Abstract
The premature increase of oxygen tension may contribute to oligodendroglial precursor cell (OPC) damage in preterm infants. Fetal OPCs are exposed to low oxygen tissue tensions not matched when cells are cultured in room air. Maturation (A2B5, O4, O1, MBP, CNP, arborization), oxidative stress (nitrotyrosine Western blot, NRF2 and SOD2 expression), apoptosis (TUNEL), proliferation (Ki67), and expression of transcription factors regulated by Hypoxia-Inducible-Factor-1-alpha (Hif-1α) expressed in OPCs (Olig1, Olig2, Sox9, Sox10) were assessed in rat OPCs and OLN93 cells cultured at 5% O2 and 21% O2. Influences of Hif-1α were investigated by Hif-1α luciferase reporter assays and Hif-1α-knockdown experiments. At 21% O2, cell proliferation was decreased and process arborization of OPCs was reduced. Expression of MBP, CNP, Olig1, Sox9 and Sox10 was lower at 21% O2, while Nrf2, SOD2, nitrotyrosine were increased. Apoptosis was unchanged. Luciferease reporter assay in OLN93 cells indicated increased Hif-1α activity at 5% O2. In OLN93 cells at 5% O2, Hif-1α knockdown decreased the expression of MBP and CNP, similar to that observed at 21% O2. These data indicate that culturing OPCs at 21% O2 negatively affects development and maturation. Both enhanced oxidative stress and reduced expression of Hif-1α-regulated genes contribute to these hyperoxia-induced changes.
Similar content being viewed by others
Introduction
In addition to its fundamental role in energy metabolism, oxygen serves as a regulator of cellular development. Cells of the central nervous system, in particular, are known to be highly susceptible to varying oxygen tensions1. While during fetal brain development, the in utero environment composes low arterial oxygen levels of 20–25 mmHg2, birth into room air causes a several fold increase of oxygen in the infant and in its brain. In preterm infants, however, this rise of oxygen occurs prematurely and may interfere with crucial cellular processes during early brain development.
In neural precursor cells, for example, higher in vitro oxygen levels (20%) induce apoptotic cell death while low oxygen (e.g. 5%) promotes the expansion of clonal precursor populations3. In astrocytes, variant oxygen tensions in vitro have been shown to result in different transcription patterns for ribosomal activity, immune responses, and cell cycle regulation4 and lower oxygen levels of 7% during reoxygenation were found to reduce cell death in astroglia after oxygen-glucose-deprivation5. In fact, the 21% O2 commonly used for cell cultures produce fairly high partial oxygen pressures of more than 150 mmHg, whereas under physiological conditions in the cerebral cortex, neural cells are exposed to much lower oxygen tensions of about 16–38 mmHg (2–5% O2)6,7.
These circumstances, however, have not yet been investigated with regards to the development of oligodendroglia. While the developmental process itself has been well described8,9,10, oligodendroglial precursor cells (OPCs) are known to have pronounced susceptibility to oxidative stress and to radicals due to their low levels of anti-oxidants and radical scavangers11,12. In primary cultured OPCs, oxidative stress caused by exposure to peroxides disrupts oligodendroglial maturation and downregulates gene expression of factors needed for oligodendroglial development13. Perturbation of immature neural cell development by high oxygen could thus be mediated by oxygen-induced oxidative stress. Alternatively, decreased hypoxia-inducible-factor-1-alpha (HIF-1α) may also disrupt cellular development.
Under hypoxic conditions, HIF-1α is stabilized and serves as a key transcriptional factor for various regulatory pathways14. High HIF-1α expression under hypoxia also coincides with increased activity of sonic hedgehog in the rat embryo heart15 and sonic hedgehog is known to promote the expansion of the oligodendroglial population during development and after injury of the CNS16,17,18. Several genes relevant to cell survival have been found to be upregulated by hypoxia via the HIF-1α pathway19 and neuroprotective pre-conditioning prior to an injurious challenge by hypoxia-ischemia has just recently been demonstrated to depend on the presence of HIF-1α20.
Hence, we hypothesize that survival, proliferation and maturation of immature oligodendroglial lineage cells may be affected by the level of environmental oxygen. In our in vitro studies, we therefore investigated whether HIF-1α and/or oxidative stress are involved in specific changes of oligodendroglial development in response to oxygen. The results may help to understand the mechanisms of oligodendroglial damage in the brains of preterm infants potentially caused by the drastic increase of oxygen levels after birth.
Results
Lower oligodendroglial cell numbers at higher oxygen levels
In order to analyze whether oxygen tensions influence oligodendroglial development we quantified total numbers of primary rat oligodendroglial lineage cells that were cultured for 48 and 96 hours in either 5% or 21% O2. We used immunocytochemistry with different oligodendroglial markers to characterize different stages of maturation. Oligodendroglial precursor cells (OPC) were labeled using antibodies against A2B5 antigen and immature oligodendroglia were marked using anti-O4 antibodies. The results show that A2B5+ cells were decreased in number in 21% oxygen when compared with 5% O2. The reduction of OPCs at 21% O2 was found after 48 hours as well as after 96 hours culture time (at 48 hours: 53.98 cells/field at 5% O2 vs. 32.39 cells/field at 21% O2, p = 0.0029; at 96 hours: 23.44 cells/field at 5% O2 vs.11.58 cells/field at 21% O2, p = 0.024) (Fig. 1a,b) A pronounced decrease in the numbers of immature O4+ oligodendroglia was observed after 48 and 96 hours (at 48 hours: 15.99 O4+ cells/field at 5% O2 vs. 4.60 O4+ cells/field at 21% O2, p < 0.0001, at 96 hours: 11.59 O4+ cells/field at 5% O2 vs.5.60 cells/field at 21% O2, p < 0.0001) (Fig. 1c,d). Similar results were found for O1+ cells after 96 hours culture time (5% O2 = 6.36+/−0.69 [SEM] cells per field vs. 21% O2 = 4.70+/−0.71 cells per field; N = 8) (Fig. 1e). This loss of cells expressing A2B5, O4 and O1 at 21% O2 could be due to increased cell death, impaired antigen expression, or decreased proliferation.
