Abstract
Gallbladder cancer represents the most common malignancy of the biliary tract and is highly lethal with less than 5% overall 5-year survival rate. Chemotherapy remains the major treatment for late-stage patients. However, insensitivity to these chemotherapeutic agents including cisplatin is common. MicroRNAs (miRNAs) have been shown as modulators of drug resistance in many cancer types. We used genome-wide gene expression analysis in clinical samples to identify miR-125b-5p down-regulated in gallbladder cancer. miR-125b-5p up-regulation promoted cell death in gallbladder cancer cells in the presence of cisplatin. In contrast, knockdown of miR-125b-5p reduced cell death in gallbladder cancer cells treated with cisplatin. Up-regulation of miR-125b-5p significantly decreased tumor growth in combination with cisplatin in a mouse model. We identified Bcl2 as a direct target of miR-125b-5p which mediates the function of miR-125b-5p in gallbladder cancer. In clinical samples, miR-125b-5p was down-regulated in gallbladder cancer whereas Bcl2 was up-regulated and their expression was inversely correlated. Moreover, low miR-125b-5p expression or high expression of Bcl2 is correlated with poor prognosis in gallbladder cancer. Taken together, our findings indicate that miR-125b-5p is a potent chemotherapy sensitizer and may function as a new biomarker for the prognosis of gallbladder cancer patients.
Similar content being viewed by others
Introduction
Gallbladder cancer (GBC) is the most common biliary tract cancer in clinic worldwide1. Chemotherapies such as cisplatin (CDDP) remain the major treatment for patients with gallbladder cancer or cholangiocarcinoma2. However, resistance to chemotherapies leads to dismal prognosis3. The overall survival is less than 12 months even with the combination of cisplatin and gemcitabine4. Therefore, it is crucial for an extensive and thorough understanding of the molecular mechanisms of chemotherapy resistance in GBC.
MicroRNAs (miRNAs) are 20–22 nucleotide long molecules that function as post-transcriptional regulators. They bind directly to the 3′ untranslated regions (3′ UTRs) of target mRNAs and mediate their degradation. MicroRNAs have been shown to play important roles in cancer development including gallbladder cancer5,6,7. However, their functions in chemotherapy resistance are not well studied.
The Bcl2 family proteins are key regulatory components of the intracellular apoptosis pathway which is critical for cancer development8. Bcl2 is uniformly expressed in chronic lymphocytic leukemia and promotes leukemia cell survival9. Additionally, it has been documented that Bcl2 promotes cell migration and invasion in colorectal cancer10 and hepatocarcinoma cells11. However, whether and how Bcl2 is involved in the development of gallbladder cancer has not been studied.
We recently found that miR-125b-5p is significantly down-regulated in gallbladder cancer from genome-wide microRNA expression profiling in GBC and neighboring normal tissues. miR-125b-5p directly suppresses Bcl2 expression and increases the sensitivity of cisplatin treatment in gallbladder cancer cells and mouse models. Because miR-125b-5p was identified in clinical samples prior to adjuvant therapy, it contributes to intrinsic resistance to chemotherapy, rather than acquired resistance. The expression of miR-125b-5p and Bcl2 is inversely correlated and predicts prognosis. These results suggest novel therapeutic targets in gallbladder cancer treatment.
Results
Identification of gallbladder cancer-related miRNAs by genome-wide miRNA expression analysis in clinical samples
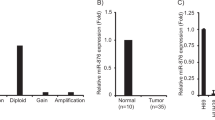
To identify potential miRNAs involved in gallbladder cancer progression, we performed a genome-wide miRNA expression analysis in six pairs of gallbladder cancer samples and their neighboring normal tissues. The expression of sixty miRNAs was found to be significantly altered between cancer and neighboring normal tissues (Fig. 1A). Among the top miRNA candidates, miR-125b-5p is highly down-regulated in tumor tissues than normal tissues. miR-125b-5p has been shown to be involved in endometriosis, myocardial infarction and chronic hepatitis B12,13,14, but its functions in cancer development have not been studied.
