Abstract
Pt/K2CO3/MgAlOx–reduced graphene oxide (Pt/K/MgAlOx–rGO) hybrids were synthesized, characterized and tested as a promising NOx storage and reduction (NSR) catalyst. Mg–Al layered double hydroxides (LDHs) were grown on rGO via in situ hydrothermal crystallization. The structure and morphology of samples were thoroughly characterized using various techniques. Isothermal NOx adsorption tests indicated that MgAlOx–rGO hybrid exhibited better NOx trapping performance than MgAlOx, from 0.44 to 0.61 mmol · g−1, which can be attributed to the enhanced particle dispersion and stabilization. In addition, a series of MgAlOx–rGO loaded with 2 wt% Pt and different loadings (5, 10, 15, and 20 wt%) of K2CO3 (denoted as Pt/K/MgAlOx–rGO) were obtained by sequential impregnation. The influence of 5% H2O on the NOx storage capacity of MgAlOx–rGO loaded with 2 wt% Pt and 10% K2CO3 (2Pt/10 K/MgAlOx–rGO) catalyst was also evaluated. In all, the 2Pt/10 K/MgAlOx–rGO catalyst not only exhibited high thermal stability and NOx storage capacity of 1.12 mmol · g−1, but also possessed excellent H2O resistance and lean–rich cycling performance, with an overall 78.4% of NOx removal. This work provided a new scheme for the preparation of highly dispersed MgAlOx–rGO hybrid based NSR catalysts.
Similar content being viewed by others
Introduction
Nitrous oxides (NOx) are known as hazardous compounds and one of the main causes for the formation of highly toxic photochemistry smog and acid rain1,2,3. For the NOx emission control from automobile exhausts, three–way catalysts are highly efficient when the engines work at stoichiometric air/fuel ratio (A/F = 14.7). However, their efficiency diminishes severely in the presence of an excess of oxygen4,5,6,7,8. Thus, up to now, the NOx abatement for lean–burn engine emissions is still widely recognized as one of the most challenging problems9,10,11.
The NOx storage and reduction (NSR) catalytic treatment appears to be a promising approach to remove NOx under the excess oxygen condition12,13. The NSR catalysts generally consist of three major components: precious metal (e.g. Pt, Pd, or Rh), alkali or alkaline earth metal oxide as NOx storage material (e.g. Ba, Sr, Ca, Li, K, or Na), and a high surface area support like γ–Al2O314,15,16,17. During lean conditions, NOx was stored as nitrates (or nitrite), while during the short rich conditions, the stored NOx will be released and then selectively reduced to N218,19,20. Up to date, several groups of NSR catalysts have been developed21. However, each type of catalysts have their own shortcomings, such as the narrow range of operating temperatures, thermal degradation and deactivation by sulfur adsorption, etc refs 22, 23. For these reasons, extensive efforts are still being made to develop novel NSR catalysts.
Recently, well mixed transition–metal oxides derived from layered double hydroxides (LDHs) have been suggested to offer potential advantages over Pt/BaO/Al2O3 in NOx storage–reduction and assessed to be the new generation of NSR catalysts24,25. LDH is a class of anionic clays25. The lamellar structure of LDH is based on positively charged brucite–like sheets with anions and water molecules intercalated between the layers26,27. For a long time, Mg–Al LDHs, which can be precursors to Mg–Al mixed oxides (MgAlOx) have received significant attention for NOx adsorption24,28,29,30. Takahashi et al.28 reported that the NOx storage material could be improved at high28,29,30 temperature by using hydrotalcite–derived MgAl2O4 spinel as support. Especially, K–supported MgAlOx (K/MgAlOx) showed improved NOx adsorption. Park et al24. reported the Pt and K promoted hydrotalcite–based catalyst displayed better adsorption capacity, the NOx storage capacity of which could be significantly increased to nearly 0.65 mmol · g−1, by using 20% CH3COOK as the precursors. Li et al.31 also found that the addition of K enhances the NOx storage capacity of hydrotalcite–derived MnMgAlO catalysts to a great extent compared with the corresponding K–free catalysts, from 0.27 to 0.92 mmol · g−1, by using 20% KNO3 as the precursor.
Recently, the growth of LDHs on various substrates has received considerable attention32. Particularly, graphene structures which possess huge surface area and can be made free–standing could provide a large number of actives sites for adsorption purpose33. Luckily, due to the negatively charged nature of graphene and positively charged nature of LDHs, the combination between these two 2D building blocks can be easily achieved by electrostatic interaction34. Various strategies have been used to fabricate LDH–GO hybrid materials, including directly mixing LDHs and GO35, layer–by–layer self–assembly36, and directly LDH formation on GO32. These LDH–GO hybrid materials have been used in supercapacitors32,35,36, biosensors37,38, water treatment39, and CO2 capture, etc refs 40, 41.
In this contribution, a novel NSR catalyst Pt/K/MgAlOx–rGO with good NOx storage capacity and excellent lean–rich cycling stability was developed by in situ grown of Mg–Al LDHs on rGO and the subsequent sequential impregnation of Pt and K2CO3. The influence of the addition of rGO on the NOx storage capacity of MgAlOx based NSR catalysts was investigated. All samples were thoroughly characterized by XRD, XPS, SEM, TEM, FT–IR, Raman and BET analyses. The influences of Pt and K doping on the NOx storage capacity of MgAlOx–GO hybrids were also evaluated. Finally, the NOx storage and reduction cycling performance and thermal stability of 2Pt/10 K/MgAlOx–GO hybrid catalyst were also tested.