Immunocytochemistry of OPC shows a significant reduction in cell numbers of A2B5+ OPCs cultured in 21% O2 compared to 5% O2 after 48 hours (a) p = 0.0029) and 96 hours (b) p = 0.024) culture time and a highly significant reduction of O4+OPC cultured in 21% O2 after 48 hours (c) p < 0.0001) and 96 hours (d) p < 0.0001) culture time. (N = 15, unpaired t-test with Welch’s correction *p < 0.05, **p < 0.01, ***p < 0.001). Numbers of O1 positive cells (e) are reduced after 96 h culture time at 21% compared to 5% (N = 6, *p < 0.05).
Lower oxygen levels lead to enhanced oligodendroglial proliferation in vitro
During oligodendrocyte development, the expansion of the oligodendroglial lineage population proceeds first by progenitor proliferation and is followed by maturation and functional refinement8,21,22. We first determined the influence of the different oxygen levels on oligodendroglial proliferation in vitro. We used immunocytochemistry for Ki67 as a widely established marker for proliferating cells in the S- and G2-phase, together with antibodies against A2B5 to identify OPCs and against O4 to analyze immature oligodendroglia.
Culture conditions at 21% oxygen for 48 hours yielded significantly lower numbers of A2B5+Ki67+ proliferating OPCs, i.e. at 5% O2 16.93% of A2B5+ were also Ki67+ compared to 10.8% in cells cultured at 21% O2) (p = 0.042) (Fig. 2a). The quantification of O4+Ki67+ cells after 48 hours culture time revealed a strong inhibitory effect of higher oxygen on proliferation of immature oligodendroglia (12.65% of O4+ cells were also Ki67+ when cultured in 5% O2in comparison to only 2.5% of O4+ cells cultured at 21% O2, p = 0.022) (Fig. 2b).
We also used the OLN93 cell line to analyze proliferation. Though the OLN93 cell line does not express A2B5 antigen23 and therefore cannot be labeled with A2B5 antibodies, it resembles bipolar O-2A-progenitor cells23. These cells were stained with DAPI and Ki67 to mark proliferating cells after 48 hours culture time. As a result, proliferation of all OLN93 cells cultured in 21% oxygen was significantly reduced compared to 5% oxygen (Ki67+ Dapi+ cells: 65.20% of Dapi+ at 5% O2 compared to 32.8% in OLN93 cultured at 21% p = 0.008) (Fig. 3).
These results clearly indicate that decreased proliferation of oligodendroglial cells represents a mechanism of cell number reduction caused by higher oxygen levels.
Developmental and maturational genes are downregulated by higher oxygen
To analyze the impact of 21% oxygen on oligodendroglial development more specifically, we used real-time PCR to quantify the expression of genes known to be important for oligodendroglial lineage progression (Olig1, Olig2), development (Sox9, Sox10) and for the function of mature oligodendrocytes (CNP, MBP). RNA was extracted from OPCs cultured at 21% and 5% O2 for 48 hours.
Interestingly, Olig1 was significantly downregulated by 25.29% in OPCs cultured at 21% as compared to OPCs kept in 5% O2 (p = 0.037) (Fig. 4a). A decline of Olig2 expression by 16.73% was also seen in OPCs at 21% but numbers were not statistically significant on statistical analysis (Fig. 4a). Remarkably, changes were more pronounced in markers of maturation and development: Sox9 and Sox10 were both drastically downregulated in OPCs cultured at 21% compared to 5% O2 (Sox 9: 66.52% of control, p = 0.030; Sox10: 60.36% of control, p = 0.039) (Fig. 4b). Sox9, in particular, is reported to be regulated by Hypoxia-Inducible-Factor, which suggests a possible mechanism of HIF-dependent response to changes in oxygen levels24. Furthermore, gene expression of CNP and of MBP was also markedly diminished in OPC cultures kept in 21% O2 down to 58.43%, (p = 0.0098) and to 41.42% of control levels (p = 0.002), respectively, as compared to OPC cultures kept at 5% O2 (Fig. 4c). Hence, the exposure to higher oxygen caused a significant downregulation of transcription factors and maturational markers in OPCs, indicating a central role of oxygen environment in the regulation of oligodendroglial development.
Realtime PCR used to quantify genexpression after 48 hours culture in 5% and 21% O2. Olig1 was significantly downregulated at 21% compared to 5% O2 (p = 0.037), Olig2 downregulation was notable. (a) Markers for oligodendroglial development (Sox9, p = 0.030; Sox10, p = 0.039) (b) and maturational markers (CNP, p = 0.0098; MBP, p = 0.002) (c) were drastically reduced in 21% compared to 5% O2. Oxidative stress response was visualized through significantly increased expression of Nrf2 (p = 0.0098) but only tended to enhance SOD2 expression (d) (N = 10, Wilcoxon signed rank test *p < 0.05, **p < 0.01, ***p < 0.001).
Oxidative stress and apoptosis in OPCs at 21% and at 5% O2
Higher oxygen levels may also induce cellular stress through reactive oxygen species and/or radicals. To assess whether cultured OPCs kept in 21% O2 are challenged by higher oxidative stress, we analyzed expression levels of factors that are needed for cellular anti-oxidant defense, i.e. superoxide dismutase 2 (SOD2) and nuclear factor erythoid 2-related factor 2 (Nrf2), which in particular represents a transcription factor that orchestrates a large set of cellular anti-oxidant enzymes. The results of realtime PCR analysis showed that OPCs kept at 21% O2 tended to have increased SOD2 expression, but numbers were not significant on statistical analysis (139.94% of controls; p = 0.0840). In contrast, Nrf2 was significantly upregulated in OPCs cultured at 21% O2 (147.87% of controls, p = 0.0098). This amplified expression underlines a pronounced anti-oxidative cellular response to oxidative stimulation (Fig. 4d).
In order to further determine oxidative stress responses in OPC cultures we used Western blot analysis of nitrotyrosine levels, which is commonly used as a biochemical marker of nitration. As a result, nitrotyrosine levels in protein lysates obtained from OPC cultures were significantly increased after incubation at the higher oxygen culture condition with 21% O2 as compared to the lower 5% O2 (Fig. 5a,b).
