the shark population information reported here will need to be contextualized to the broader biotic and abiotic features of Palmyra; however, our finding that an unexploited location with high quality habitat has surprisingly low, but stable, shark abundance implies that population size limitations resulting from density dependence may be as important in explaining shark vulnerability to overexploitation as the slow life history characteristics that are primarily cited.
Marine wildlife management has an opportunity unparalleled in terrestrial systems to reconstruct historical baselines for many species using the few remaining large unexploited marine ecosystems. However, monitoring mobile species in complex ecosystems presents a suite of technical challenges that demand the application of appropriately sophisticated analytics. By employing an assessment method that directly addresses the potential biases associated with counting mobile species, we find that the conclusions that were drawn from methodologically and analytically simplistic methods can be strikingly at odds with reality. Ultimately, overestimates of shark density and biomass represent a double-edged sword that can weaken shark conservation. Our far lower estimates of shark abundance and density suggest that shark recovery targets should be adjusted: present targets are likely unrealistic and therefore impossible to achieve. At the same time, harvest quotas should be similarly downgraded to prevent continued overexploitation of these important predators.
Methods
Ethics Statement
This project was certified and all sampling protocols were approved by the Institutional Animal Care and Use Committee (IACUC), University of California, Santa Barbara, protocol no. 856 (date of IACUC approval: 5/31/2012) and under U.S. Fish and Wildlife Service (USFWS) special use permits (permit numbers #12533-14011, #12533-13011, #12533-12011, #12533-11007, #12533-10011, #12533-09010, #12533-08011, and #12533-07006). All methods were carried out in accordance with relevant IACUC and USFWS guidelines and regulations.
Study Site
Palmyra atoll is a U.S. incorporated territory that was established as a Fish and Wildlife Refuge in 2001 and is currently managed by The Nature Conservancy and the U.S. Fish and Wildlife Service. Palmyra is located in the central Pacific (5°54′N; 162°05′W); the associated marine refuge and marine national monument, within which commercial fishing is banned, extends out 50 nautical miles. Shark fishing within the refuge is scientific and non-extractive.
Field sampling
Minimally invasive tags with unique number IDs were applied to individual grey reef sharks during 12 separate sampling occasions in the spring, summer, and/or fall from October 2006 through October 2014 (Fig. 2; Supplementary Fig. 1). Between 2006 and 2012, tag application was primarily via use of roto tags (also called fin tags), which are applied with an applicator through a hole punched in the leading edge of the first dorsal fin. Starting in 2010, 15 cm stainless steel head dart tags (Hallprint Co.) were introduced and applied using stainless steel tag applicators, with the head of the tag implanted in the dorsal musculature near the base of the first dorsal fin. Tag loss has been reported for dart tags used on reef sharks48, which can significantly bias capture-recapture estimates. To determine rate of tag loss, we double-tagged 79 individuals (from all sampled habitats: forereef, backreef, lagoons), applying a uniquely numbered dart tag to each side of the animal near the first dorsal fin. Of these double-tagged individuals, 18 animals were recaptured at least once in a subsequent sampling period (a minimum of 70 days and a maximum of 495 days after initial tagging). All recaptured individuals that were initially double-tagged had both tags intact and in good condition upon recapture, indicating that dart tags had minimal tag loss during the study period.
Fishing effort (days spent scientific fishing) varied in intensity at each sampling occasion (Supplementary Fig. 1). Sequential sampling occasions were a minimum of 58 days apart to decrease the likelihood of behavioral effects (e.g. attraction, habituation, aversion to fishing activity). Sampling was unstructured, and fishing locations were chosen opportunistically to cover Palmyra’s forereef habitat and select channel, lagoon, and backreef habitats (Fig. 1). When fishing in forereef and offshore habitats, the boat was allowed to drift with currents (note drift patterns in Fig. 1). Chum (tuna, wahoo, and/or mackerel) was used to attract sharks to the boat, where they were caught using hand lines baited with a single barbless circle hook and restrained at the side of the boat. For each individual, we recorded length (precaudal, fork, and total), sex, and capture/recapture location (latitude and longitude). Given the highly variable oceanographic conditions at individual locations around the atoll, we were unable to accurately estimate the area of attraction for the bait.