Results and Discussion
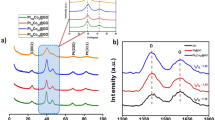
Figure 1(a) shows the Raman spectra of GO and the GO treated by hydrothermal method at 120 °C under pH = 10 for 12 h (designated as hGO). It is well known that graphene exhibits two main characteristic peaks: the D band at ~1350 cm−1, arising from a breathing mode of k–point photons of A1g symmetry and the G band at ~1575 cm−1, arising from the first order scattering of E2g phonon of sp2 C atoms42. In our present study, it can be seen that both GO and hGO exhibited a D band at 1330 cm−1 and a G band at 1593 cm−1. It is also found that the hGO showed a relatively higher intensity of D to G band (1.01) than that of GO (0.93). These observations confirmed the formation of new graphitic domains after the hydrothermal treatment process43. The XRD patterns of dried GO and hGO were also shown in Fig. 1(b). As it is expected that the dried GO displayed a layered structure with an interlayer spacing of 0.86 nm corresponding to the feature diffraction peak at 10.2°, revealing the introduction of oxygen functional groups on the graphite sheets44,45. However, after treated with hydrothermal method under pH = 10, the hGO exhibited the major peak at about 23–24°. This gives an interlayer spacing of approximately 0.37–0.38 nm. This interlayer spacing is much smaller than that for GO (0.86 nm), and is closer to the (002) graphite peak of 0.336 nm46. Taken together, both Raman and XRD data suggested the evidence to support the formation of rGO under hydrothermal condition at pH = 10. Therefore, the obtained hybrid material was denoted as LDH/rGO.
The XRD patterns of neat LDH, and the as synthesized LDH/rGO are shown in Fig. 2(a). Neat LDH exhibited a hexagonal structure with sharp symmetric peaks for the (003), (006) and (009) planes and broad asymmetric peaks for (015) and (018) planes which are characteristic of hydrotalcites. The diffraction lines at about 60.5° and 61.5° are ascribed to the (110) and (113) reflections47. In the case of LDH/rGO, the diffraction peaks are similar to those of pristine LDH, but no shift in basal reflection peaks was observed. This suggests the similarity in the intercalation of  anions into the layer galleries between the pristine LDH and the distributed LDH grown on rGO37. Figure 2(b) shows the XRD patterns of K2CO3 doped MgAlOx–rGO (K/MgAlOx–rGO) samples with different loadings of K2CO3. These diffractograms showed two intense lines (43°, and 62.5°), which are typical to MgO–like phase or likely magnesia–alumina solid solution (JCPDS 45–946). No diffraction peaks corresponding to K–related species were observed. Many similar results have been reported that the K–containing species could hardly be recognized by XRD analysis48,49,50,51. So, it was deduced that the K2CO3 species were well dispersed on the support and probably existed in amorphous phase that may be below the XRD detection limit48.
anions into the layer galleries between the pristine LDH and the distributed LDH grown on rGO37. Figure 2(b) shows the XRD patterns of K2CO3 doped MgAlOx–rGO (K/MgAlOx–rGO) samples with different loadings of K2CO3. These diffractograms showed two intense lines (43°, and 62.5°), which are typical to MgO–like phase or likely magnesia–alumina solid solution (JCPDS 45–946). No diffraction peaks corresponding to K–related species were observed. Many similar results have been reported that the K–containing species could hardly be recognized by XRD analysis48,49,50,51. So, it was deduced that the K2CO3 species were well dispersed on the support and probably existed in amorphous phase that may be below the XRD detection limit48.
ATR‒FTIR spectra of dried GO, neat LDH, and LDH/rGO hybrids are shown in Fig. 3(a). The absorption peaks at 3400 cm−1 for the GO correspond to the stretching mode of O–H. The characteristic features for GO are the stretching vibrations of C=O (carboxylic acid) at 1677 cm−1, C–O–C (epoxy) and C–O (alkoxy) at 925–1445 cm−1, and C=C at 1575 cm−1, originating from the skeletal vibrations of un–oxidized graphitic domains52,53,54,55,56. In all the spectra of neat LDH samples, a strong broad vibration band in the 3200 to 3600 cm−1 range can be observed, which can be attributed to the O–H stretching vibration of water molecules and hydroxyl groups that belong to brucite layers. The band at 1624 cm−1 was due to the H–O–H bending vibration. A strong band at 1360 cm–1 was attributed to the carbonate group. The bands below 800 cm−1 were ascribed to Al–O and Mg–O vibrations57. When combined, the peaks in the spectra of LDH/rGO are consistent with those of the LDH and GO, indicating the successful hybridization of rGO with LDH. As no information about K species was given by XRD results, thus ATR FT–IR technique was employed to investigate the states of K species, as shown in Fig. 3(b). The bulk K2CO3 used as the precursor of potassium salt shows the characteristic IR bands in the region of 1800–1000 cm−1. When K was introduced, the K2CO3 species was clearly detected, with the characteristic peak located at 1385 cm−158. The intensity of this characteristic peak became stronger with the increase in K2CO3 loading.
The BET specific surface areas of MgAlOx, MgAlOx–rGO, 10 K/MgAlOx, and 10 K/MgAlOx–rGO were also investigated. The specific surface area of MgAlOx–rGO hybrid was 230.6 m2 · g−1, which was larger than that of neat MgAlOx (207.5 m2 · g−1). It has been proven that the addition of rGO can decrease the aggregation of MgAlOx, resulting in more exposed MgAlOx nanosheets and enlarged specific surface area59. After loading K2CO3, the specific surface area decreased a little for both 10 K/MgAlOx (199.1 m2 · g−1) and 10 K/MgAlOx–rGO (129.2 m2 · g−1), probably due to the pore blocking by K2CO3.
The morphologies and structures of the GO and LDH were investigated by XRD and HR–TEM, as shown in Fig. 4. The SEM image of pure LDH (Fig. 4(a)) shows that the sample consist of a large number of LDH nanoplates, which have aggregated in a disordered fashion with a definite shape. The image in Fig. 4(b) shows that the pure LDH consists of abundant particles with a definite shape, which was of high quality in terms of crystallinity, and the mean size is determined to be ca. 100–200 nm. However, because of the high surface energy and strong van der Waals interactions, aggregated LDHs were observed. Figure 4(c) reveals that the GO nanosheets are curled and wrinkled just like a transparent corrugated voile due to the intrinsic nature of the GO nanosheets60. Closer observation of the HR–TEM image (Fig. 4(d)) shows the fringes at the edge of a flake, indicating the stacking of 2–3 monolayers. The inset SAED pattern of the GO shows the typical hexagonal crystal lattice which has a six–fold symmetry.