Western blot analysis of nitrotyrosine intensity in protein lysates obtained from OPC cultures at 5% O2 compared to those at 21% O2 (a). Statistical analysis using paired ttest to directly compare the two culture conditions after 48 hours incubation time revealed a significant increase of nitrotyrosine in proteins obtained from OPCs at the higher oxygen level. (b) (N = 5, paired ttest *P = 0.013). Immunocytochemistry for TUNEL (red) and for A2B5 (green) in OPC cultures do not show differences in the numbers of TUNEL+ apoptotic cells at 21% O2 as compared to 5% O2 (dapi = blue) (c,d). The majority of dying TUNEL+ cells does not reveal co-expression of A2B5.
To measure a potential impact of oxidative stress on apoptotic death, we performed TUNEL stainings in the OPC cultures together with A2B5 immunocytochemistry. However, the number of TUNEL+ cells was not increased in cell cultures kept in 21% O2 (3.18+/−0.54 cells per field) as compared to those at 5% O2 (2.84+/−0.41 cells per field; N = 4) (Fig. 5c,d). At both culture conditions, the vast majority of TUNEL+ nuclei were without co-localization for A2B5.
Morphological changes in O4+ oligodendroglia
We used Sholl analysis to designate morphological differences of OPCs cultured at 5% vs. 21% oxygen. The number of intersections of cell appendices with the concentric circles was used as a parameter of morphological complexity and the ending radius was used as a representation of cell size. O4+ cells cultured for 48 hours showed a drastic difference in cell size depending on the amount of environmental oxygen used in the cultures. The ending radius of cells cultured at 21% O2 only amounted to 49% of that of OPCs cultured at 5% O2 (after 48 hours culture time: 2.96 vs. 6.03 arbitrary units, p < 0.0001) (Fig. 6a). The assessment of morphological features in terms of sum of intersections of OPC branches with concentric circles produced by the software-plugin showed similar differences: The numbers differed from a mean of 1.663.066 intersections in cells cultured at 5% to 356.360 intersections in O4+ cells cultured for 48 hours at 21% O2 (p < 0.0001) (Fig. 6a), hence demonstrating a much higher morphological complexity of OPCs cultured at 5% O2. Sholl analysis of O4+ cells cultured for 96 hours showed similar results. Again, the mean ending radius of cells at 5% significantly exceeded that of cells cultured in 21% O2 (6.33 vs. 4.27 arbitrary units, respectively, p = 0.0009) (Fig. 5b), and the arborization analysis revealed a significantly more complex structure as indicated by higher numbers of intersections (1.206.300 vs. 680.938, p < 0.0237) (Fig. 6b).
Analysis of morphology using the ImageJ PlugIn Sholl analysis resulted in a highly significant decrease in cell size and arborization of primary O4+ immature oligodendroglia cultured at 21% O2 compared to 5% O2 after 48 hours (a) p < 0.0001 for both) and 96 hours (b) ending radius: p = 0.0009, sum of intersections: p = 0.0237) (N = 8, analyzing 4 sections in 5 plates/N, unpaired t-test with Welch’s correction *p < 0.05, **p < 0.01, ***p < 0.001).
Taken together, 21% oxygen consistently and over different exposure times, impaired the morphological development of O4+ immature oligodendroglia in our in vitro experiments.
HIF-1α activity is reduced in oligodendroglial cells at 21% O2
Many cellular signaling responses to oxygen are mediated by hypoxia-inducible-factor-1 alpha (HIF-1α) activity14,25. HIF-1α is also known to be a relevant factor for adequate neuronal cell development26. In chondrocytes, some specific transcription factors, including Sox9, have been reported to be regulated via HIF-1α activity. Since in our experiments, using oligodendroglial cultures, there was an effect of oxygen levels on the expression of transcription factors Sox9 and Sox10, we aimed to determine the influence of HIF-1α activity on the development of oligodendroglial cells cultured at 5% and at 21% oxygen. For the analysis of HIF-1α activity in cells, reporter gene assay by transient transfection would provide important information. Unfortunately, transfection of plasmid DNA is described to have detrimental effects in primary OPCs, which was also confirmed in our preliminary experiments, with OPCs barely surviving the transfection procedures (data not shown). We therefore used cells of the oligodendroglia cell line OLN93 and subjected them to the two different oxygen levels for 48 hours to determine HIF-1α activity by luciferase expression. Luciferase activity in the OLN93 cultures exposed to 5% was more than 3 times higher than that of the cultures kept at 21% O2 (3.30 vs. 1.00, respectively; p < 0.0001) (Fig. 7). Based on these results, the culture condition providing 5% O2 increases the activity of Hif-1a by about threefold, and 21% O2 may be altering cellular gene expression as a result of decreased Hif-1α activity.
Gene expression in HIF-1α knockdown cells is mostly decreased compared to wildtype
To clarify whether oligodendroglial development is directly influenced by HIF activity we performed a HIF-1α knockdown in OLN93 cells by utilizing siRNA binding to the HIF-1α mRNA to prevent its expression. To confirm knockdown efficiency, we measured luciferase activity in HIF-1a knockdown cells compared to control siRNA transfected (scrambled) cells incubated at 5% O2, indicating 40 pmol as an appropriate siRNA concentration.
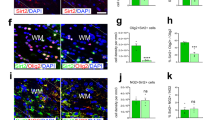
We compared real-time PCR analysis of Sox9, Sox10, MBP and CNP in scrambled transected cells cultured at 5% with that in HIF-1α knockdown cells cultured at 5% O2, to see whether the upregulation of these genes under the lower oxygen level would be mitigated by the induced HIF-1α deficiency. Surprisingly, Sox9 was significantly upregulated by HIF-1α knockdown (380.25% vs. 100%; p < 0.0001) and Sox 10 showed no significant difference in expression (92.66% vs. 100%; p = 0.337) (Fig. 8a). The expression of MBP and CNP genes, however, was significantly decreased after HIF-1α knockdown cells in comparison to scrambled transfected OLN93 cultured (Fig. 8b, MBP: 62.95% of controls, p = 0.044; CNP: 50.65% of controls; p < 0.0001) indicating a downregulation of maturation signals by HIF-1α at 5% O2.