Spatial Capture-Recapture Models
We carried out a Bayesian analysis of our spatial capture-recapture (SCR) model to relate the known observation process (capture-recapture encounter data) to the unknown ecological process (population size, population density, movement)49 and allow for temporary emigration50. SCR models differ from traditional capture-recapture models in that they introduce a random effect, which corresponds to the location of an individual’s center of activity during the sampling occasion. Individuals that reside primarily in non-sampled areas will therefore have a low probability of capture, while individuals with activity centers close to sampled areas will have a higher probability of capture; the distance between sampled areas (a grid cell center) and an individual’s activity center ultimately determines the probability of capture. Importantly, SCR models make the assumption that an individual is more likely to be captured in areas closest to its activity center, which is assumed to be fixed through the study period (for a full list of SCR model assumptions see Supplementary Methods).
Given our spatially opportunistic survey method and lack of fixed capture/recapture locations, we estimated a SCR model with methods from Thompson et al.51 and Russell et al.52. In their modification to traditional SCR models – which use traps set at fixed locations – they impose a spatial sampling grid on the survey area51, and grid cells are used as conceptual traps with capture locations assigned to the center point of the grid cell in which the individual was captured52. Grid cell size was selected to allow adequate modeling of spatial heterogeneity due to habitat features and the location of individuals within those habitats51. Although grey reef sharks have a relatively high movement capacity, Palmyra has highly variable habitat (Fig. 1). Therefore, we used a grid that encompassed our study area with 84, 2 km × 2 km grid-cells (Fig. 1). If no research fishing occurred in a grid cell during a sampling occasion, we imposed the constraint that the probability of encountering an individual was necessarily 0. We also transformed capture/recapture data into binary encounters for each individual during each sampling occasion to minimize the effects of spatial autocorrelation between grid cells given our relatively small grid cell size. Encounter histories take the form yijk for an individual i, grid-cell j, and sampling occasion k, where yijk = 1 if individual i was encountered in grid-cell j during sampling occasion k, otherwise yijk = 0. Encounter probabilities are a function of distance and they are grid-cell specific53 where

 is the expected number of captures for an individual.
is the expected number of captures for an individual.
We used a Gaussian hazard model that assumes a circular bivariate normal activity space to model the effect of distance on detection probability as  28. The distance between an individual’s activity center and grid-cell center d is Euclidean, and σ is a scaling parameter53. An advantage of the bivariate normal encounter model is that it allows direct estimation of activity space as a function of σ49. We included four covariates we expected would influence baseline encounter probability: distance from activity center, effort (fishing days), sex, and size (fork length). As sampling intensity varied across sampling occasions, we included fishing effort (days) as a covariate. Sex and size were also included, because they are known to affect activity space in grey reef sharks31,32 and will therefore impact capture probability. Handline fishing for sharks (the method used in the present study) also tends to result in a female biased sex ratio for captured individuals (Bradley, unpublished data), indicating that capture probability is likely not equivalent in male and female sharks. We therefore ran several parameterizations of the SCR model, introducing each covariate as a linear effect on the linear predictor for detection probability:
28. The distance between an individual’s activity center and grid-cell center d is Euclidean, and σ is a scaling parameter53. An advantage of the bivariate normal encounter model is that it allows direct estimation of activity space as a function of σ49. We included four covariates we expected would influence baseline encounter probability: distance from activity center, effort (fishing days), sex, and size (fork length). As sampling intensity varied across sampling occasions, we included fishing effort (days) as a covariate. Sex and size were also included, because they are known to affect activity space in grey reef sharks31,32 and will therefore impact capture probability. Handline fishing for sharks (the method used in the present study) also tends to result in a female biased sex ratio for captured individuals (Bradley, unpublished data), indicating that capture probability is likely not equivalent in male and female sharks. We therefore ran several parameterizations of the SCR model, introducing each covariate as a linear effect on the linear predictor for detection probability:

λ0ijk is then the expected number of captures for an individual i in a grid cell j given the individual’s activity center at each sampling occasion k. All sampled grid-cells have a constant baseline encounter rate λ0 for the average fishing effort. Both sex and size are unknown for unobserved individuals and were modeled as latent variables with uninformative priors (for model code see Supplementary Methods). We define sexfemale = 0 and sexmale = 1 and report ψsex as an estimate of the proportion of the population that is male. To estimate population abundance, we defined a state space S that encompassed our sampling area with individual potential activity centers si assigned a prior uniform distribution si ~ Uniform(S)28,29,53.
Within the SCR modeling framework, the activity centers of all individuals are estimated as the realization of a point process, and the number of activity centers in a given region represents the population size of that region. We estimated the density of sharks by dividing the total abundance of sharks in the sampled region by the area occupied by those individuals, where area occupied is a function of the activity space of individuals in the population. Changes in population abundance and density were also assessed through time using a subset of data from a region of the island (the western forereef) that was consistently sampled across all sampling occasions (Supplementary Fig. 3). Population time series data were analyzed for the first 9 sampling occasions (2006–2013), because sampling effort was comparable during these occasions (Supplementary Fig. 1).
