Abstract
Many endemic fish species are threatened with extinction. Conservation strategies and the restoration of endemic fish after extinction must therefore be investigated. Although sperm cryopreservation is indispensable for the conservation of endangered fishes, the limited number of mature fish and limited availability (volume and period) of sperm from small endemic fish hinders the optimization and practical use of this material. In this report, we demonstrate the in vitro differentiation of fertile sperm from cryopreserved spermatogonia of juveniles of the endangered small cyprinid honmoroko (Gnathopogon caerulescens), which is endemic to Lake Biwa in Japan. The entire process of spermatogenesis was recapitulated in vitro using cryopreserved spermatogonia of non-spawning adult and juvenile fish. The differentiation of sperm from spermatogonia was captured as a time-lapse video and confirmed by 5-ethynyl-2′-deoxyuridine (EdU) incorporation into sperm. Fertility was demonstrated by artificial insemination. These results suggest that the combination of cryopreservation of spermatogonia and in vitro sperm differentiation will provide a new and promising strategy for the preservation of paternal genetic materials.
Similar content being viewed by others
Introduction
Animal populations are sustained by a balance among various species, and the loss of biodiversity influences entire ecosystems1. Many endemic fishes have been classified as endangered species, and the threats to these species have recently become more immediate2. Important technical approaches to restoring these species involve the preservation of sperm, eggs or embryos. Although sperm cryopreservation has been reported in large fish such as salmonids and in experimental model fish such as zebrafish (Danio rerio) and medaka (Oryzias latipes)3, few reports have described sperm cryopreservation in small endangered fish species. Systematic studies of cryopreservation in small endangered fish are prevented by their small volume, the short durations of their spawning periods4, inter-individual variation in quality, and the limited number of mature fish available. In addition, eggs, which are required for testing the fertility of cryopreserved sperm, are not available except during the spawning period5, and cryopreservation methods for eggs have not been established because of their large size, high sensitivity to chilling, and low membrane permeability6,7. Although transplantation of germ cells (primordial germ cells8, spermatogonia9, and oogonia10) into surrogates has been reported for producing both eggs and sperm, allogeneic surrogates will not be available after the extinction of an endangered fish, and validation of suitable xenogeneic surrogates takes time. Therefore, it is important to prepare multiple strategies for the conservation of critically endangered fishes.
The use of spermatogenic cells other than sperm is beneficial for the preservation of critically endangered fish species because it expands the possibilities for preservation and allows the use of non-spawning males and even juveniles as genetic resources. Furthermore, the preservation of undifferentiated male germ cells, spermatogonia, provides a unique advantage because, after transplantation into a surrogate, these cells can potentially differentiate into eggs as well as sperm11. Therefore, the use of spermatogonia is a practical method for the preservation of both sperm and eggs. In vitro production of sperm from spermatogonia has been reported for three fish species, Japanese eel (Anguilla japonica)12, medaka13, and zebrafish14,15. However, such sperm has only been confirmed to be fertile for zebrafish, using sperm differentiated from freshly prepared spermatogonia14 or using spermatogonial stem cells cultured for up to 1 month15. No report has described the in vitro differentiation of fertile sperm from the cryopreserved spermatogonia of juveniles or non-spawning adult fish. Here, we describe the establishment of culture and cryopreservation conditions for spermatogonia of the critically endangered species Gnathopogon caerulescens and demonstrate the differentiation of fertile sperm from cryopreserved spermatogonia using juveniles and non-spawning adults.