Realtime PCR in HIF-1α knockdown cells of the OLN93 cell line showed a surprisingly strong increase of Sox9 expression (p < 0.0001) and no difference between wildtype and knockdown in Sox10 expression (p = 0.337) (a) CNP (p < 0.0001) and MBP (p = 0.044) were significantly downregulated in HIF-1α knockdown cells (b) control (c) (N = 8, unpaired ttest * < 0.05, ** < p0.01, ***p < 0.001).
Discussion
In this study, we investigated the mechanisms through which oligodendroglial development may be regulated by the level of environmental oxygen. In our in vitro experiments, low oxygen improved cell survival and morphological complexity compared with 21% oxygen. Moreover, high oxygen decreased cell proliferation and the expression of transcription factors important for the regulation of oligodendroglial development. Both, increased oxidative stress and reduced HIF-1α were identified as potential mediators of the inhibitory effects of higher environmental oxygen.
Oxygen has previously been demonstrated to represent a signal of cell differentiation in stem cells. Chen and co-workers reported that 5% oxygen enhanced clonal and long-term expansion of mouse fetal cortical precursors in vitro as compared to 21% oxygen3. Low oxygen was shown to promote the development of neural progenitor and stem cells by increasing proliferation and neuronal differentiation under low oxygen27,28,29,30,31 suggesting a possible role for increased HIF-1α expression28,29,30.
The increased production of ROS and oxidative stress induced by 21% O2 may impair OPC development and maturation. Oxidative stress has been shown to impair development and survival in neurons32 and oligodendroglia13 and has moreover been defined as a major cause of brain injury in neonates33,34. In our study, we detected elevated nitrotyrosine production in protein samples of oligodendroglial cultures kept at 21% oxygen, indicating oxidative stress. Furthermore, the increased expression of the transcription factor Nrf2 and of SOD2 indicated an anti-oxidant response to oxidative stress. Immature oligodendroglia in vitro have been shown to be very susceptible to apoptotic cell death induced by drastically increased oxygen at 80% O235. However, the oxidative challenge at 21% O2 in comparison to 5% O2 does not result in apoptotic activity, as shown by TUNEL analysis in primary OPC cultures. Instead, oxidative stress at 21% oxygen causes changes in central cellular processes, such as proliferation and oligodendroglial differentiation, but does not lead to apoptotic cell death, which is similar to the finding of French et al.13 and Pistollato et al.36.
A well-described cellular response to low oxygen concentrations is the upregulation of HIF-1α14,25,37 e.g. in neovascularization38 and chondrogenesis24,39, immune reactions40 and different cells of the central nervous system41. Recently, oligodendroglial maturation in the immature brain has been found to be strongly regulated by HIF1/2α stabilization and to be reversed by loss of HIF function42. It was moreover demonstrated that HIF signaling regulates oligodendroglial development with coupling of angiogenesis and the onset of myelination via paracrine stimulation by oligodendroglia42. We hypothesized that changes of oligodendroglial function in response to differences in oxygen concentrations may also be mediated via HIF-1α. Indeed, HIF-1α activity was decreased in our oligodendroglial cultures at 21% O2 in comparison to cultures at 5% O2. Moreover, in our cell culture experiments using cell line OLN93, at 5% O2 environment, HIF-silencing caused a downregulation in MBP and CNP expression to levels similar to 21% oxygen. This data indicates that maturation of oligodendroglia at 5% O2 is at least partially regulated by HIF, which represents a novel finding. The regulation of oligodendroglial maturation by HIF should in our view be further investigated.
It should be mentioned that we have also found contradictory results with regards to the expression of Sox9 because Sox9 expression was lower at 21% O2 in OPCs and in OLN93 cells but silencing of HIF-1α in OLN93 cells led to upregulation of Sox9. Apparently, in oligodendroglia, Sox9 is not directly regulated via HIF-1α which contrasts with the Hif-1 α dependent cell regulation described in cultured chondrocytes24,43. Distinct regulatory pathways of HIF-1α and HIF-2 have been reported to provide possible mechanisms of compensation44. Given that specific silencing of HIF-1α in our experiments does not fully mimic a broader suppression of HIF activities that is likely to be caused by increased oxygen it is possible that compensatory HIF-2 activity may induce Sox9 up-regulation in the absence of HIF-1.
In our view, the results of our study have technical implications for in vitro experiments. Physiological O2 levels at which OPCs and oligodendroglia are naturally developing in the cerebral tissue in vivo are much lower at about 16–38 mmHg (amounting to 2–5% O2)7,45, oligodendroglial cell cultures maintained at 21% O2, are therefore in fact being exposed to a relatively high O2 concentration conditions that interfere with oligodendroglial development. Indeed, we have found that conventional observations of neural cell processes and mechanisms made at 21% O2 contain oxygen-mediated growth inhibitory effects. It remains to be determined whether oxygen concentrations of lower than 10% should be used for in vitro experiments in OPCs and oligodendroglia to adapt the experimental conditions more closely to the natural situation and avoid oxygen-related impairment. Our results suggest the possibility that lower oxygen levels applied at the beginning of mixed glial culture procedure even before enrichment or purification of OPCs may benefit development.
Our results further indicate that oxidative stress and HIF-related dysregulation of oligodendroglial genes can be avoided by using lower oxygen such as 5% in oligodendroglial cell cultures. This is in agreement with results in neural stem cells, which show improved survival under low oxygen conditions in vitro46 and in human mesenchymal stem cells in which proliferation was increased at 2% compared to 20% oxygen47. Interestingly, Akundi et al. have made similar observations by using OPC cultures at 4% O2, however, they defined 4% O2 as hypoxia and interpreted their results as an injurious effect of too little oxygen48. The possibility has not been previously considered that room air oxygen levels used for in vitro experiments might represent supraphysiological oxygen levels for the cellular environment. The degree of vulnerability to the higher oxygen condition will vary between cell types, however, OPCs and immature oligodendroglia are well described to be highly susceptible to oxygen toxicities.
As the primary role of oligodendroglial cells in myelination is the wrapping of oligodendroglial membranes around neuronal axons49,50 the marked difference in morphological features such as the decreased cell size and substantially less arborization of O4+ cells kept at 21% may lead to myelination deficit. This is a subject for further investigation.