(A) Heatmap demonstrating microRNA expression of six paired human gallbladder cancer and neighboring normal tissues. The heatmap analysis was performed by R software with gplots package (p value < 0.05 and log10 fold change >2 or <0.5). (B) Validation of miR-125b-5p expression of different stages (All stages; Stage I&II; III; IV) in human gallbladder cancer and neighboring normal tissues. miR-125b-5p expression is down-regulated in clinical gallbladder cancer samples (*p < 0.05, ***p < 0.001). (C–F) Gallbladder cancer patient survival was analyzed by Kaplan-Meier analysis. P value was calculated using log-rank test. MicroRNA expression which exceeds the average level (mean value) is defined as “high” while microRNA expression which is less than the average level (mean value) is defined as “low”. (C) Lower miR-125b-5p expression in tumor is correlated with poor prognosis. (D) miR-376a-3p expression is not correlated with survival in gallbladder cancer. (E) miR-3145-5p expression is not correlated with survival in gallbladder cancer. (F) miR-3174 expression is not correlated with survival in gallbladder cancer.
To validate the down-regulation of miR-125b-5p expression in gallbladder cancer, we performed qPCR in 82 paired human gallbladder cancer and their corresponding neighboring normal tissues. The expression of miR-125b-5p was down-regulated in cancer tissues when compared with normal tissues (Fig. 1B). To determine whether the expression of microRNA candidates is correlated with prognosis, we selected two most down-regulated microRNAs, miR-125b-5p and miR-376a-3p (Fig. 1A), and two most up-regulated microRNAs, miR-3145-5p and miR-3174 (Fig. 1A), in gallbladder cancer to determine the correlation of their expressions and survival in clinical gallbladder cancer samples. Low expression of miR-125b-5p is correlated with poor prognosis (Fig. 1C), whereas the expression of miR-376a-3p (Fig. 1D), miR-3145-5p (Fig. 1E), and miR-3174 (Fig. 1F) is not correlated with prognosis. These data suggested that miR-125b-5p expression is significantly altered in gallbladder cancer and its low expression is correlated with poor prognosis.
miR-125b-5p sensitize gallbladder cancer to cisplatin treatment
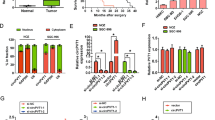
miR-125b-5p expression was determined in three gallbladder cancer cell lines NOZ, GBC-SD, and SGC-996 by qPCR. NOZ has the lowest miR-125b-5p expression whereas SGC-996 has the highest (Fig. 2A). It has been shown that NOZ is highly resistant to chemotherapy treatment15, thus we determined whether miR-125b-5p played any role in the sensitivity of cisplatin treatment in gallbladder cancer. miR-125b-5p mimics, antisense oligos, control mimics, and control antisense oligos were transfected into NOZ, GBC-SD, and SGC-996 cells which were subsequently treated with cisplatin or with no treatment. Cell death was measured by Annexin V staining and FACS analysis. Over-expression or knock-down of miR-125b-5p did not affect cell death (Fig. 2B). Overexpression of miR-125b-5p in these cells led to significant cell death when cells were treated with cisplatin (Fig. 2C). In contrast, downregulation of miR-125b-5p led to resistance in cells treated with cisplatin (Fig. 2C). IC50s of cisplatin were reduced in NOZ, GBC-SD, and SGC-996 cells transfected with miR-125b-5p mimics (Fig. 2D), but increased in these cells transfected with miR-125b-5p antisense oligos (Fig. 2D). These results indicated that miR-125b-5p is involved in cisplatin sensitivity in gallbladder cancer cells.
(A) miR-125b-5p expression in human gallbladder cancer cell lines, NOZ, GBC-SD, SGC-996, was quantified by qPCR. (B–C) NOZ, GBC-SD, SGC-996 cells were transfected with miR-125b-5p mimic or control mimic oligos, antisense miR-125b-5p or control anti-sense oligos. Cells were treated with cisplatin for 24 hours or with no treatment. Apoptosis were measured using Annexin V/PI staining. Student t test was used to calculate p value. *P < 0.05; **P < 0.01; ***P < 0.001; n.s, no significance. (B) miR-125b-5p mimics or antisense oligos did not affect cell death. (C) miR-125b-5p mimics sensitized gallbladder cells to cisplatin treatment and miR-125b-5p antisense oligos increased resistance to cisplatin treatment in gallbladder cancer cells. (D) IC50s of NOZ, GBC-SD, SGC-996 cells treated with cisplatin were calculated using cell viability data in Fig. 2B. Ectopic expression of miR-125b-5p decreased IC50 when compared with that of cells transfected with control oligos. Inhibition of miR-125b-5p by anti-sense oligos increased IC50 when compared with that of cells transfected with control anti-sense oligos.