The LDH platelets are adsorbed on the surface of rGO nanosheets due to the electrostatic interaction between rGO and LDH61. Both LDH and rGO nanosheets were clearly observed was showed in Fig. 5, as indicated by the white arrows and black arrows, respectively. Form Fig. 5(b), it is clear that the LDH/rGO nanoplates are distributed on the surface of rGO nanosheets. Some LDH grew with ab–planes of the crystallites parallel to rGO, whereas some LDH grew with ab–planes perpendicular to rGO surface. Figure 5(c) reveals a marked contrast between the LDH nanoparticles and the underlying rGO. The thickness and the average particle size of the LDH nanoparticles were found to be ca. 10 and 50 nm, respectively. At higher magnification (Fig. 5(d)), it can be seen more clearly. The distribution of the LDH is not uniform because the rGO sheets capture the LDH mainly via reactive groups, and the active sites of rGO are not homogeneously distributed on the nanosheets32.
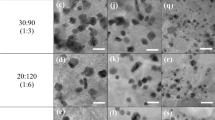
As noble metals, such as Pt, plays a key role in the NOx storage and reduction cycles, it is of great interest to investigate its existence in detail. No relevant changes are introduced in the diffraction patterns with Pt incorporation (Fig. 6(a)), which might due to the high dispersion of Pt in the catalyst62. To further elucidate the existence of Pt, SEM–EDS was performed on the catalyst, and the images are displayed in Fig. 6(b). The SEM image is fully consistent with the XRD results. No Pt–containing crystals could be observed, suggesting Pt species that are so small and highly dispersed. However, the EDS analysis confirmed the existence of Pt with an average Pt loading of 2.87 ± 0.38 wt%. This value is somehow higher than that of the theoretical amount used for the preparation, which can be attributed to the detection limitation of EDS. Figure 6(c) and (d) show the HR–TEM images of the catalyst. In all cases the Pt nanoparticles are dispersed on the support surface (Fig. 6(c)), with an average of particle size of 2.7–3.5 nm being determined from the HR–TEM images (Fig. 5(d)). The d–spacing value of 0.227 nm coincides with that of fcc Pt (111)63.
The NOx storage amount on MgAlOx was first measured by the isothermal storage of NO+O2 at 250, 300, 350, and 400 °C, respectively, as shown in Fig. 7(a). The NOx concentration in the outlets first showed a sharp decrease during the first several min and then followed by a gradual increase as a function of time. The lowest values were reached after about 10–15 min for all temperature range (250–400 °C). The difference between inlet and outlet NOx concentration corresponded to the NOx stored on the catalyst. It took around 100 min for MgAlOx to become saturated. Particularly, the sample can quickly capture the NOx within the first ca. 10 min, reaching the lowest point (~250 ppm) at 350 °C. Upon calculation, a maximum value of adsorbed NOx species close to 0.44 mmol · g−1 catalyst was achieved at 350 °C. Since the reaction time was only 100 min and the reaction balance has not been reached yet. Both NO2 storage and NO conversion to NO2 occurred at the same time, therefore the NO conversion to NO2 could not be calculated accurately. But for MgAlOx, the NO to NO2 conversion could be approximately estimated by the outlet NO2/NOx value because the reaction was close to equilibrium. As shown in Fig. 7(b), it is clear that the conversion increased with the increase in testing temperature from 250 to 400 °C. However, pure MgAlOx without noble metal such as Pt showed low NO oxidation activity.
In our previous work, the addition of 7 wt% GO showed better NOx storage capacity due to decreasing the aggregation of LDO and resulting in more exposed LDO nanosheets62. Therefore, in this study rGO was also investigated as a supporting material to optimized the NOx storage capacity of neat LDH. Figure 7(c) exhibits the isothermal NOx storage performance of MgAlOx and MgAlOx–rGO at 350 °C. For MgAlOx–rGO hybrid, it exhibited quicker NOx uptake and higher NOx capture capacity than those of MgAlOx. The NOx adsorption rate is very high and the reachable lowest NOx concentration was about 200 ppm. A maximum NOx storage value around 0.61 mmol·g−1 was obtained, even much higher than that of MgAlOx (0.44 mmol · g−1). Therefore, these data suggest that the absolute NOx storage capacity of MgAlOx can be markedly improved by adding 7 wt% GO, which was used in the hydrothermal process for its functional surfaces, worked as a template for the nucleation of Mg–Al LDHs and provided anchoring sites to the LDH nanosheets64. For LDH, the addition of K could remarkably improve their performance for NOx storage28,65.
The presence of K increased the mobility of surface oxygen species on the catalysts66 and enhanced the formation of low melting–point compounds67. Therefore, the influence of K doping has been investigated over MgAlOx–rGO at 350 °C, as shown in Fig. 7(d). In this contribution, K2CO3 was regarded as the precursor material. For the samples with increasing the loading of K2CO3, the overall amount of NOx stored was enhanced significantly and the reachable lowest NOx concentration was about 100 ppm when the K loading was increased to 10 wt%. Meanwhile, the NOx storage became a rather slow process and the highest NOx storage amount was improved to 0.81 mmol · g−1. However, the additional K did not provide further enhancement in the amount of stored NOx. At K loading of 15 and 20 wt%, the overall NOx storage activity decreased slightly. The presence of more bulk or bulk–like K2CO3 in these samples makes NOx storage more difficult due to the gradually increased surface to bulk diffusion resistance. Finally, the NOx storage amount of neat MgAlOx can be significantly promoted from 0.44 to 0.81 mmol · g−1 with the addition of 7 wt% GO and 10 wt% K2CO3.