Our results may also provide insights into oxygen-dependent regulation of oligodendroglial development in preterm infants. The intrauterine environment in which the fetus resides is at very low arterial oxygen levels of 20–25 mmHg (or 70–75% oxygen saturation)2. Birth into room air exposes the infant to a several-fold increase of oxygen levels, however the preterm brain has poorly developed anti-oxidant defense capacity10,11,12,51,52,53. Cellular differentiation and development in the brain have been shown to be influenced by oxygen54. Immature glial cells and progenitor cells are also sensitive to increases of oxygen levels52 which can lead to perturbation of the developmental program and to cellular damage. As shown by our data, decreased activity of HIF by higher oxygen concentrations can inhibit transcriptional regulation of oligodendroglial development and maturation.
In conclusion, it is possible that the findings of hypomyelination and diffuse white matter injury in survivors of preterm birth might at least in part be caused by the premature increase in oxygen tension in these patients, leading to oligodendroglial dysregulation by increased oxidative stress and lower HIF activity. The impact of oxygen on the oligodendroglial developmental program should be further investigated in order to understand the causes of white matter injury.
Methods
Cell cultures
Primary mixed glial cultures were prepared from E19 pregnant Sprague-Dawley rats by mechanical dissociation according to the method of McCarthy and de Vellis55, as previously described56,57. Mixed cultures (7–10 days old) were shaken for 4 h to detach microglia followed by aspiration of the media to remove the microglia from mixed cultures. After addition of new media, flasks were shaken overnight to detach oligodendrocyte progenitor cells (OPCs) from the astrocyte monolayer. To further minimize contamination by microglial cells, the detached OPC suspension was incubated in succession for 45 min. in 60 mm dishes. OPCs enriched by this method contained >95% GD31 cells labeled by the LB1 monoclonal antibody58,59, with <0.05% GFAP+ astrocytes and <0.05% Ox42+ microglia. Mixed glial cultures were used for one shake to produce OPC cultures which were then used for the distinct experiments in a way that N reflects the number of different litters and the number of experiments.
All procedures were approved by the state animal welfare authorities Berlin, Germany, (LAGeSo T-0124/08) and followed institutional guidelines.
OLN93 cells
We received cells of the oligodendroglia linage cell line OLN9323 from Dr. C. Richter-Landsberg (Oldenburg, Germany). Cells were cultured as described before (Gerstner et al.,)35,60. Briefly, for immunocytochemistry, cells were transferred on poly-lysine-coated coverslips kept in 12-well plates at a density of 1.5 × 105 cells per well and incubated at 5% and 21% oxygen for 48 hours.
For Hif-1α gene reporter assay 0.5 × 105 cells per 24-well were transfected with the commercial available HIF-1α reporter assay (Qiagen) by lipofection (Metafectene Pro, Biontex) according to the manufactures guide lines and incubated at 5% and 21% O2. After 48 hours incubation, cells were harvested and preceded with the Dual-Luciferase Reporter Assay System (Promega). Luminescence was measured by the Lumat LB 9501 Luminometer (Berthold).
For gene expression analyses, 1.5 × 105 cells per 12-well were incubated at 5% and 21% oxygen. After 48 hours incubation cells were preceded for Real-time PCR analyses.
Gene silencing by Hif-1α siRNA (Santa Cruz Biotechnology) was performed in 0.5 × 105 cells per 24-well. siRNA transfection and co-transfection with the Hif-1α reporter assay was performed by lipofection (Metafectene Pro, Biontex) according to the manufactures guide lines and incubated at 5% and 21% O2 for 48 hours.
Western blot
Whole cells lysates were homogenized in 4 °C RIPA buffer solution for protein extraction. Protein concentration was measured by the Pierce BCA kit (Pierce, Rockford IL). Total proteins were equally loaded using 20 μg per lane on a 12% mini precast Tris-glycine gel. Proteins were transferred onto PVDF membranes at 4 °C overnight and blocked in 3% BSA in TBST. Primary antibodies were diluted 1:1,000 to 1:1,250 in 3% BSA/TBST. Horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit and anti-mouse, BD Biosciences Pharmingen, San Diego CA) were diluted 1:2,000 in 3% BSA/TBST. Chemiluminescent detection was performed using the ECL Plus (Amersham) kit according to manufacturers’ directions. Positive signals were visualized using enhanced chemiluminescence (Amersham Biosciences, Freiburg, Germany) and quantified using a ChemiDoc XRSþ system and the software Image Lab (Bio-Rad). The antibodies used were monoclonal mouse anti-β-actin 1:1,250 (Millipore/Chemicon, Temecula CA), polyclonal rabbit anti-nitrotyrosine 1:1,000 (Millipore/Upstate, Temecula CA).
Immunocytochemistry
Live staining for cell surface antigens with A2B5 and O4 antibodies61 was performed as described elsewhere62. Briefly, live cells were incubated at room temperature for 1 hour with primary antibodies diluted 1:10 in DMEM, followed by fluorescein-conjugated goat anti-mouse IgM for 45 min. After three washes in phosphate-buffered saline solution (PBS), cells were fixed in 4% paraformaldehyde (PFA) in PBS for 10 min. at room temperature and washed in PBS. Coverslips were then mounted in DAPI (4′,6-diamidino-2-phenylindole)-Containing Vectashield. For double staining with Ki67, cells after live staining, fixation and washing were blocked again in 10% normal goat serum (NGS) in DMEM containing 0.1% Triton X-100 for permeabilization during 20 min. at room temperature. Incubation with Ki67 rabbit antibody (1:500, Dako) followed for 1 hour at room temperature. After washing, the cells were incubated with rhodamine anti-rabbit IgG antibodies (1:200, JacksonImmuno, West Grove PA). The cells were then washed and mounted in Vectashield with DAPI. For TUNEL staining, a commercially available kit (RocheDiagnostics, Mannheim, Germany) was used according to the manufacturer’s instructions after live-staining, fixation and washing of the cells.