Results
Histological analysis of spermatogenic cells in G. caerulescens
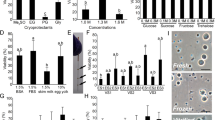
To identify spermatogenic cells such as spermatogonia, spermatocytes, spermatids, and sperm in G. caerulescens, testicular sections of matured spawning (May) and non-spawning (September) adults, as well as juvenile fish (approx. 5 months old, ~4 cm in body length), were analysed histologically. Based on the histological identification of spermatogenic cells, flow cytometric analyses were also performed using propidium iodide (PI) staining and expression of the germ cell marker Vasa16. Testes from spawning adults contained all developmental stages of spermatogenic cells17, including spermatogonia (0.1%), spermatocytes (11.2%), spermatids, and flagellated sperm (82.2%) (Fig. 1 and Supplementary Fig. S1). Intense Vasa signal was observed in the spermatogonial cytoplasm; Vasa signal was also present in primary spermatocytes, but no signal was detected in secondary spermatocytes, spermatids, or spermatozoa. In the non-spawning testis, spermatogonia with strong Vasa signal were predominant in the seminiferous tubules (Fig. 1). Based on flow cytometry, the population of spermatogenic cells was estimated as follows: spermatogonia, 32.4%; spermatocytes, 1.7%; and somatic cells, 59.2% (Supplementary Fig. S1). Spermatids and sperm were not observed. The proliferation of spermatogonia was detected using PCNA staining. In juvenile fish, spermatogonia with single nucleoli and strong Vasa signal were predominant (49.9%), similar to the non-spawning adult testis (Fig. 1 and Supplementary Fig. S1). However, they showed higher levels of heterochromatin and little PCNA signal in the nucleus, unlike the spermatogonia in the non-spawning testis (Fig. 1). No spermatocytes, spermatids or sperm were detected, and somatic cells composed 39.5% of the samples (Supplementary Fig. S1).
Testes were sampled from spawning (May) and non-spawning (September) adults and from juvenile fish. Images show the gross morphology of the fish and testes; adjacent testicular sections are stained with haematoxylin-eosin (HE) and for Vasa (green) and proliferating cell nuclear antigen (PCNA, red). Nuclei were stained with DAPI (blue). The images shown are representative of ten different male G. caerulescens per group. The insets in each panel show high-magnification images of spermatogonia. Spawning testes (May) were thick and plump with ivory white coloration, whereas non-spawning adult (September) and juvenile (5-month-old) testes were thin and threadlike with translucent white coloration. Experiment reproduced ten times. Scales are indicated in the figure.
In vitro sperm differentiation from spermatogonia
Enzymatically dissociated testicular cells were grown in either adherent or suspension culture using testicular cell culture medium (TCCM)18 supplemented with growth factors and hormones under humidified air at 18 °C.
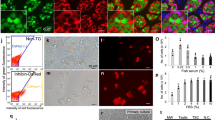
In the adherent culture of spawning testis, all stages of spermatogenic cells were observed on the fibroblast-like testicular somatic cells during the first week of culture (Fig. 2a). These germ cell colonies underwent spermatogenesis, and motile flagellated sperm were observed at the beginning of the first week in culture. Flagellated sperm were then released into the culture medium. The morphology of these spermatogenic cell colonies in vitro was quite similar to that observed in testicular sections (Figs 1 and 2a). Flagellated sperm were produced continuously for approximately 1 month, and the size and number of spermatogenic cell colonies decreased accordingly. Adherent somatic cells proliferated to confluence and detached from the dish, forming a cell sheet that wrapped around the germ cell colonies and terminated sperm production.
Testicular cells isolated from spawning (May) and non-spawning (September) adult and juvenile testes (5-month-old) were cultured. (a) Bright-field images of testicular cell cultures. The insets in each panel represent high-magnification images of the most advanced germ cell types. In the testicular cell cultures from spawning adults, spermatogenic cells of all developmental stages were observed at days 7, 11, and 21. In the testicular cell cultures from non-spawning adults, germ cell colonies of spermatogonia, spermatocytes, and flagellated sperm were observed at days 7, 11, and 18. In testicular cell cultures from juveniles, colonies of spermatogonia, spermatocytes, and flagellated sperm were observed at days 4, 13, and 19. Sperm flagella are indicated by arrowheads. Experiment reproduced six times. Bars represent 100 μm. (b) Bright-field images (top) of juvenile testicular cell cultures and fluorescent images for 5-ethynyl-2′-deoxyuridine (EdU, green) and Vasa (red). Nuclei were stained with DAPI (blue). EdU-positive and EdU-negative cells are indicated by the arrows/solid lines and arrowheads/dotted lines, respectively. EdU-positive spermatogonia, spermatocytes, and sperm were observed at days 7, 14, and 21, respectively. Experiment reproduced four times. Bars represent 50 μm.