We constructed the SCR model using data augmentation54 with Markov chain Monte Carlo (MCMC) sampling in the rjags55 package in R56. We ran the MCMC algorithm 12,000 times with three chains and discarded the first 2,000 runs as burn-in. Model convergence was assessed using the Gelman-Rubin  statistic where values < 1.1 indicate convergence57. We report only the full model, but results from all model parameterization are reported in Supplementary Table 1.
statistic where values < 1.1 indicate convergence57. We report only the full model, but results from all model parameterization are reported in Supplementary Table 1.
Passive Acoustic Telemetry
SCR models make the assumption that activity centers are stationary through the study period. To test the validity of this assumption, and to ground truth SCR model activity space estimates, we examined residency patterns by estimating activity space independent of capture-recapture effort using long-term passive acoustic monitoring data available for a subset of the population. In 2010–2012, 45 grey reef sharks were surgically implanted with acoustic transmitters (69 kHz, V16, Vemco Ltd., Nova Scotia, Canada; for surgical implantation methods see Papastamatiou et al.58). Passive acoustic telemetry was used to monitor the movement of grey reef sharks via an array of over 70 individual underwater acoustic receivers (VR2W, Vemco) that cover all of Palmyra’s unique forereef and backreef habitats (Supplementary Fig. 2). We calculated 99% bivariate normal kernel utilization distributions (KUDs)59 for each acoustically tagged individual with >100 detections and a minimum of 10 months of location data (N = 37) in the package adehabitatHR60 in R56.
Additional Information
How to cite this article: Bradley, D. et al. Resetting predator baselines in coral reef ecosystems. Sci. Rep. 7, 43131; doi: 10.1038/srep43131 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Vitousek, P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. Human domination of earth’s ecosystems. Science 277, 494–499 (1997).
Sanderson, E. W. et al. The Human Footprint and the Last of the Wild. Bioscience 52, 891 (2002).
Haberl, H. et al. Quantifying and mapping the human appropriation of net primary production in earth’s terrestrial ecosystems. Proc. Natl. Acad. Sci USA 104, 12942–12947 (2007).
Estes, J. A. et al. Trophic downgrading of planet Earth. Science 333, 301–6 (2011).
Ceballos, G. et al. Accelerated modern human – induced species losses: entering the sixth mass extinction. Sci. Adv. 1, 1–5 (2015).
Dulvy, N. K., Sadovy, Y. & Reynolds, J. D. Extinction vulnerability in marine populations. Fish Fish. 4, 25–64 (2003).
Webb, T. J. & Mindel, B. L. Global patterns of extinction risk in marine and non-marine systems. Curr. Biol. 25, 506–511 (2015).
Jackson, J. B. C. Ecological extinction and evolution in the brave new ocean. Proc. Natl. Acad. Sci. USA 105, 11458–11465 (2008).
Costello, C. et al. Global fishery futures under contrasting management regimes. Proc. Natl. Acad. Sci. USA 113, 5125–5129 (2016).
Carlton, J. T. Apostrophe to the ocean. Conserv. Biol. 12, 1165–1167 (1998).
Clark, J. S. et al. Ecological forecasts: an emerging imperative. Science 293, 657–60 (2001).
Lotze, H. K. & Worm, B. Historical baselines for large marine animals. Trends Ecol. Evol. 24, 254–62 (2009).
Roberts, C. M. Our shifting perspectives on the oceans. Oryx 37, 166–177 (2003).
Scott Baker, C. & Clapham, P. J. Modelling the past and future of whales and whaling. Trends Ecol. Evol. 19, 365–71 (2004).
Baum, J. K. & Myers, R. A. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 7, 135–145 (2004).
Rosenberg, A. A. et al. The history of ocean resources: Modeling cod biomass using historical records. Front. Ecol. Environ. 3, 84–90 (2005).
Alter, S. E., Newsome, S. D. & Palumbi, S. R. Pre-whaling genetic diversity and population ecology in eastern pacific gray whales: Insights from ancient DNA and stable isotopes. PLoS One 7, 1–12 (2012).
Ruegg, K. et al. Long-term population size of the North Atlantic humpback whale within the context of worldwide population structure. Conserv. Genet. 14, 103–114 (2013).
Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430 (1995).