Precious metals represent an obvious choice for both the NO oxidation to NO2 during lean condition and the adsorbed NOx reduction during rich condition for the NSR catalysts, due to their unparalleled red–ox activity, and Pt has been the primary oxidation catalyst choice for NSR samples to date. Thus, the influence of Pt doping on 10 K/MgAlOx and 10 K/MgAlOx–rGO hybrids was also studied, as shown in Fig. 8(a). In comparison to Pt unloaded samples, all Pt loaded samples show much higher NOx storage activity at 350 °C, which was increased from 0.44 and 0.61 mmol · g−1 to 0.85 and 1.12 mmol · g−1, respectively. This improvement in NOx storage capacity can be ascribed to the promoted NO oxidation to NO2 by Pt, and highly dispersed Pt in the catalyst. Moreover, the containing Mg would help Pt to be well dispersed on the supports, leading to the increase in NOx storage performance8. Figure S1 further confirmed the excellent NOx storage capacity and NO oxidation in obtained 2Pt/10 K/MgAlOx–rGO catalyst during the whole reaction through plotting the NO, NO2, NOx, and the ratio of NO/NO2 in the outlet.
H2O is one of the main components in the vehicle exhausts and often affects the storage and regeneration performance of NSR catalysts. Previous investigations have shown that the presence of H2O affect the NOx storage capacity68,69. Therefore, the H2O poisoning effect on Pt/K/MgAlOx–rGO catalyst was then studied. The influence of 5% H2O on NOx storage over Pt/K/MgAlOx–rGO catalyst at 350 °C is shown in Fig. 8(b). There is an almost complete capture of NOx for both conditions at the beginning. However, the saturation concentration is lower and the stability is higher in the absence of H2O. From the inset of Fig. 8(b), it is evident that only slightly less NOx is stored when introduced 5% H2O, from 1.12 to 1.02 mmol · g−1, which is consistent with previous studies68,69. The common agreement in the literature is that the water vapor reduces the NOx storage capacity and leads to catalyst deactivation by decreasing the number of available active sites. The results demonstrated that although Pt/K/MgAlOx–rGO was also slightly effected by 5% H2O, its performance is still much better than that of Pt/K/MgAlOx.
Figure 9(a) depicts the NOx concentration (ppm) profiles of the inlet and outlet gases over 2Pt/10 K/MgAlOx–rGO catalyst during the NOx storage and reduction cyclic reaction at 350 °C. At beginning of the lean phase (6 min), a sharp NOx removal peak appeared immediately after the 2Pt/10 K/MgAlOx–rGO contact was in contact with the gas mixture of NO+O2, which indicated a nearly complete adsorption and/or conversion of NOx on the catalyst. The NOx profiles obtained during the lean cycle showed almost the same shape, and the NOx level reached a more or less constant value afterwards. Meanwhile, the NOx concentration decreased slowly with time and reached 150 ppm at the end of the lean phase. This is far below the inlet NOx concentration of 700 ppm, showing a large amount of NOx was stored. When it was switched to the rich phase (1 min), a quick increase in NOx concentration was observed in the first few seconds. This may be attributed to two reasons. One is that the rate of adsorption of NOx decreased when oxygen was cut off. The other is that in the first few seconds of rich phase, a certain amount of oxygen remained on the Pt70. Because of the high reductive activity of Pt, the main product was N2 (~230 ppm) during rich cycles (Fig. 9(b)). Due to the limit of experimental condition, the N2O could not be calibrated accurately. But the intensity of N2O was very low compared with N2 in the present results (as shown in Fig. S2) and the similar discussion has been reported by other literature6,7. Overall, more than 78.40% of NOx was removed during the whole lean–rich cycling tests.
The nature of the active species present on the surface is important for establishing the properties of the catalyst. For this purpose, the XPS analysis has been performed in order to obtain information about the surface composition of the obtained catalysts. Figure 10 shows the typical XPS spectra of 2Pt/10 K/MgAlOx–rGO. In Fig. 10(a), the spectrum was deconvoluted into three components labelled as 1, 2 and 3 with respective binding energies of 71.55, 74.0, and 78.16 eV. The relative intensities (%) of the three components were 9.9, 84.7 and 5.4%, respectively. The Pt-4f7/2 signal at 71.55 eV can be assigned to zero-valent platinum. The relative shift from the value of 70.8 eV for the bulk metal is probably due to a contribution from metal-support interaction or small cluster-size effects71. The signal at 74.0 and 78.1 eV could be ascribed to Pt2+ and Pt4+ oxidation states72. Oxygen chemisorption easily occurs at step and kink sites present on the surface of Pt clusters73. For the calcined catalyst, the Al 2p signals labelled as 1 and 2 were adjusted by using two components, as shown in Fig. 10(b). The first one, occurring around at 74.23 eV, is assigned to octahedrally coordinated Al3+, whereas the second one, occurring around 73.41 eV must be assigned to tetrahedrally coordinated Al3+74. The intensity of the octahedrally coordinated Al3+ (81.68%) is higher than that of tetrahedrally coordinated Al3+ (16.10%). That may be assigned to the “memory effect” of LDH after exposure in the air for a long time75. Figure 10(c) displayed the binding energy of Mg 1 s in the obtained catalyst. The peak located around 1304.28 eV must be assigned to Mg2+ in periclase MgO structure76.
The thermal stability of catalyst is another important issue. To investigate this, GO, MgAlOx, MgAlOx–GO and 2Pt/10 K/MgAlOx–GO were tested in the TGA in the temperature range of 50–700 °C and in the presence of air, as shown in Fig. 11. The data indicates that GO is only thermally stable up to 400 °C. When the temperature was higher than 400 °C, GO started to decompose quickly, with a total weight loss of ca. 84.8% at 650 °C. This data suggested that neat GO is not thermally stable. The weight decrease of MgAlOx was only 16.92% in the temperature range of 50–700 °C, which was due to the loss of loosely held water in the interlayer pace77. While once GO was hybridized with MgAlOx, its thermal stability was significantly improved. Moreover, the 2Pt/10 K/MgAlOx–rGO catalyst also showed excellent thermal stability, the decrease of which was approximately 14% in the temperature range of 50–700 °C. There was only a slight decrease in weight with increasing the calcination temperature from 50 to 700 °C. So the thermal stability of the catalyst is not considered as a problem when the operating temperature of this novel catalyst is only ca. 350 °C. Taking its thermal and cycling stability into consideration, 2Pt/10 K/MgAlOx–rGO displayed great potential to work as a NSR catalyst with improved NOx storage ability.