In the cultures of non-spawning adult and juvenile testes (Fig. 2), only spermatogonia were observed on the somatic cells during the first week. Then, the spermatogonia differentiated to form spermatocyte colonies during the second week of culture. The differentiation of spermatogonia proceeded via a similar time course in both cultures, and motile, flagellated sperm were observed during the third week (Supplementary movie S1). Thereafter, sperm production decreased gradually as described in the culture of spawning testes. Differentiation of spermatogonia was also monitored using Vasa staining during the culture period (Supplementary Fig. S2).
To confirm the production of sperm from spermatogonia in vitro, 5-ethynyl-2′-deoxyuridine (EdU) incorporation was evaluated. EdU-positive spermatogonia, spermatocytes, and flagellated sperm were detected during the first, second, and third weeks of culture when using juvenile testes, respectively (Fig. 2b).
Cystic spermatogenesis on the surfaces of cell aggregates in suspension culture
We next tested whether suspension culture could improve spermatogenesis. Dissociated testicular cells autonomously assembled to form spherical aggregates. When non-spawning adult and juvenile testicular cells were cultured, many flagellated sperm appeared on the surfaces of the aggregates during the third week of culture (Fig. 3a and Supplementary movie S2), in a time course similar to that in adherent culture. The flagellated sperm then detached from the aggregates and were released into the culture medium. Cell aggregates grew to 500 μm in diameter in the third week and actively produced sperm for approximately 1 month. Thereafter, the size of the aggregates and the number of flagellated sperm in the culture medium gradually decreased, and the sperm production ceased after approximately 2 months.
Testicular cells isolated from non-spawning adults and juveniles were cultured in suspension. (a) Bright-field images of testicular cell cultures. The insets in each panel represent high-magnification images of the selected area. Sperm flagella are indicated by arrowheads. Experiment reproduced four times. Bars represent 200 μm. (b) Histological sections of cell aggregates formed in juvenile testicular cell cultures. Adjacent sections were stained with haematoxylin-eosin (HE) (top) and for Vasa (green) and PCNA (red). The insets in each panel represent high-magnification images of the most advanced germ cell types: spermatogonia, spermatocytes, and spermatozoa in the first, second, and third weeks of culture, respectively. Colonies of spermatogonia (arrows), spermatocytes (solid lines), and sperm (dotted lines) are indicated. Experiment reproduced three times. Bars represent 200 μm.
Immunohistochemical analysis of the aggregates revealed that a small number of Vasa-positive spermatogonia were scattered on the surfaces of the cell aggregates in the first week, and the spermatogonia then differentiated into spermatocytes, which covered the entire surface. This is the opposite of the cell configuration in the testes, in which Vasa-positive germ cells are located outside the aggregates, with Vasa-negative somatic cells located inside (Fig. 3b). Similar to the testicular germ cells, however, immunostaining for Vasa and PCNA showed the formation of cysts and the synchronous differentiation of spermatogenic cells in the cysts (Fig. 3b).
As Sertoli cells interact directly with germ cells and are prerequisite for spermatogenesis in vivo19, we investigated the localization of Sertoli cells in in vitro culture, by immunostaining of cell aggregate sections with Sox9 antibody20,21. In spawning testes, Sox9-positive Sertoli cells were frequently found at the periphery of the spermatogonial cysts characterized by the higher level of Vasa-expression and large nuclei, while in non-spawning testes many Sox9-positive Sertoli cells were detected adjacent to the spermatogonia (Fig. 4). In cell aggregates, Sox9-positive cells were observed in close proximity to the spermatogonial cysts throughout the culture period. This result suggests that the spatial arrangement of spematogonia and Sox9-positive Sertoli cells in vitro was similar to that of in vivo.
Testes were sampled from spawning (May) and non-spawning (September) adults. Cell aggregates formed in suspension culture of non-spawning adult testicular cells were sampled at first, second, and third week of culture. Sections were stained for Sox9 (green) and Vasa (red). Nuclei were stained with DAPI (blue). Arrowheads indicate Sox9-positive cells. The insets represent high-magnification images of Sox9-positive cells. Experiment reproduced three times. Bars represent 50 μm.