Cox, S. P. et al. Reconstructing ecosystem dynamics in the central Pacific Ocean, 1952–1998. II. A preliminary assessment of the trophic impacts of fishing and effects on tuna dynamics. Can. J. Fish. Aquat. Sci. 59, 1736–1747 (2002).
Friedlander, A. M. & DeMartini, E. E. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 230, 253–264 (2002).
Stevenson, C. et al. High apex predator biomass on remote Pacific islands. Coral Reefs 26, 47–51 (2007).
Sandin, S. A. et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One 3, e1548 (2008).
McCauley, D. J., McLean, K. A., Bauer, J., Young, H. S. & Micheli, F. Evaluating the performance of methods for estimating the abundance of rapidly declining coastal shark populations. Ecol. Appl. 22, 385–392 (2012).
Nadon, M. O. et al. Re-creating missing population baselines for Pacific reef sharks. Conserv. Biol. 26, 493–503 (2012).
Trebilco, R., Baum, J. K., Salomon, A. K. & Dulvy, N. K. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol. Evol. 28, 423–431 (2013).
Ward-Paige, C., Mills Flemming, J. & Lotze, H. K. Overestimating fish counts by non-instantaneous visual censuses: consequences for population and community descriptions. PLoS One 5, e11722 (2010).
Efford, M. Density estimation in live-trapping studies. Oikos 106, 598–610 (2004).
Royle, J. A. & Young, K. V. A hierarchical model for spatial capture-recapture data. Ecology 89, 2281–2289 (2008).
Royle, J. A., Karanth, K. U., Gopalaswamy, A. M. & Kumar, N. S. Bayesian inference in camera trapping studies for a class of spatial capture-recapture models. Ecology 90, 3233–3244 (2009).
Espinoza, M., Heupel, M. R., Tobin, A. J. & Simpfendorfer, C. A. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar. Biol. 162, 343–358 (2014).
Heupel, M. R. & Simpfendorfer, C. A. Importance of environmental and biological drivers in the presence and space use of a reef-associated shark. Mar. Ecol. Prog. Ser. 496, 47–57 (2014).
Mourier, J. et al. Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Curr. Biol. 26, 2011–2016 (2016).
Sala, E. et al. The structure of mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS One 7 (2012).
Guidetti, P. et al. Large-scale assessment of mediterranean marine protected areas effects on fish assemblages. PLoS One 9 (2014).
Trebilco, R., Dulvy, N. K., Anderson, S. C., Salomon, A. K. & Trebilco, R. The paradox of inverted biomass pyramids in kelp forest fish communities. Proc. R. Soc. B Biol. Sci. 283, 20160816 (2016).
McCauley, D. J. et al. Assessing the effects of large mobile predators on ecosystem connectivity. Ecol. Appl. 22, 1711–1717 (2012).
Smith, S. E., Au, D. W. & Show, C. Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Freshw. Res. 49, 663 (1998).
Field, I. C., Meekan, M. G., Speed, C. W., White, W. & Bradshaw, C. J. A. Quantifying movement patterns for shark conservation at remote coral atolls in the Indian Ocean. Coral Reefs 30, 61–71 (2011).
Barnett, A., Abrantes, K. G., Seymour, J. & Fitzpatrick, R. Residency and spatial use by reef sharks of an isolated seamount and its implications for conservation. PLoS One 7, e36574 (2012).
Vianna, G. M. S., Meekan, M. G., Meeuwig, J. J. & Speed, C. W. Environmental influences on patterns of vertical movement and site fidelity of grey reef sharks (Carcharhinus amblyrhynchos) at aggregation sites. PLoS One 8, e60331 (2013).
Heupel, M. R. & Simpfendorfer, C. A. Long-term movement patterns of a coral reef predator. Coral Reefs 34, 679–691 (2015).
Robbins, W. D., Hisano, M., Connolly, S. R. & Choat, J. H. Ongoing collapse of coral-reef shark populations. Curr. Biol. 16, 2314–9 (2006).
McCook, L. J. et al. Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. Proc. Natl. Acad. Sci. USA 107, 18278–85 (2010).
Graham, N. A. J., Spalding, M. D. & Sheppard, C. R. C. Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat. Conserv. Mar. Freshw. Ecosyst. 20, 543–548 (2010).
Davidson, L. N. K., Krawchuk, M. A. & Dulvy, N. K. Why have global shark and ray landings declined: improved management or overfishing? Fish Fish. 17, 438–458 (2015).
Costello, C. et al. Status and solutions for the world’s unassessed fisheries. Science 338, 517–20 (2012).