OLN93 cells were fixed with 4% PFA in PBS for 15 minutes. After washing with PBS, cells were permeabilized with 0.1% Triton X 100 and 0.1% Tween 20 in PBS for 10 minutes. After three washing steps, the cells were blocked with 0.5% BSA in PBS for 1 hour at room temperature, followed by the incubation with the rabbit anti Ki67 (Leica, NCL-Ki67p) 1:200 in 0.5% BSA in PBS for one hour. After several washing steps, the cells were incubated with a goat-anti-rabbit IgG Alexa Fluor 594 (Molecular Probes) 1:200 for 30 minutes. The cells were then washed and mounted in Vectashield with DAPI.
Images were taken at 20 fold magnification. Five images were taken per 24 mm cover slip covering north/south/east/west/center regions of the each cover slip. The average of the five images was used as n = 1.
Sholl analysis
After live staining for cell surface antigens with O4 was conducted, evaluation of cell size and morphology was mastered using Sholl Analysis, an ImageJ plugin used to describe neuronal arbors63. Images were first converted into binary 8-bit images and the center of each cell was marked using the ImageJ point selection tool. Sholl analysis was then conducted scaled to pixels, the minimum radius being 0.00 and the radius step being set to 0.032 pixels.
Real-time PCR
Total cellular RNA was isolated by acidic phenol/chloroform extraction and DNase I treatment (Qiagen, Hilden Germany). Prior to reverse transcription, DNA contamination was ruled out by running RNA samples on a 2% agarose gel subsequently developed with ethidium bromide. For each sample, 1 μg of RNA was reverse transcribed at 42 °C for 1 h with 200 U of Moloney murine leukemia virus reverse transcriptase and random-primer (Promega, Madison, WI) in 35 μl of reaction mixture including DNaseI treatment. For qPCR analysis, 3 μL of 1:10 diluted cDNA samples were used. The PCR products of Sox-9, Sox-10, Olig1, Olig2, 2′3′ cyclic nucleotide phosphodiesterase (CNP), myelin basic protein (MBP), superoxide dismutase 2 (SOD2) and NF-E2-like basic leucine zipper transcriptional activator(NRF2) were quantified in real-time, using dye-labeled fluorogenic reporter oligonucleotide probes (Sox-9 F 5′cggaggaagtcggtgaagaa 3′, R 5′tgcagcgccttgaagatg 3′, probe 5′acagactcacatctctccta3 3′, NM_001109181.1, Sox-10 F 5′ ccgcacctccacaatgct 3′, R 5′ ggtacttgtagtccggatggtcttt 3′, probe 5′ ttgctgaacgagagtgacaa 3′, NM_019193.1; Olig1 F 5′ ccctcgcgtcctggatct 3′, R 5′ ggaagaaggcgccctacag 3′, probe 5′ aaaggaggacatttccagac 3′, NM_021770;Olig2 F 5′ tgcgcaagctctccaagat 3′, R 5′ tctcgctcaccagtctcttcatc 3′, probe 5′ cgaaactacatcctgatgct 3′, NM_001100557.1;CNP F 5′ ggcgtgctgcactgtacaac 3′, R 5′ aagatctcctcaccacatcctgtt 3′, probe 5′ aattctgtgactacgggaag 3′, NM_012809.1; MBP F 5′ gagccctctgccttctcatg 3′, R 5′ agggagccgtagtgggtagttc 3′, probe 5′ acatgtacaaggactcacac 3′, NM_001025291.1, SOD2 F 5′gacctacgtgaacaatctgaacgt 3′, R 5′ aggctgaagagcaacctgagtt 3′, probe 5′accgaggagaagtaccacga 3′, NM_017051.2, NRF2 F5′actcccaggttgcccacat 3′, R 5′gcgactcatggtcatctacaaatg 3′, probe F 5′ctttgaagactgtatgcagc 3′, NM_017051.2).
The probes were labeled at the 5′ end with the reporter dye 6-carboxy-fluoresceine (FAM) and at their 3′ ends with the quencher dye, 6-carboxy-tetramethylrhodamine (TAMRA).Hypoxanthine-guanine phosphoribosyl-transferase (HPRT, F 5′ ggaaagaacgtcttgattgttgaa 3′, R 5′ccaacacttcgagaggtcctttt 3′, probe 5′ ctttccttggtcaagcagtacagcccc 3′, NM_013556.2) was used as internal standard. The FAM spectral data was collected from reactions carried out in separate tubes using the same stock of cDNA to avoid spectral overlap among FAM/TAMRA and limitations of reagents. Real-time PCR was performed in three replicates and repeated two times of each sample using a total reactive volume of 11 μl which contained 5 μl of 2x KAPA PROBE FAST qPCR Mastermix (PEQLAB Biotechnologie GMBH, Erlangen, Germany), 2.5 μl of 2 μM oligonucleotide mix (forward and reverse primer, BioTeZ Berlin-Buch, Germany), 0.5 μM probe, and 9 ng of cDNA template (diluted in RNase-free water to 3 μl). The PCR amplification was performed in 96-well optical reaction plates for 40 cycles with each cycle at 94 °C for 15 sec and 60 °C for 1 min. Each plate included at least three “No Template Controls (NTC)”. The reaction was carried out with the StepOnePlus™ Real-Time PCR System (Applied Biosystems) according to the 2−ddCT method, and fluorescent data were converted into cycle threshold (CT) values. Sox-9, Sox-10, Olig1, Olig2, CNP, MBP, SOD2, NRF2 levels were normalized to HPRT levels. Results were normalized in per cent with the 5% oxygen culture group used as control group.
Statistical analysis
All of the results were analyzed by student’s t-test, RT-PCR was analyzed using Wilcoxon signed rank test, P < 0.05 was defined as statistically significant. All values are given as mean ± standard error of the mean (SEM).
Additional Information
How to cite this article: Brill, C. et al. Oxygen impairs oligodendroglial development via oxidative stress and reduced expression of HIF-1α. Sci. Rep. 7, 43000; doi: 10.1038/srep43000 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12 May 2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
12 May 2017
Scientific Reports 7: Article number: 43000; published online: 23 February 2017; updated: 12 May 2017 This Article contains an error in Figure 8: the oxygen percentage for the black columns is incorrectly labeled as 21% instead of the correct value of 5%. The correct Figure 8 appears below as Figure1 along with the accompanying legend.