Conclusion
In this contribution, a novel NSR catalyst Pt/K/MgAlOx–rGO with good NOx storage capacity and excellent lean–rich cycling stability was developed by in situ grown of Mg–Al LDHs on rGO and the subsequent sequential impregnation of Pt and K2CO3. Mg–Al LDHs were grown on rGO via in situ hydrothermal crystallization. The Pt nanoparticles were well dispersed with an average particle size of ca. 2.7–3.5 nm. Isothermal NOx storage tests demonstrated that the optimal operating temperature for MgAlOx was 350 °C. By introducing only 7 wt% of GO, the NOx storage capacity of neat MgAlOx was significantly improved from 0.44 to 0.61 mmol · g−1, which can be attributed to the enhanced particle dispersion and stabilization. By impregnating 2 wt% Pt and 10 wt% K2CO3, the NOx storage capacity was further increased to 1.12 mmol · g−1. We also demonstrated that our newly developed 2Pt/10 K/MgAlOx–rGO catalyst possesses excellent H2O resistance and thermal stability. The obtained Pt/K/MgAlOx–rGO catalyst also exhibited excellent lean–rich cycling performance, with an overall 78.4% of NOx removal.
Methods
Preparation of Mg–Al–CO3 LDH
LDH with Mg/Al = 3 was prepared using a hydrothermal procedure. In the method, 100 mL aqueous salt solution containing a mixture of 0.075 mol Mg(NO3)2 · 6H2O and 0.025 mol Al(NO3)3 · 9H2O was added drop–wise into a 100 mL aqueous solution containing 0.05 mol Na2CO3. In the meantime, the pH of the precipitation was controlled at 10 using a 4 M NaOH solution. The resulting mixture was hydrothermal treated at 120 °C for 12 h. It was then filtered and washed with deionized water until pH of the wash water became 7, and washed with acetone for several times, followed by drying at 60 °C.
Preparation of Mg–Al LDH/rGO hybrid
Firstly, the GO used in this study were prepared by modified Hummers method as described in our previous work62. The Mg–A1–CO3 LDH/rGO hybrids were prepared via a hydrothermal method. In the method, 50 mL aqueous salt solution containing a mixture of 0.0375 mol Mg(NO3)2·6H2O and 0.0125 mol Al(NO3)3·9H2O was added drop‒wise to another 250 mL solution containing 0.025 mol Na2CO3 and 200 mL GO dispersion (1 g · L−1) under vigorous stirring at room temperature. The pH of the mixture was kept constant at 10 by adding a 4 M NaOH solution. The resulting mixture was hydrothermal treated at 120 °C for 12 h. It was then filtered and washed with deionized water until pH of the wash water became 7, and washed with acetone for several times, followed by dring at 60 °C in an oven. The obtained samples were designated as LDH/rGO.
Preparation of Pt/K/MgAlOx–rGO
The Pt/K/MgAlOx–rGO catalysts were prepared using a successive impregnation method. LDH/rGO was first pretreated at 400 °C in air for 5 h to obtain MgAlOx–rGO hybrids. Next, the obtained MgAlOx–rGO support was first impregnated with H2PtCl6 · 6H2O ethanol solution (100 g · L−1), dried at 60 °C for overnight, and calcined in air at 400 °C for 5 h. The sample was then impregnated with K2CO3 ethanediol solution (10 g · L−1), followed by drying at 60 °C and calcining at 400 °C for 5 h. The loading of Pt was 2 wt%, and the loading of K2CO3 was controlled to be 5, 10, 15, and 20 wt%, respectively. The obtained catalysts were denoted as 2Pt/XK/MgAlOx–rGO, where 2 and X represent the weight loading of Pt and K2CO3, respectively. XK/MgAlOx–rGO samples were also prepared similarly for comparison.
Characterization
The phase structure and morphology of the samples were characterized by powder X–ray diffractometer (XRD, Shimadzu XRD–7000) with Cu Kα radiation and a power of 40 kV × 30 mA, X–ray photoelectron spectra (XPS) were recorded on a Thermo escalab 250Xi spectrometer equipped with A1 Kα X–ray source, a scanning electron microscope (FE–SEM, SU–8010), and a high resolution transmission electron microscopy (HR–TEM, JEOL 2010). Raman spectra were obtained on a Renishaw inVia Raman spectrometer with 532 nm wavelength incident laser light. Attenuated total reflectance‒Fourier transform infrared spectroscopy (ATR‒FTIR, Bruker VERTEX 70) was used to identify the functional groups of samples. BET specific surface areas were measured with a physisorption analyzer (SSA–7000, Builder). The weight loss of samples were measured using a Q50 TGA analyser (TA Instruments, N2 flow rate = 60 mL · min−1) in the temperature range of 50–700 °C.
NOx storage and reduction reaction
For NOx storage reaction, experiments were carried out in a fixed bed reactor (10 mm internal diameter) under atmospheric pressure. All gases except H2O were introduced into the reactor via several mass flow controllers (Brooks Instruments). Liquid water was continuously added using a syringe pump (Lead fluid, TYD01) into a stainless steel tube wrapped with a temperature–controlled heating tape, in which the water vapor was generated. An on–line NO–NO2–NOx analyser (Thermo Scientific 42i–HL, USA) was used to measure the concentration of NO, NO2 and NOx in the outlet gas. The NOx storage capacity (NSC) was calculated by integrating the concentration curves of NOx when steady state was reached according to the following equation (1).