Bcl2 is a direct target of miR-125b-5p and mediates its function in gallbladder cancer
To identify the direct targets of miR-125b-5p, we used microRNA target prediction program TargetScan 7.016 and find Bcl2 is one of the potential targets which contain miR-125b-5p binding site at its 3′UTR region. Bcl2 has been shown to be involved in cell death17,18 that is highly relevant to the cisplatin sensitivity phenotype. Immunoblotting was used to determine the expression of Bcl2 in gallbladder cancer cells. Introduction of miR-125b-5p mimics in NOZ, GBC-SD, and SGC-996 cells substantially decreased Bcl2 protein expression whereas miR-125b-5p antisense oligos increased Bcl2 expression (Fig. 3A,B, Supplementary Figure 1). To test whether Bcl2 is a direct target of miR-125b-5p, we constructed reporter plasmids containing the 3′UTR of Bcl2 (Fig. 3C). Co-transfection of miR-125b-5p and the reporter plasmids showed that the introduction of miR-125b-5p significantly suppressed the expression of a luciferase gene containing the 3′UTR of Bcl2 but not the one containing the 3′UTR of Bcl2 with the mutation in the miR-125b-5p binding site (Fig. 3D). Conversely, knockdown of miR-125b-5p by antisense oligos increased the luciferase signal containing 3′UTR of Bcl2 but not 3′UTR of Bcl2 with mutant miR-125b-5p binding site (Fig. 3D). These results suggested that Bcl2 is directly regulated by miR-125b-5p.
(A,B) Human gallbladder cancer cell lines, NOZ, GBC-SD, SGC-996, were tranfected with miR-125b-5p mimics, or control mimics, anti-sense miR-125b-5p, or control anti-sense oligos. Bcl2 mRNA expression in these cells were quantified by qPCR (A) and Bcl2 protein expression were measured by immunoblotting (B). (C) Bcl2-luciferase reporter plasmid construction. Seed sequence of miR-125b-5p targeting Bcl2 was indicted in grey. Mutant sequence of the reporter plasmid was indicted in white. (D) NOZ, GBC-SD, SGC-996 cells were transfected with wildtype or mutant Bcl2-luciferase reporter plasmid and miR-125b-5p mimics or control mimics or miR-125b-5p anti-sense oligos or control anti-sense oligos. Firefly luciferase activity was measured and normalized to the Renilla luciferase activity. (E) Bcl2 mediates the function of miR-125b-5p in apoptosis. NOZ, GBC-SD, SGC-996 cells were transfected with a Bcl2 cDNA plasmid without the 3′UTR and miR-125b-5p mimics or control mimics. Apoptosis were measured using Annexin V/PI staining. Student t test was used to calculate p value. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether Bcl2 mediates the function of miR-125b-5p in cisplatin sensitivity, we co-transfected miR-125b-5p and Bcl2 cDNA without the 3′UTR which contains the miR-125b-5p binding site into NOZ, GBC-SD, and SGC-996 cells. Introduction of both miR-125b-5p and Bcl2 significantly reduced cell death in these cells treated with cisplatin when compared with miR-125b-5p and a control vector (Fig. 3E). These results suggested that Bcl2 is a critical mediator in the cisplatin sensitivity function of miR-125b-5p.
miR-125b-5p enhances cisplatin therapy in a mouse model
To further confirm the function of miR-125b-5p in cisplatin sensitivity, we transplanted GBC-SD cells into mice which were then treated with cisplatin or saline control. Tumor volume was measured to determine tumor growth. Introduction of miR-125b-5p did not affect tumor growth in vivo (Fig. 4A,B). Bcl2 expression was decreased in tumor cells with miR-125b-5p (Fig. 4C,D), which further confirmed that miR-125b-5p down-regulates Bcl2. Apoptosis of the tumor cells were similar in cells with or without miR-125b-5p overexpression (Fig. 4E,F), which is consistent with our observation that miR-125b-5p alone does not affect cell death or proliferation in vitro. miR-125b-5p markedly sensitize tumor cells to cisplatin treatment (Fig. 4A,B). Bcl2 expression in the tumor cells were further decreased in cells with miR-125b-5p and cisplatin treatment (Fig. 4C,D). Apoptosis of transplanted tumor cells were significantly increased in cells which were introduced with miR-125b-5p and treated with cisplatin when compared with cells with a control vector and cisplatin treatment (Fig. 4E,F). Taken together, these results further confirmed the function of miR-125b-5p in cisplatin sensitivity in vivo.