Chin, A., Tobin, A. J., Heupel, M. R. & Simpfendorfer, C. A. Population structure and residency patterns of the blacktip reef shark Carcharhinus melanopterus in turbid coastal environments. J. Fish Biol. 82, 1192–210 (2013).
Royle, J. A., Chandler, R. B., Sollmann, R. & Gardner, B. Spatial capture-recapture. (Academic Press, Oxford, United Kingdom, 2014).
Kendall, W. L., Nichols, J. D. & Hines, J. E. Estimating temporary emigration using capture – recapture data with Pollock’s robust design. Ecology 78, 563–578 (1997).
Thompson, C. M., Royle, J. A. & Garner, J. D. A framework for inference about carnivore density from unstructured spatial sampling of scat using detector dogs. J. Wildl. Manage. 76, 863–871 (2012).
Russell, R. E. et al. Estimating abundance of mountain lions from unstructured spatial sampling. J. Wildl. Manage. 76, 1551–1561 (2012).
Gardner, B., Royle, J. A., Wegan, M. T., Rainbolt, R. E. & Curtis, P. D. Estimating black bear density using DNA data from hair snares. J. Wildl. Manage. 74, 318–325 (2010).
Royle, J. A., Dorazio, R. M. & Link, W. A. Analysis of multinomial models with unknown index using data augmentation. J. Comput. Graph. Stat. 16, 67–85 (2007).
Plummer, M. M. rjags: Bayesian graphical models using MCMC. R Package version 3–14. URL https://CRAN.R-project.org/package=rjags (2014).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2013).
Gelman, A. & Hill, J. Data analysis using regression and multilevel/hierarchical models (Cambridge University Press, New York, New York, USA, 2007).
Papastamatiou, Y. P., Lowe, C. G., Caselle, J. E. & Friedlander, A. M. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 90, 996–1008 (2009).
Worton, B. J. Using Monte Carlo simulation to evaluate kernel-based home range estimators. J. Wildl. Manage. 59, 794–800 (1995).
Calenge, C. The package ‘adehabitat’ for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519 (2006).
Kahle, D. & Wickham, H. ggmap: Spatial visualization with ggplot2. R Journal 5, 144–161 (2013).
Acknowledgements
We thank The Nature Conservancy staff for support at the Palmyra Atoll research station, the Palmyra Atoll Research Consortium, our volunteer field assistants including J. Calhoun, R. Carr, J. Eurich, A. Filous, J. Giddens, M. Hutchinson, S. Larned, R. Most, R. Pollock, K. Stamoulis, J. Schem, M. Shepard, R. Sylva, Y. Watanabe, and T. White. Thanks to K. Weng for use of Palmyra’s passive acoustic receiver array. Funding was provided by the Koaniani Foundation (via The Nature Conservancy), Georgia and Deke Welles (via The Nature Conservancy), the Marisla Foundation (JEC), and the Benioff Ocean Initiative (DJM); the Center for Scientific Computing from the CNSI/MRL at UCSB provided access to a high-performance computer cluster (NSF CNS-0960316 and MRSEC DMR-1121053). DB was supported by a NSF Graduate Research Fellowship (DGE-114408), a Dr. Daniel Vapnek Fellowship (UCSB Bren School), and an AAUS Kathy Johnston English Scholarship. This is publication number PARC-0131 from the Palmyra Atoll Research Consortium (PARC).
Author information
Authors and Affiliations
Contributions
D.B., E.C., Y.P.P., D.J.M., K.P., A.P., J.E.C. designed the study and conducted the field sampling and data collection. D.B. and B.E.K. performed statistical analyses and interpretation of data. D.B. prepared the figures and wrote the manuscript text with significant input from S.D.G. All authors reviewed the manuscript and gave final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bradley, D., Conklin, E., Papastamatiou, Y. et al. Resetting predator baselines in coral reef ecosystems. Sci Rep 7, 43131 (2017). https://doi.org/10.1038/srep43131
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43131
This article is cited by
-
Recent expansion of marine protected areas matches with home range of grey reef sharks
Scientific Reports (2021)
-
Habitat-specific inter and intraspecific behavioral interactions among reef sharks
Oecologia (2020)
-
Activity seascapes highlight central place foraging strategies in marine predators that never stop swimming
Movement Ecology (2018)
-
A unifying theory for top-heavy ecosystem structure in the ocean
Nature Communications (2018)
-
First estimates of Greenland shark (Somniosus microcephalus) local abundances in Arctic waters
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.