References
Corcoran, A. & O’Connor, J. J. Hypoxia-inducible factor signalling mechanisms in the central nervous system. Acta physiologica (Oxford, England) 208, 298–310, doi: 10.1111/apha.12117 (2013).
Hoffman, J. In Rudolph’s Pediatrics 21 (ed C. D. Rudolph et al.) 1750 (McGraw-Hill Professional, 2002).
Chen, H. L. et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem cells (Dayton, Ohio) 25, 2291–2301, doi: 10.1634/stemcells.2006-0609 (2007).
Chadwick, W. et al. Multiple oxygen tension environments reveal diverse patterns of transcriptional regulation in primary astrocytes. PloS one 6, e21638, doi: 10.1371/journal.pone.0021638 (2011).
Danilov, C. A. & Fiskum, G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia 56, 801–808, doi: 10.1002/glia.20655 (2008).
Dings, J., Meixensberger, J., Jager, A. & Roosen, K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 43, 1082–1095 (1998).
Erecinska, M. & Silver, I. A. Tissue oxygen tension and brain sensitivity to hypoxia. Respiration physiology 128, 263–276 (2001).
Miller, R. H. Regulation of oligodendrocyte development in the vertebrate CNS. Progress in neurobiology 67, 451–467 (2002).
Rivkin, M. J. et al. Oligodendroglial development in human fetal cerebrum. Annals of neurology 38, 92–101, doi: 10.1002/ana.410380116 (1995).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet. Neurology 8, 110–124, doi: 10.1016/s1474-4422(08)70294-1 (2009).
Baud, O. et al. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. The European journal of neuroscience 20, 29–40, doi: 10.1111/j.0953-816X.2004.03451.x (2004).
Back, S. A., Gan, X., Li, Y., Rosenberg, P. A. & Volpe, J. J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. The Journal of neuroscience: the official journal of the Society for Neuroscience 18, 6241–6253 (1998).
French, H. M., Reid, M., Mamontov, P., Simmons, R. A. & Grinspan, J. B. Oxidative stress disrupts oligodendrocyte maturation. Journal of neuroscience research 87, 3076–3087, doi: 10.1002/jnr.22139 (2009).
Jiang, B. H., Semenza, G. L., Bauer, C. & Marti, H. H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. The American journal of physiology 271, C1172–1180 (1996).
Hwang, J. M. et al. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Molecular and cellular biochemistry 311, 179–187, doi: 10.1007/s11010-008-9708-6 (2008).
Bouslama-Oueghlani, L. et al. Purkinje cell maturation participates in the control of oligodendrocyte differentiation: role of sonic hedgehog and vitronectin. PloS one 7, e49015, doi: 10.1371/journal.pone.0049015 (2012).
Bambakidis, N. C. et al. Intravenous hedgehog agonist induces proliferation of neural and oligodendrocyte precursors in rodent spinal cord injury. Neurosurgery 67, 1709–1715; discussion 1715, doi: 10.1227/NEU.0b013e3181f9b0a5 (2010).
Ferent, J., Zimmer, C., Durbec, P., Ruat, M. & Traiffort, E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. The Journal of neuroscience: the official journal of the Society for Neuroscience 33, 1759–1772, doi: 10.1523/jneurosci.3334-12.2013 (2013).
Wellmann, S. et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. Journal of cell science 117, 1785–1794, doi: 10.1242/jcs.01026 (2004).
Sheldon, R. A., Lee, C. L., Jiang, X., Knox, R. N. & Ferriero, D. M. Hypoxic preconditioning protection is eliminated in HIF-1alpha knockout mice subjected to neonatal hypoxia-ischemia. Pediatric research 76, 46–53, doi: 10.1038/pr.2014.53 (2014).
Noll, E. & Miller, R. H. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development (Cambridge, England) 118, 563–573 (1993).
Baron, W., Metz, B., Bansal, R., Hoekstra, D. & de Vries, H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Molecular and cellular neurosciences 15, 314–329, doi: 10.1006/mcne.1999.0827 (2000).
Richter-Landsberg, C. & Heinrich, M. OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. Journal of neuroscience research 45, 161–173, doi: 10.1002/(SICI)1097-4547(19960715)45:2<161::AID-JNR8>3.0.CO;2-8 (1996).
Amarilio, R. et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development (Cambridge, England) 134, 3917–3928, doi: 10.1242/dev.008441 (2007).
Roitbak, T., Surviladze, Z. & Cunningham, L. A. Continuous expression of HIF-1alpha in neural stem/progenitor cells. Cellular and molecular neurobiology 31, 119–133, doi: 10.1007/s10571-010-9561-5 (2011).
Tomita, S. et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Molecular and cellular biology 23, 6739–6749 (2003).
Yuan, L. L., Guan, Y. J., Ma, D. D. & Du, H. M. Optimal concentration and time window for proliferation and differentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours. Neural regeneration research 10, 1516–1522, doi: 10.4103/1673-5374.165526 (2015).
Rodrigues, C. A., Diogo, M. M., da Silva, C. L. & Cabral, J. M. Hypoxia enhances proliferation of mouse embryonic stem cell-derived neural stem cells. Biotechnology and bioengineering 106, 260–270, doi: 10.1002/bit.22648 (2010).
Zhang, K. et al. Reduced Cerebral Oxygen Content in the DG and SVZ In Situ Promotes Neurogenesis in the Adult Rat Brain In Vivo . PloS one 10, e0140035, doi: 10.1371/journal.pone.0140035 (2015).
Zhang, C. P. et al. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1Alpha. Neuro-Signals 15, 259–265, doi: 10.1159/000103385 (2006).
Stacpoole, S. R. et al. Neural precursor cells cultured at physiologically relevant oxygen tensions have a survival advantage following transplantation. Stem cells translational medicine 2, 464–472, doi: 10.5966/sctm.2012-0144 (2013).
Saito, A. et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Molecular neurobiology 31, 105–116, doi: 10.1385/mn:31:1-3:105 (2005).