(A,B) Human gallbladder cancer GBC-SD cells expressing miR-125b-5p or a control vector were transplanted in mice subcutaneously. Mice were treated with cisplatin or saline. Tumor volume (A) and tumor weight (B) were measured. P value was determined by student t test. **P < 0.01; ***P < 0.01; n.s, no significance. Tumor growth was significantly suppressed in mice expressing miR-125b-5p treated with cisplatin. (C,D) Representative images of Bcl2 expression in tumor tissues in mouse model (C). Bcl2 expression was determined using immunohistochemistry (D). Bcl2 expression was decreased in tumors expressing miR-125b-5p. (E,F) Representative images of apoptosis in tumor tissues in mouse model (E). Apoptosis was determined using TUNEL staining (F). Apoptosis was increased in tumor cells expressing miR-125b-5p and treated with cisplatin.
miR-125b-5p and Bcl2 expressions are biomarkers for prognosis in human gallbladder cancer
We have shown that miR-125b-5p was down-regulated in gallbladder cancer samples (Fig. 1A,B) and suppressed Bcl2 expression in vitro and in vivo. To investigate whether miR-125b-5p negatively regulates Bcl2 in clinical samples, we determined the Bcl2 expression in human gallbladder cancer samples. The expression of Bcl2 is higher in gallbladder cancer tissues than in neighboring normal tissues (Fig. 5A). Immunohistochemistry staining of Bcl2 showed higher Bcl2 expression in gallbladder cancer when compared with that in neighboring normal tissues (Fig. 5B). The expression of miR-125b-5p and Bcl2 are inversely correlated in gallbladder cancer samples (Fig. 5C). Low miR-125b-5p expression is inversely correlated with survival (Fig. 1C). Interestingly, high Bcl2 expression is correlated with poor survival in gallbladder cancer (Fig. 5D). Moreover, the combination of miR-125b-5p low expression and Bcl2 high expression is highly correlated with poor prognosis in clinical samples. These results further supported the regulation of Bcl2 by miR-125b-5p and demonstrated the significance of miR-125b-5p and Bcl2 as biomarkers in gallbladder cancer progression.
(A,B) Bcl2 mRNA (A) expression and protein expression (B) in clinical gallbladder cancer tissues and neighboring normal tissues was determined by qPCR and immunohistochemistry. Bcl2 expression was up-regulated in gallbladder cancer tissues than neighboring normal tissues. (C) Correlation of miR-125b-5p expression and Bcl2 expression in human gallbladder cancer tissues was analyzed using Pearson’s correlation analysis. r = −0.594, P < 0.001. The expression of miR-125b-5p and Bcl2 were inversely correlated. (D,E) Gallbladder cancer patient survival was analyzed by Kaplan-Meier analysis. P value was calculated using log-rank test. Bcl2 expression which exceeds the average level (mean value) is defined as “high” while Bcl2 expression which is less than the average level (mean value) is defined as “low”. (D) Higher Bcl2 expression in tumors is correlated with poor prognosis. (E) Patients with both lower miR-125b-5p expression and higher Bcl2 expression in tumors exhibit poor prognosis.
Discussion
Cisplatin resistance is common among gallbladder cancer patients with cisplatin treatment as adjuvant therapy. Discovery of molecules and therapies that sensitize cisplatin in gallbladder cancer is urgently needed. We used a genome-wide microRNA expression profiling to identify miR-125b-5p markedly down-regulated in gallbladder cancer. Lower miR-125b-5p expression in GBC is correlated with poor prognosis. Moreover, miR-125b-5p sensitizes gallbladder cancer cells to cisplatin treatment. Bcl2 is a direct target of miR-125b-5p and mediates its functions. These findings suggest a novel therapeutic target for gallbladder cancer.
Although microRNAs have been implicated in gallbladder cancer development5,19, their functions in the sensitization of chemotherapies have not been well studied. We recently found that miR-145 is up-regulated in gallbladder cancer tissues and sensitize cisplatin treatment by suppressing MRP120. In this work, we found that miR-125b-5p is down-regulated in human gallbladder cancer and has different mechanisms to enhance cisplatin sensitivity. It contributes to intrinsic resistance to chemotherapy after surgery rather than acquired resistance. These results indicate that cisplatin resistance in gallbladder cancer is due to multiple mechanisms. Therapeutics that targeting multiple molecules is required to improve therapeutic efficiency of cisplatin in GBC.
In summary, we showed for the first time that miR-125b-5p-Bcl2 pathway is potentially a therapeutic target for gallbladder cancer. MicroRNA mimics have been developed for therapeutics21,22,23,24, although delivery remains a major issue for their use in the clinic. Bcl2 small molecule inhibitors have also been developed and are currently in clinical trials for chronic lymphocytic leukemia patients25,26. Although Bcl2 inhibitors are easier to be delivered in patients, miR-125b-5p mimics potentially have advantages in therapy because miR-125b-5p target molecules in addition to Bcl2 may also contribute to resistance. In combination with cisplatin, these therapeutics would potentially overcome cisplatin resistance and improve clinical outcomes in gallbladder cancer.