For each test, the time span is about 100 min and 0.2 g sample that was pre–calcined at 400 °C for 5 h in air was employed. Alternative lean/rich cyclic NOx storage and reduction was performed in the same reactor described as above under cyclic operation. Totally 8 cycles of 6 min lean and 1 min rich were applied, and 0.2 g of sample was used to measure the activity. NOx concentrations in both inlet and outlet were measured with an on–line quadrupole mass spectrometer (QGA, Hidden, UK). The mixture gas consisted of a continuous flow of 700 ppm NOx (430 ppm NO and 270 ppm NO2), 10% O2 (for lean condition only), 3.5% H2 (for rich condition only), 5% H2O (for evaluating the poisoning effect of H2O only) and Ar as the balance gas, with a flow rate of 100 mL · min−1 (GHSV = 15000 h−1). The reaction temperature was controlled at 250, 300, 350, and 400 °C, respectively.
Additional Information
How to cite this article: Mei, X. et al. Synthesis of Pt/K2CO3/MgAlOx–reduced graphene oxide hybrids as promising NOx storage–reduction catalysts with superior catalytic performance. Sci. Rep. 7, 42862; doi: 10.1038/srep42862 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Yoon, D. Y. et al. NO oxidation activity of Ag-doped perovskite catalysts. J. Catal. 319, 182 (2014).
Heo, I. et al. Activity and thermal stability of Rh-based catalytic system for an advanced modern TWC. Appl. Catal. B: Environ. 121, 75 (2012).
Heo, I. et al. Combination of photocatalysis and HC/SCR for improved activity and durability of DeNOx catalysts. Environ. Sci. Technol. 47, 3657 (2013).
Park, J.-H., Park, S. J. & Nam, I.-S. Fast colorimetric assay for screening NSR catalyst. Catal. Surv. Asia 14, 11 (2010).
Farrauto, R. J. & Heck, R. M. Catalytic converters: state of the art and perspectives. Catal. Today 51, 351 (1999).
Wang, Q., Sohn, J. H. & Chung, J. S. Thermally stable Pt/K2Ti2O5 as high-temperature NOx storage and reduction catalyst. Appl. Catal. B: Environ. 89, 97 (2009).
Wang, Q. & Chung, J. S. NOx storage and reduction over Cu/K2Ti2O5 in a wide temperature range: Activity, characterization, and mechanism. Appl. Catal. A: Gen. 358, 59 (2009).
Jeong, S., Youn, S. & Kim, D. H. Effect of Mg/Al ratios on the NOx storage activity over Pt-BaO/Mg–Al mixed oxides. Catal. Today 231, 155 (2014).
Ogura, M. et al. Stabilization of bare divalent Fe(II) cations in Al-rich beta zeolites for superior NO adsorption. J. Catal. 315, 1 (2014).
Peng, Y., Si, W., Li, J., Crittenden, J. & Hao, J. Experimental and DFT studies on Sr-doped LaMnO3 catalysts for NOx storage and reduction. Catal. Sci. Technol. 5, 2478 (2015).
Gogurla, N., Sinha, A. K., Santra, S., Manna, S. & Ray, S. M. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 4, 6483 (2014).
Matsumoto, S. DeNOx catalyst for automotive lean-burn engine. Catal. Today 29, 43 (1996).
Shinjoh, H., Takahashi, N., Yokota, K. & Sugiura, M. Effect of periodic operation over Pt catalysts in simulated oxidizing exhaust gas. Appl. Catal. B: Environ. 15, 189 (1998).
Liu, Y., Meng, M., Li, X.-G., Guo, L.-H. & Zha, Y.-Q. NOx storage behavior and sulfur-resisting performance of the third-generation NSR catalysts Pt/K/TiO2–ZrO2 . Chem. Eng. Res. Des. 86, 932 (2008).
Xiao, J., Li, X., Deng, S., Wang, F. & Wang, L. NOx storage-reduction over combined catalyst Mn/Ba/Al2O3–Pt/Ba/Al2O3 . Catal. Commun. 9, 563 (2008).
Casapu, M. et al. Comparative study of structural properties and NOx storage-reduction behavior of Pt/Ba/CeO2 and Pt/Ba/Al2O3 . Appl. Catal. B: Environ. 78, 288 (2008).
Piacentini, M., Maciejewski, M. & Baiker, A. NOx storage-reduction behavior of Pt-Ba/MO2 (MO2 = SiO2, CeO2, ZrO2) catalysts. Appl. Catal. B: Environ. 72, 105 (2007).
Elbouazzaoui, S., Corbos, E. C., Courtois, X., Marecot, P. & Duprez, D. A study of the deactivation by sulfur and regeneration of a model NSR Pt/Ba/Al2O3 catalyst. Appl. Catal. B: Environ. 61, 236 (2005).
Sedlmair, C., Seshan, K., Jentys, A. & Lercher, J. A. Studies on the deactivation of NOx storage-reduction catalysts by sulfur dioxide. Catal. Today 75, 413 (2002).
Engström, P., Amberntsson, A., Skoglundh, M., Fridell, E. & Smedler, G. Sulphur dioxide interaction with NOx storage catalysts. Appl. Catal. B: Environ. 22, L241 (1999).
Roy, S. & Baiker, A. NOx Storage-Reduction Catalysis: From Mechanism and Materials Properties to Storage-Reduction Performance. Chem. Rev. 109, 4054 (2009).
Castoldi, L., Nova, I., Lietti, L. & Forzatti, P. Study of the effect of Ba loading for catalytic activity of Pt–Ba/Al2O3 model catalysts. Catal. Today 96, 43 (2004).
Piacentini, M., Maciejewski, M. & Baiker, A. Pt-Ba/alumina NOx storage-reduction catalysts: Effect of Ba-loading on build-up, stability and reactivity of Ba-containing phases. Appl. Catal. B: Environ. 59, 187 (2005).