Haploid formation in vitro
To monitor meiosis and haploid formation in vitro, the DNA content of pre-culture (freshly dissociated testicular cells) and post-culture cells was analysed by flow cytometry with PI staining (Fig. 5). Haploid cells, sperm and spermatids, were observed only in the spawning testes, not in the non-spawning adult and juvenile testes (Fig. 5 and Supplementary Fig. S1). After the culture, a clear haploid peak was detected in the cultures of non-spawning adult and juvenile testes, as well as those of spawning adults, suggesting that spermatogenic cells underwent successful meiosis in vitro. The proportions of haploid cells among cultures from non-spawning adult and juvenile testes were 9.7 and 7.3% in adherent culture and 40.7 and 34.2% in suspension culture, respectively. This suggests that spermatogenesis progressed more efficiently in suspension culture than in adherent culture.
The DNA content of the spawning and non-spawning adult and juvenile testes was analysed using flow cytometry before and after culture. Flow cytometric profiles of PI intensity, presented as histograms (FL2-A). Histogram of freshly dissociated testicular cells of spawning and non-spawning adult and juvenile testes (top). Histogram of testicular cells cultured for longer than three weeks in adherent (middle) and suspension (bottom) cultures. The percentages of haploid cells are indicated. The DNA content is indicated by c, 2c, and 4c, representing haploid, diploid, and tetraploid, respectively. Experiment reproduced three times.
Efficient sperm production from cryopreserved spermatogonia in vitro
The cryopreservation of germ cells is a prerequisite and a key technology for conserving endemic fish. Therefore, the cryopreservation of spermatogonia prepared from non-spawning adults and juvenile testes was examined using rapid (vitrification) cooling method. As spermatogonia are the unipotent stem cells and shares some characteristics with pluripotent stem cells (e.g., self-renewal, high nuclear cytoplasmic ratio, and presence of prominent nucleoli), we used commercially available freezing medium for human iPS cells. After 6-months to 3-years of storage, cell survival rates and differentiation ability of the cryopreserved testicular cells were examined. The survival rates of cryopreserved testicular cells of non-spawning adult and juvenile fish were 64.7 ± 9.1% and 52.2 ± 9.5% (n = 6), respectively. Vitrified cells in either adherent or suspension culture formed many germ cell colonies and differentiated into sperm with motile flagella. The entire process of spermatogenesis was recorded as a time-lapse video (Supplementary movie S3), showing active migration, cell division, and differentiation of spermatogonia of G. caerulescens, as in mouse seminiferous tubules22. Because uniform somatic cells23 are known to proliferate in adherent culture, we attempted to separate the germ cells, which produce less side-scattered light than the somatic cells, using only PI staining and flow cytometry. The germ cells formed 20.9% of the adherent culture (spermatids and sperm: 2.9%, spermatogonia and spermatocytes: 18.0%), with the somatic cells forming 78.3% (Supplementary Fig. S3a). However, in the suspension culture of cryopreserved non-spawning testes, germ cells were predominant, forming 87.6% of the cells (spermatids and sperm: 25.4%, spermatogonia and spermatocytes: 62.2%) (Supplementary Fig. S4b). Accordingly, the proportion of somatic cells in suspension culture was much lower than that in adherent culture (9.9 vs 78.3%, respectively). The suspension culture of vitrified juvenile testes also showed efficient differentiation of sperm (32.0%) and a high proportion of germ cells (75.2%) (Supplementary Fig. S3b). The total number of sperm produced in suspension culture (17.85 ± 1.95 × 105, n = 4) was larger than that of adhesion culture (4.98 ± 1.37 × 105, n = 4) (P < 0.05), when cryopreserved non-spawning testicular cells (6.8 × 105 cells) were cultured for 30-days.
Motility of the sperm differentiated in vitro
Initiation of motility of the sperm differentiated in vitro was triggered at hypotonic conditions. The sperm in the culture medium were activated immediately by diluting the medium with water more than 10 times (Supplementary movie S4 and S5). The physical parameters of sperm motion were compared to those of in vivo-differentiated sperm (Supplementary Fig. S4 and Table S1). The mean values of VCL (curvilinear velocity) and VSL (straight line velocity) in in vitro-differentiated sperm (129.6 ± 40.5 μm/s and 106.4 ± 45.8 μm/s, respectively) were both lower than those of in vivo-differentiated sperm (269.8 ± 60.3 μm/s and 257.2 ± 69.1 μm/s, respectively) (P < 0.05). In addition, linear pattern of movement (LIN = VSL/VCL × 100) of in vitro-differentiated sperm (81.7 ± 20.7%) was also lower than that of in vivo-sperm (94.8 ± 10.4%) (P < 0.05). These results suggest that in vitro-sperm have acquired motility at 28 days of culture, but their motility is inferior to that of in vivo-sperm regarding the velocity and linearity.