Materials and Methods
Tissue samples
Formalin-fixed, paraffin-embedded (FFPE) cancer tissues were collected from 82 patients harboring histologically-confirmed GBC who underwent surgical resection of the gallbladder and postoperative adjuvant chemotherapy at the Department of Pathology (Renji Hospital) from January 2004 to December 2013 retrospectively. Fresh GBC tissues and the neighboring noncancerous gallbladder tissues were also obtained from 82 GBC patients. All fresh tumor samples were collected immediately after the surgical removal and snap-frozen in liquid nitrogen, then stored at −80 °C until total RNA was extracted. Postoperative survival was calculated from time of surgery to time of last follow-up or death. The collection and analysis of patient samples were approved by the Ethical Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all patients. All methods and experiments were carried out in accordance with the approved guidelines and regulations.
miRNA microarray
Total RNA samples were spiked using the MicroRNA Spike-In Kit (Agilent Technologies) to assess the labeling and hybridization efficiencies. After the spiked total RNA was treated with alkaline calf intestine phosphatase, a labeling reaction was initiated with 100 ng of total RNA per sample. T4 RNA ligase, which incorporates cyanine 3-cytidine biphosphate (miRNA Complete Labeling and Hyb Kit; Agilent Technologies), was used to label the dephosphorylated RNA. The cyanine 3-labeled miRNA samples were subsequently prepared for one-color hybridization (miRNA Complete Labeling and Hyb Kit). The labeled miRNA samples were hybridized to the Agilent Human miRNA Microarray 8 × 60 K Release 16.0 (Release 16.0, 8 × 60 K format; Agilent Technologies) for 20 h at 55 °C. After washing the microarray slides with buffers with increasing stringency (Gene Expression Wash Buffers; Agilent Technologies), the slides were dried with acetonitrile. Fluorescent signal intensities were detected on an Agilent Microarray Scanner (Agilent Technologies) with the Scan Control A.8.4.1 Software (Agilent Technologies) and were extracted from the images using the Feature Extraction 10.7.3.1 Software (Agilent Technologies). All of the steps described above were performed according to the manufacturer’s instructions. The raw miRNAs microarray data were normalized using the GeneSpring GX software version 12.0 (Agilent Technologies). The signal values were transformed to the log base 10, and then quantile and percentile shift was applied to obtain an equal distribution of probe signal intensities. The comparative analysis of the gallbladder cancer and normal control group samples was performed using the t-test (p-values) and SAM (http://www-stat.stanford.edu/~tibs/SAM/). Compared with the expression level of the reference RNA, the miRNAs were described as differentially expressed if the p-values were <0.05, and the fold change (FC) was greater than 2 or less than 0.5.
Cell culture
The human embryonic kidney 293 cells (HEK293FT) and two human GBC cell lines, NOZ and GBC-SD were maintained in Dulbecco’s Modified Eagle Medium (DMEM), Another human GBC cell line SGC- 996 was maintained in RPMI-1640 medium, with all media containing 10% fetal bovine serum (FBS) and antibiotics (Gibco, Grand Island, NY, USA). Cells were maintained at 37 °C in a humidified atmosphere consisting of 5% CO2. NOZ was purchased from the Health Science Research Resources Bank (Osaka, Japan). GBC-SD and SGC-996 cells were provided by the Academy of Life Sciences, Tongji University (Shanghai, China). HEK293FT cells were purchased from Invitrogen (MD, USA) and were used for adenovirus amplification. Cisplatin was dissolved in dimethyl sulfoxide (DMSO). GBC cells were treated with cisplatin (40 μM in NOZ and 4 μM in GBC-SD or SGC-996 cells) or control DMSO.