Park, S. J. et al. Hydrotalcite as a support for NOx trap catalyst. Top. Catal. 53, 57 (2010).
Yang, R., Gao, Y., Wang, J. & Wang, Q. Layered double hydroxide (LDH) derived catalysts for simultaneous catalytic removal of soot and NOx . Dalton T. 43, 10317 (2014).
Wang, Q. & O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 112, 4124 (2012).
Wang, Z. et al. Co-Mn-Al Nonstoichiometric Spinel-Type Catalysts Derived from Hydrotalcites for the Simultaneous Removal of Soot and Nitrogen Oxides. Sci. Adv. Mater. 5, 1449 (2013).
Takahashi, N., Matsunaga, S. I., Tanaka, T., Sobukawa, H. & Shinjoh, H. New approach to enhance the NOx storage performance at high temperature using basic MgAl2O4 spinel support. Appl. Catal. B: Environ. 77, 73 (2007).
Silletti, B. A. et al. A novel Pd/MgAlOx catalyst for NOx storage-reduction. Catal. Today 114, 64 (2006).
Fornasari, G. et al. Novel low temperature NOx storage-reduction catalysts for diesel light-duty engine emissions based on hydrotalcite compounds. Catal. Today 75, 421 (2002).
Li, Q. et al. Multifunctional hydrotalcite-derived K/MnMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Chem. Eng. J. 184, 106 (2012).
Huang, S. et al. Immobilization of Co–Al layered double hydroxides on graphene oxide nanosheets: growth mechanism and supercapacitor studies. ACS Appl. Mater. Inter. 4, 2242 (2012).
Chabot, V. et al. A review of graphene and graphene oxide sponge: material synthesis and applications to energy and the environment. Energy Environ. Sci. 7, 1564 (2014).
Wang, Y., Zhang, D., Bao, Q., Wu, J. & Wan, Y. Controlled drug release characteristics and enhanced antibacterial effect of graphene oxide–drug intercalated layered double hydroxide hybrid films. J. Mater. Chem. 22, 23106 (2012).
Wang, L. et al. Layered assembly of graphene oxide and Co–Al layered double hydroxide nanosheets as electrode materials for supercapacitors Chem. Commun. 47, 3556 (2011).
Dong, X., Wang, L., Wang, D., Li, C. & Jin, J. Layer-by-layer engineered Co–Al hydroxide nanosheets/graphene multilayer films as flexible electrode for supercapacitor. Langmuir: the ACS journal of surfaces and colloids 28, 293 (2012).
Li, M. et al. Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine. Nanoscale 3, 4240 (2011).
Wang, Y. et al. Enhanced conductivity of a glassy carbon electrode modified with a graphene-doped film of layered double hydroxides for selectively sensing of dopamine. Microchim. Acta 174, 41 (2011).
Wu, X.-L., Wang, L., Chen, C.-L., Xu, A.-W. & Wang, X.-K. Water-dispersible magnetite-graphene-LDH composites for efficient arsenate removal. J. Mater. Chem. 21, 17353 (2011).
Wang, J. et al. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energ. Chem. 24, 127 (2015).
Garcia-Gallastegui, A. et al. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 24, 4531 (2012).
Guo, Y. et al. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: synthesis and host− guest inclusion for enhanced electrochemical performance. ACS Nano. 4, 4001 (2010).
Liu, S., Tian, J., Wang, L., Luo, Y. & Sun, X. One-pot synthesis of CuO nanoflower-decorated reduced graphene oxide and its application to photocatalytic degradation of dyes. Catal. Sci. Technol. 2, 339 (2012).
Hu, Z.-A. et al. Synthesis and electrochemical characterization of mesoporous CoxNi1−x layered double hydroxides as electrode materials for supercapacitors. Electrochim. Acta 54, 2737 (2009).
Xu, J. et al. A sandwich-type three-dimensional layered double hydroxide nanosheet array/graphene composite: fabrication and high supercapacitor performance. J. Mater. Chem. A 2, 1022 (2014).
Park, S. et al. Hydrazine-reduction of graphite-and graphene oxide. Carbon 49, 3019 (2011).
Palomares, A. E., Uzcátegui, A. & Corma, A. NOx storage/reduction catalysts based in cobalt/copper hydrotalcites. Catal. Today 137, 261–266, 2007.12.137 (2008).
Büchel, R., Strobel, R., Baiker, A. & Pratsinis, S. E. Flame-Made Pt/K/Al2O3 for NOx Storage–Reduction (NSR) Catalysts. Top. Catal. 52, 1799 (2009).
Zou, Z.-Q., Meng, M. & He, J.-J. Surface distribution state and storage performance of the potassium species in the lean-burn NOx trap catalyst Pt/K/Al2O3-TiO2-ZrO2 . Mater. Chem. Phys. 124, 987 (2010).
Castoldi, L. et al. Alkaline-and alkaline-earth oxides based Lean NOx Traps: Effect of the storage component on the catalytic reactivity. Chem. Eng. J. 161, 416 (2010).
Prinetto, F., Manzoli, M., Morandi, S., Frola, F. & Ghiotti, G. J. Pt-K/Al2O3 NSR Catalysts: Characterization of Morphological, Structural and Surface Properties. Phys. Chem. C 114, 1127 (2009).
Kim, I. Y. et al. A strong electronic coupling between graphene nanosheets and layered titanate nanoplates: a soft-chemical route to highly porous nanocomposites with improved photocatalytic activity. Small 8, 1038 (2012).
Lee, Y. R., Kim, I. Y., Kim, T. W., Lee, J. M. & Hwang, S.-J. Mixed colloidal suspensions of reduced graphene oxide and layered metal oxide nanosheets: useful precursors for the porous nanocomposites and hybrid films of graphene/metal oxide. Chem. Eur. J. 18, 2263 (2012).