Fertility of the sperm differentiated in vitro
To validate the fertility and developmental competence of the sperm differentiated in vitro, the sperm were used for in vitro fertilization with unfertilized eggs obtained from female G. caerulescens by the injection of human chorionic gonadotropin (HCG). As shown in Table 1, in vitro differentiated sperm from both fresh and cryopreserved testicular cells were able to fertilize eggs, and most of the fertilized embryos hatched. Within the sperm differentiated from cryopreserved cells, an interaction between the effects of culture methods (adhesion and suspension cultures) and origin of testicular cells (non-spawning adult and juvenile fish) on the fertilization and hatching rates was observed (P < 0.05). Suspension culture of cryopreserved spermatogonia yielded higher rates of fertilization and hatching (24.7 and 21.7% in non-spawning adults and 4.8 and 4.1% in juveniles, respectively) than did adherent culture (both values are 0.7% in non spawning adults and 0.6% in juveniles, P < 0.05), regardless of the origin of testicular cells. The fertilization and hatching rates of non-spawning adult were both higher than those of juvenile in suspension culture (P < 0.05), but not in adherent culture. No fertilized embryos were obtained using sperm subjected to repeated freeze-thaw cycles or to more than 27 days in culture, suggesting that fertilized embryos were not parthenogenotes activated by unknown factor(s) in the culture medium, nor were they the result of contamination with sperm from the spawning male. Offspring were obtained from cryopreserved spermatogonia of juveniles and from non-spawning testes, and these offspring developed normally (Fig. 6). Obvious anomalies, such as the abnormally small eyes24 that are a known characteristic of artificially induced gynogenetic haploid G. caerulescens, were not observed. In addition, yolk sac absorption and feeding behaviour appeared to be normal.
Fertilized embryos, hatched larvae, and juveniles developed from G. caerulescens (top) and D. rerio (bottom) eggs that were artificially inseminated with in vitro differentiated sperm from cryopreserved juvenile testicular cells of G. caerulescens. The fertilized eggs of G. caerulescens reached the somite stage at 24 h post-insemination and hatched at approximately 7 days. The fish grew to approximately 6 cm in body length by 7 months. Fertilized D. rerio eggs reached the 4-cell stage at 3 h post-insemination. Asterisks indicate unfertilized eggs. Experiment reproduced nine (G. caerulescens) and eight (D. rerio) times. Bars represent 1 mm.
Because G. caerulescens eggs are only available during the spawning period, it is impossible to examine fertility during most of the year, even with cultures of freshly prepared spermatogonia from non-spawning adult and juvenile testes. To overcome this problem, we investigated whether zebrafish eggs, which also belong to the family Cyprinidae and have the same chromosomal number (2n = 50; zebrafish25, G. caerulescens26), can be used to validate the fertility of G. caerulescens sperm. Both in vivo differentiated sperm collected from spawning G. caerulescens and in vitro differentiated sperm from non-spawning adults were able to fertilize zebrafish eggs at high rates: 79.1 and 61.4%, respectively. The fertilized eggs also developed and hatched (Fig. 6 and Supplementary Table S2), but the hatching rates were lower than that with allogeneic eggs (35.2 and 11.4% vs 76.5%). In vitro differentiated sperm from cryopreserved spermatogonia of non-spawning adults and juveniles were also able to fertilize zebrafish eggs (at 0.3 and 3.5%, respectively). Although the hatched larvae did not display a short and stocky body27, which is the most common feature of gynogenetic haploid zebrafish, many of them showed a truncated body axis with severe anomalies in the circulatory system, and they did not survive longer than 4 days.