Cell transfection
Human miR-125b-5p expression construct was generated by insertion of the coding sequence (CDS) of miR-125b-5p into pCDHCMV- MCS-EF1-copGFP (System Biosciences, CA, USA). Recombinant lentiviruses were produced by transient transfection of HEK293FTcells, along with package vectors, using Lipofectamine 2000 (Invitrogen). After transfection for 48 h, the viruses were harvested and viral titers were determined. Then, GBC-SD cells were infected with lentiviruses in the presence of 4 μg/ml polybrene (Sigma), followed by puromycin selection (2 μg/ml). Bcl2 expression vector was constructed by cloning Bcl2 cDNAinto a pcDNA 3.1 vector (Invitrogen). NOZ, GBC-SD and SGC-996 cells were transfected with the Bcl2 expression plasmid using Lipofectamine 2000 (Invitrogen) transfection reagent according to the protocols. The pcDNA3.1 empty vector was used as negative control (vector). The miR-125b-5p mimic, miR-125b-5p inhibitor, and siRNA of Bcl2 were purchased from GenePharma (Shanghai, China). Cells were cultured to 60–70% confluence in six-well plates and then transfected using Lipofectamine 2000 (Invitrogen).
Quantitative
Real-time PCR analysis Total RNA and miRNA were isolated from fresh tissues and cells using TRIzol reagent (Invitrogen) and miRNeasy Mini Kit (Qiagen, Hilden, Germany), and miRNAs were extracted from FFPE samples using miRNeasy FFPE Kit (Qiagen) according to the manufacturer’s instructions. After synthesizing cDNAs with Reverse Transcriptase M-MLV kit, the expression levels of miR-125b-5p and Bcl2 were analyzed using SYBR Premix Ex Taq (Takara, Shiga, Japan) and run with Applied Biosystems ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA). Data were analyzed by 2−ΔΔCT method27 and presented relative to the expression of GAPDH for Bcl2 and in relation to the expression of small nuclear U6 RNA for miR-125b-5p. The primer sequences are listed in Supplementary Table 1.
Cytotoxicity, cell apoptosis, cell proliferation assays
Cell viability (NOZ, GBC-SD and SGC-996) was identified by 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2H-tetrazolium assay (MTS; Promega, Madison, WI, USA). Briefly, cells were plated (5 × 103 cells/well) on 96-well plates and incubated overnight to allow cell attachment. Then, NOZ cells were treated with cisplatin at a series of concentrations of 1, 2, 4, 8, 16, 32, 64, 128 and 256 μM while GBC-SD and SGC-996 cells were treated with cisplatin at concentrations of 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μM for 24 h, respectively. Subsequently, the MTS reagent (20 μl) was added to each well, followed by incubation at 37 °C in a humidified, 5% CO2 atmosphere for 2 h. Finally, the absorbance was read at 490 nm by using a Synergy 2 (BioTek, VT, USA) plate reader. The cell viability was indicated as a percentage relative to control. Cell proliferation was also analyzed using MTS assay (Promega) when NOZ, GBC-SD and SGC-996 cells were seeded into a 96-well plate (1 × 103 cells/well) and cultured for 72 h. Apoptosis of gallbladder cancer cell lines were analyzed using Annexin V/PI Apoptosis Detection Kit (BD Biosciences, MA, USA) according to the manufacturer’s instructions. Cells were seeded in six-well plates and grown to approximately 60% confluence, followed by treating with cisplatin (40 μM in NOZ and 4 μM in GBC-SD or SGC-996) for 48 h. Cells were harvested and incubated with Annexin V/PI for 15 min in the dark, followed by fluorescence-activated cell sorting (FACS) analysis. All the assays were carried out four times.
Reporter vector constructs and dual luciferase reporter assay
The fragment from Bcl2-3′-UTR containing the predicted miR-125b-5p binding site was amplified by PCR and then cloned into a pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega) to form the reporter vector Bcl2-3′-UTR wild type. The putative binding site of miR-125b-5p in the Bcl2- 3′-UTR was mutated by using a site-directed mutagenesis kit from Fast Mutagenesis System (TransGen Biotech, Beijing, China), and the mutant reporter vector was named as Bcl2-3′-UTR mutant. The miR-125b-5p mimic and vector were co-transfected into 3 human gallbladder cancer cells, and Renilla luciferase reporter plasmid (pRL-TK) was also co-transfected as the internal reference. After transfection for 48 h, cells were lysed in passive lysing buffer, and then firefly and Renilla luciferase activities were analyzed using the Dual-Luciferase Reporter Assay System (Promega). The results of firefly luciferase activity were normalized to the Renilla luciferase activity.
Western blot analysis
For protein isolation from NOZ, GBC-SD and SGC-996 cells, RIPA buffer supplemented with proteinase inhibitor cocktail was used. The protein concentration was determined using the BCA assay. Equal amounts of cell lysates were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Millipore, IL, USA). The membranes were blocked for 1 h at room temperature using Tris-bufferred saline with 0.05% Tween 20 (TBST) and 5% skimmed milk, and then the following primary antibodies were applied overnight at 4 °C: anti-Bcl2 (Santa Cruz, CA, USA) and anti-β-actin (Sigma). After washing three times with TBST, the membranes were incubated with secondary antibody at room temperature for 2 h and washed again with TBST. Images of target proteins were detected by chemiluminescence HRP substrate kit (Millipore).
Immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling assays
All specimens from patients and subcutaneous xenografts fixed in 10% buffered formalin were embedded in paraffin blocks. Consecutive 4-μm thick sections were analyzed using a standard immunohistochemistry protocol and stained by antibodies of Bcl2 (1:100, Santa Cruz). Positive staining cells were visualized by DAB systems and counterstained with hematoxylin. The stained sections were photographed and converted to a digital image using light microscopy equipped with camera (Olympus, Tokyo, Japan). The scoring of immunohistochemistry (IHC) is based upon the staining intensity (I) and the proportion of stained quantity (q) of tumor cells to obtain a final score (Q) defined as the product of I × q and was performed by two independent pathologists. The scoring system for I was 0 = negative, 1 = low, 2 = moderate, 3 = intense immunostaining; and for q was 0 = negative, 1 = 1–9% positive, 2 = 10–39% positive, 3 = 40–69% positive, and 4 = 70–100% positive cells. Cell apoptosis of xenograft sections was also detected by using In Situ Cell Death Detection Kit, POD (Roche, Basel, Switzerland) according to the manufacturer’s instruction. The sections were visualized with DAB and counterstained with hematoxylin. The number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells was randomly counted in five fields, and the apoptosis index for each field was calculated as the percent of TUNEL-positive cells relative to the total cells.
In vivo studies
Animal maintenance and experimental procedures were strictly performed following the guidelines of the Animal Care and Use Committee of Shanghai Jiao Tong University and approved by IACUC committee of Shanghai Jiao Tong University. A total 1 × 106 GBC-SD/pcDNA3.1 miR-125b-5p or GBC-SD/pcDNA3.1 empty vector cells in 60 μl medium were subcutaneously transplanted into 4-week-old male nude mice of each group (group 1, vector; group 2, miR-125b-5p; group 3, vector + cisplatin; group 4, miR-125b-5p + cisplatin; n = 6/group). When the average tumor size reached approximately 0.1 cm3, cisplatin was administered via intraperitoneal injection at a dose of 4 mg/kg at Day 14 and Day 24. Saline was used as a control. Tumor volumes were examined using external caliper once every 4 days and were calculated based on the equation: V = (length × width2)/228. All mice were sacrificed at the 44th day, and the tumors were dissected out for hematoxylin and eosin (H&E) staining, IHC staining, and TUNEL staining. All methods and experiments were carried out in accordance with the approved guidelines and regulations.
Statistics
Data are expressed as mean ± SEM. Two-group comparisons were performed with unpaired two-tailed Student’s t test. Survival probabilities were determined using Kaplan-Meier analyses and compared by the log-rank test. Each experiment consisted of at least four replicates per condition. SPSS 17.0 software was used for all statistical analysis. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Yang, D. et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci. Rep. 7, 43109; doi: 10.1038/srep43109 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kanthan, R., Senger, J. L., Ahmed, S. & Kanthan, S. C. Gallbladder Cancer in the 21st Century. Journal of oncology 2015, 967472, doi: 10.1155/2015/967472 (2015).
Stein, A. et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC cancer 15, 564, doi: 10.1186/s12885-015-1498-0 (2015).
Wang, W. et al. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncology reports 26, 1143–1148, doi: 10.3892/or.2011.1390 (2011).
Rho, Y. S., Barrera, I., Metrakos, P. & Kavan, P. Complete Resolution of Metastatic Gallbladder Cancer after Standard Gemcitabine-Cisplatin Combination Therapy. Cureus 7, e415, doi: 10.7759/cureus.415 (2015).
Chang, Y. et al. MiR-20a triggers metastasis of gallbladder carcinoma. Journal of hepatology 59, 518–527, doi: 10.1016/j.jhep.2013.04.034 (2013).
Ma, M. Z. et al. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell death & disease 6, e1583, doi: 10.1038/cddis.2014.541 (2015).
Li, Z. et al. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget 6, 13914–13921, doi: 10.18632/oncotarget.4227 (2015).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews. Molecular cell biology 15, 49–63, doi: 10.1038/nrm3722 (2014).