Huang, Z., Wu, P., Gong, B., Fang, Y. & Zhu, N. Fabrication and photocatalytic properties of a visible-light responsive nanohybrid based on self-assembly of carboxyl graphene and ZnAl layered double hydroxides. J. Mater. Chem. 2, 5534 (2014).
Li, L. et al. Layer-by-layer assembly and spontaneous flocculation of oppositely charged oxide and hydroxide nanosheets into inorganic sandwich layered materials. J. Am. Chem. Soc. 129, 8000 (2007).
Gunjakar, J. L., Kim, I. Y., Lee, J. M., Lee, N. S. & Hwang, S. J. Self-assembly of layered double hydroxide 2D nanoplates with graphene nanosheets: an effective way to improve the photocatalytic activity of 2D nanostructured materials for visible light-induced O2 generation. Energy Environ. Sci. 6, 1008 (2013).
Wang, Q. et al. Polypropylene/layered double hydroxide nanocomposites. J. Mater. Chem. 22, 19113 (2012).
You, R. et al. A series of ceria supported lean-burn NOx trap catalysts LaCoO3/K2CO3/CeO2 using perovskite as active component. Chem. Eng. J. 260, 357 (2015).
Fang, J. et al. Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim. Acta 85, 248 (2012).
Zhou, W. et al. One-step synthesis of Ni3S2 nanorod@Ni(OH)2 nanosheet core–shell nanostructures on a three-dimensional graphene network for high-performance supercapacitors. Energy Environ. Sci. 6, 2216 (2013).
Zhao, X., Cao, J.-P., Zhao, J., Hu, G.-H. & Dang, Z.-M. A hybrid Mg–Al layered double hydroxide/graphene nanostructure obtained via hydrothermal synthesis. Chem. Phys. Lett. 605, 77 (2014).
Mei, X., Wang, J., Yang, R., Yan, Q. & Wang, Q. Synthesis of Pt doped Mg-Al layered double oxide/graphene oxide hybrid as novel NOx storage-reduction catalyst RSC Adv. 5, 78061 (2015).
Li, Y., Gao, W., Ci, L., Wang, C. & Ajayan, P. M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 48, 1124 (2010).
Williams, G., Seger, B. & Kamat, P. V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano. 2, 1487 (2008).
Zhang, Z., Mou, Z., Yu, P., Zhang, Y. & Ni, X. Diesel soot combustion on potassium promoted hydrotalcite-based mixed oxide catalysts. Catal. Commun. 8, 1621 (2007).
Ura, B. et al. Effect of potassium addition on catalytic activity of SrTiO3 catalyst for diesel soot combustion. Appl. Catal. B: Environ. 101, 169 (2011).
Becerra, M. E. et al. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4 . Appl. Catal. B: Environ. 102, 260 (2011).
Lietti, L., Forzatti, P., Nova, I. & Tronconi, E. NOx Storage Reduction over Pt/Ba/γ-Al2O3 Catalyst J. Catal. 204, 175 (2001).
Epling, W. S., Campbell, G. C. & Parks, J. E. The Effects of CO2 and H2O on the NOx Destruction Performance of a Model NOx Storage/Reduction Catalyst. Catal. Lett. 90, 45 (2003).
Huang, H. Y., Long, R. Q. & Yang, R. T. A highly sulfur resistant Pt-Rh/TiO2/Al2O3 storage catalyst for NOx reduction under lean-rich cycles. Appl. Catal. B: Environ. 33, 127 (2001).
Eberhardt, W. et al. Photoemission from mass-selected monodispersed Pt clusters. Phys. Rev. Lett. 64, 780 (1990).
Bera, P., Patil, K. C., Jayaram, V., Subbanna, G. N. & Hegde, M. S. Ionic dispersion of Pt and Pd on CeO2 by combustion method: Effect of metal–ceria interaction on catalytic activities for NO reduction and CO and hydrocarbon oxidation. J. Catal. 196, 293 (2000).
Arico, A. S. et al. An XPS study on oxidation states of Pt and its alloys with Co and Cr and its relevance to electroreduction of oxygen. Appl. Surf. Sci. 172, 33 (2001).
Rao, M. M., Reddy, B. R., Jayalakshmi, M., Jaya, V. S. & Sridhar, B. Hydrothermal synthesis of Mg–Al hydrotalcites by urea hydrolysis. Mater. Res. Bull. 40, 347 (2005).
Pavel, O. D. et al. The activity of Mg/Al reconstructed hydrotalcites by “memory effect” in the cyanoethylation reaction. Catal. Commun. 9, 1974 (2008).
Zhao, Y. et al. Enhanced antimicrobial properties, cytocompatibility, and corrosion resistance of plasma-modified biodegradable magnesium alloys. Acta Biomater. 10, 544 (2014).
Wang, Q. et al. High temperature adsorption of CO2 on Mg–Al hydrotalcite: effect of the charge compensating anions and the synthesis pH. Catal. Today 164, 198 (2011).
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2016ZCQ03), the National Natural Science Foundation of China (51622801, 51572029, and 51308045), and the Beijing Excellent Young Scholar (2015000026833ZK11).
Author information
Authors and Affiliations
Contributions
X.M. carried out most of the experiments and the analysis of data, and prepared the manuscript. Q.Y. helped with some of the experiments reported; P.L., J.W., Y.C., Y.N. and U.A. assisted with data analysis and discussion, and manuscript preparation. Q.W. designed the experiments, analyzed the data, and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mei, X., Yan, Q., Lu, P. et al. Synthesis of Pt/K2CO3/MgAlOx–reduced graphene oxide hybrids as promising NOx storage–reduction catalysts with superior catalytic performance. Sci Rep 7, 42862 (2017). https://doi.org/10.1038/srep42862
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42862
This article is cited by
-
Aqueous miscible organic-layered double hydroxides with improved CO2 adsorption capacity
Adsorption (2020)
-
In situ surface formation of TiO2/Ti(NO2) hybrid nanocomposites with N2 APPJ treatment for efficient C2H4 photodegradation
Journal of Food Science and Technology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.