Ikonomidou, C. & Kaindl, A. M. Neuronal death and oxidative stress in the developing brain. Antioxidants & redox signaling 14, 1535–1550, doi: 10.1089/ars.2010.3581 (2011).
Bendix, I. et al. Hyperoxia changes the balance of the thioredoxin/peroxiredoxin system in the neonatal rat brain. Brain research 1484, 68–75, doi: 10.1016/j.brainres.2012.09.024 (2012).
Gerstner, B. et al. Maturation-dependent oligodendrocyte apoptosis caused by hyperoxia. Journal of neuroscience research 84, 306–315, doi: 10.1002/jnr.20880 (2006).
Pistollato, F., Chen, H. L., Schwartz, P. H., Basso, G. & Panchision, D. M. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Molecular and cellular neurosciences 35, 424–435, doi: 10.1016/j.mcn.2007.04.003 (2007).
Lukyanova, L. D. & Kirova, Y. I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Frontiers in neuroscience 9, 320, doi: 10.3389/fnins.2015.00320 (2015).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology 16, 4604–4613 (1996).
Kanichai, M., Ferguson, D., Prendergast, P. J. & Campbell, V. A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. Journal of cellular physiology 216, 708–715, doi: 10.1002/jcp.21446 (2008).
Hellwig-Burgel, T., Stiehl, D. P., Wagner, A. E., Metzen, E. & Jelkmann, W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 25, 297–310, doi: 10.1089/jir.2005.25.297 (2005).
Chavez, J. C., Agani, F., Pichiule, P. & LaManna, J. C. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. Journal of applied physiology (Bethesda, Md.: 1985) 89, 1937–1942 (2000).
Yuen, T. J. et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158, 383–396, doi: 10.1016/j.cell.2014.04.052 (2014).
Robins, J. C. et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 37, 313–322, doi: 10.1016/j.bone.2005.04.040 (2005).
Barteczek, P. et al. Neuronal HIF-1alpha and HIF-2alpha deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism, doi: 10.1177/0271678x15624933 (2016).
Liu, K. J. et al. Assessment of cerebral pO2 by EPR oximetry in rodents: effects of anesthesia, ischemia, and breathing gas. Brain research 685, 91–98 (1995).
Clarke, L. & van der Kooy, D. Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem cells (Dayton, Ohio) 27, 1879–1886, doi: 10.1002/stem.96 (2009).
Dos Santos, F. et al. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. Journal of cellular physiology 223, 27–35, doi: 10.1002/jcp.21987 (2010).
Akundi, R. S. & Rivkees, S. A. Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PloS one 4, e4739, doi: 10.1371/journal.pone.0004739 (2009).
Simons, M. & Lyons, D. A. Axonal selection and myelin sheath generation in the central nervous system. Current opinion in cell biology 25, 512–519, doi: 10.1016/j.ceb.2013.04.007 (2013).
Zuchero, J. B. et al. CNS myelin wrapping is driven by actin disassembly. Developmental cell 34, 152–167, doi: 10.1016/j.devcel.2015.06.011 (2015).
Back, S. A. et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of neuroscience: the official journal of the Society for Neuroscience 21, 1302–1312 (2001).
Husain, J. & Juurlink, B. H. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain research 698, 86–94 (1995).
Schneider, H. Oxygenation of the placental-fetal unit in humans. Respiratory physiology & neurobiology 178, 51–58, doi: 10.1016/j.resp.2011.05.009 (2011).
Studer, L. et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. The Journal of neuroscience: the official journal of the Society for Neuroscience 20, 7377–7383 (2000).
McCarthy, K. D. & de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85, 890–902 (1980).
Gallo V. & Armstrong, R. C. Developmental and growth factor induced regulation of nestin in oligodendrocyte lineage cells. J Neurosci 15, 394–406 (1995).
Schmitz, T., Endesfelder, S., Chew, L. J., Zaak, I. & Buhrer, C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. Journal of neuroscience research 90, 933–944, doi: 10.1002/jnr.22824 (2012).
Levi, G., Gallo, V. & Ciotti, M. T. Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and “neuron-like” GABA transport. Proc Natl Acad Sci USA 83, 1504–1508 (1986).
Curtis, R. et al. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J Neurocytol 17, 43–54 (1988).
Gerstner, B. et al. Glutaric acid and its metabolites cause apoptosis in immature oligodendrocytes: a novel mechanism of white matter degeneration in glutaryl-CoA dehydrogenase deficiency. Pediatric research 57, 771–776, doi: 10.1203/01.pdr.0000157727.21503.8d (2005).
Bansal, R., Warrington, A. E., Gard, A. L., Ranscht, B. & Pfeiffer, S. E. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb use in the analysis of oligodendrocyte development. J Neurosci Res 24, 548–557 (1989).
Yuan X., Eisen A. M., McBain C. J. & Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914 (1998).
Ferreira, T. A. et al. Neuronal morphometry directly from bitmap images. Nature methods 11, 982–984, doi: 10.1038/nmeth.3125 (2014).
Acknowledgements
Financial support was received by the “Deutsche Forschungsgemeinschaft (DFG)” (grant no. SCHM3007/2–3), “Buchwald Stiftung”, and “Verein für Frühgeborene an der Charité e.V.”. We thank Mrs. Ruth Herrmann for her help with cell cultures and immunocytochemistry.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the paper. C. Brill prepared the manuscript, performed experiments and data analysis, and generated figures. T. Schmitz was involved in manuscript writing, supervision and analysis of experiments. T. Scheuer conducted Luciferase assay and Hif-1a knockout experiments and contributed the corresponding Method sections. C. Bührer advised and contributed parts of the main text. S. Endesfelder prepared cell cultures, helped with qPCRs and included the PCR section of Methods. All authors constantly discussed and reviewed experiments and text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Brill, C., Scheuer, T., Bührer, C. et al. Oxygen impairs oligodendroglial development via oxidative stress and reduced expression of HIF-1α. Sci Rep 7, 43000 (2017). https://doi.org/10.1038/srep43000
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43000
This article is cited by
-
Comparative Study of HIF-1α- and HIF-2α-Immunopositive Neurons and Capillaries in Rat Cortex under Conditions of Tissue Hypoxia
Bulletin of Experimental Biology and Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.