Tam, C. S., Seymour, J. F. & Roberts, A. W. Progress in BCL2 inhibition for patients with chronic lymphocytic leukemia. Seminars in oncology 43, 274–279, doi: 10.1053/j.seminoncol.2016.02.014 (2016).
Koehler, B. C. et al. Beyond cell death - antiapoptotic Bcl-2 proteins regulate migration and invasion of colorectal cancer cells in vitro . PloS one 8, e76446, doi: 10.1371/journal.pone.0076446 (2013).
Sun, T. et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology (Baltimore, Md.) 54, 1690–1706, doi: 10.1002/hep.24543 (2011).
Cosar, E. et al. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertility and sterility, doi: 10.1016/j.fertnstert.2016.04.013 (2016).
Jia, K. et al. Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Molecular medicine reports, doi: 10.3892/mmr.2016.5246 (2016).
Ninomiya, M. et al. The expression of miR-125b-5p is increased in the serum of patients with chronic hepatitis B infection and inhibits the detection of hepatitis B virus surface antigen. J Viral Hepat 23, 330–339, doi: 10.1111/jvh.12522 (2016).
Iwase, R. et al. Combination chemotherapy of nafamostat mesylate with gemcitabine for gallbladder cancer targeting nuclear factor-kappaB activation. J Surg Res 184, 605–612, doi: 10.1016/j.jss.2013.06.003 (2013).
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature reviews. Cancer 2, 647–656, doi: 10.1038/nrc883 (2002).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442, doi: 10.1038/335440a0 (1988).
Kono, H. et al. High expression of microRNA-155 is associated with the aggressive malignant behavior of gallbladder carcinoma. Oncology reports 30, 17–24, doi: 10.3892/or.2013.2443 (2013).
Zhan, M. et al. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, doi: 10.1007/s13277-016-4957-6 (2016).
Rupaimoole, R., Calin, G. A., Lopez-Berestein, G. & Sood, A. K. miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov 6, 235–246, doi: 10.1158/2159-8290.cd-15-0893 (2016).
Ma, L. et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 28, 341–347, doi: 10.1038/nbt.1618 (2010).
Kasinski, A. & Slack, F. J. Small RNAs deliver a blow to ovarian cancer. Cancer Discov 3, 1220–1221, doi: 10.1158/2159-8290.cd-13-0667 (2013).
Tyagi, N. et al. Exploiting Nanotechnology for the Development of MicroRNA-Based Cancer Therapeutics. Journal of biomedical nanotechnology 12, 28–42 (2016).
Anderson, M. A. et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 127, 3215–3224, doi: 10.1182/blood-2016-01-688796 (2016).
Roberts, A. W. et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20 + lymphoid malignancies. British journal of haematology 170, 669–678, doi: 10.1111/bjh.13487 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25, 402–408, doi: 10.1006/meth.2001.1262 (2001).
Tomayko, M. M. & Reynolds, C. P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24, 148–154 (1989).
Acknowledgements
This work was supported by National Science Foundation of China (81272748, 81472240, 81072011), Science and Technology Commission of Shanghai Municipality, 16411952700, 10411955400, 09411960800, Ministry of Science and technology of the People’s Republic of China (2012BAI06B01) Chinese Scholarship Council (CSC) Scholarship (DY), NIH/NCI R01CA148759, NIH/NCI 1R21CA198558-01A1.
Author information
Authors and Affiliations
Contributions
J.W., Q.H. designed the study. D.Y., M.Z., T.C., W.C., Y.Z., S.X. performed the experiments. J.Y. provided materials. D.Y., M.Z., T.C., J.W., Q.H. analyzed the data. D.Y., M.Z., Q.H., J.W. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, D., Zhan, M., Chen, T. et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep 7, 43109 (2017). https://doi.org/10.1038/srep43109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43109
This article is cited by
-
miR-125b-5p upregulation by TRIM28 induces cisplatin resistance in non-small cell lung cancer through CREB1 inhibition
BMC Pulmonary Medicine (2022)
-
Tanshinone I restrains osteosarcoma progression by regulating circ_0000376/miR-432-5p/BCL2 axis
Molecular and Cellular Biochemistry (2022)
-
Overview of current targeted therapy in gallbladder cancer
Signal Transduction and Targeted Therapy (2020)
-
miR-125b-5p inhibits cell proliferation, migration, and invasion in hepatocellular carcinoma via targeting TXNRD1
Cancer Cell International (2019)
-
A thiosemicarbazone derivative induces triple negative breast cancer cell apoptosis: possible role of miRNA-125a-5p and miRNA-181a-5p
Genes & Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.