To confirm resultant larvae are true hybrid of G. caerulescens and zebrafish, we performed genotyping and ploidy analysis using genomic PCR and flow cytometry. Amplification of C-terminal region of Sox9b ortholog gave a unique band in G. caerulescens (328 bp) and zebrafish (248 bp), and both bands were detected in hybrid larvae (Supplementary Fig. S5). In addition, flow cytometry revealed that the DNA content of resultant larvae cells was different from that of the diploid zebrafish embryo and zebrafish sperm. It showed a good agreement with the sum of the DNA of G. caerulescens and zebrafish sperm (Supplementary Fig. S6). These results clearly indicate that the resultant larvae were truly hybrids of G. caerulescens and zebrafish, not parthenogenotes nor gynogenetic haploid.
Discussion
This study demonstrates that in the critically endangered endemic cyprinid G. caerulescens28, spermatogonia from juvenile fish and from non-spawning adults can be cryopreserved by vitrification, and fertile sperm can be differentiated in vitro. To the best of our knowledge, this is the first report of in vitro differentiation of fertile sperm from fresh and cryopreserved spermatogonia from juvenile and non-spawning adult fish. Furthermore, we reconstituted three-dimensional cystic spermatogenesis in vitro using suspension culture, which is more suitable for efficient sperm production than is the adherent culture reported for zebrafish14. Of interest, spermatogenesis occurred only on the surface of cell aggregates in suspension culture. This can be explained by the availability of factors, which are required for spermatogenesis such as nutrients, hormones, and growth factors. These factors were provided through the medium in in vitro culture, and therefore spermatogenesis could take place in the region with close proximity to these factors. In addition, similarity of the cystic localization of Sox9-positive Sertoli cells and spermatogonia in spawning testes and cell aggregates suggests that in vitro spermatogenesis proceeded in a similar manner to that of in vivo with regard to the interaction with Sertoli cells, and the presence of Sox9-expressing Sertori cell in the cysts would be necessary for spermatogenesis. This result was consistent with the Sox9b expression reported in medaka29, in which Sox9b is more strongly expressed near spermatogonia compared to more developmentally advanced germ cells. Therefore, the reason that Sox9 signal was not detected in the cysts of later stages of development might be attributed to the lower level of Sox9-expression to detect with this antibody.
The use of spermatogonia permits the use of non-spawning fish, including juveniles, which could not otherwise be used for sperm collection, and it avoids sacrificing spawning males for sperm preservation. In addition, the successful cryopreservation of spermatogonia can be confirmed simply in culture, without the fertilization of eggs from a spawning female. The production of fertile sperm from juvenile testes is particularly advantageous for the conservation of critically endangered fish, given that the cold winters faced by G. caerulescens cause the loss of most juveniles before sexual maturity30.
Here, we have demonstrated efficient in vitro spermatogenesis, which requires only 3 weeks from spermatogonia to flagellated sperm, even in juveniles. Spermatogenic cells observed in juvenile testes are mitotically inactive spermatogonia. Therefore, this culture condition represents an entire stage of meiosis. G. caerulescens in the wild requires approximately one year to reach sexual maturity31, and the differentiation of sperm from spermatogonia requires approximately 6 months in vivo (from September to March)32. Despite such rapid spermatogenesis in vitro, differentiated sperm were fertile and showed no detrimental effects on further development, suggesting that this culture can recapitulate normal meiosis in vitro. Shortening the sperm production using in vitro spermatogenesis from juvenile fish will be an advantage for the propagation of endangered species.
High fertility and hatching rates (24.7 and 21.7%, respectively) were achieved using suspension cultures with high haploid rates (25.4%) from tissues of non-spawning adults (Supplementary Fig. S3b). The low rates of fertilization and hatching (less than 1%) using adherent culture of vitrified cells may be attributable to the small number of sperm used for in vitro fertilization (Supplementary Fig. S3a). Sperm differentiation appears to be favored by the formation of three-dimensional cysts on the surfaces of the cell aggregates and the limited proliferation of somatic cells in the suspension culture. Similar reciprocal proliferation of germ cells and somatic cells has also been reported in testicular cell culture for the rainbow trout Oncorhynchus mykiss33. These high rates of fertilization and hatching in the suspension culture of cryopreserved spermatogonia were similar to those reported in zebrafish using sperm differentiated from adult testes in vitro (15% fertilization rate)34 and spermatocytes of medaka (30% hatching rate)35. Further, these rates were comparable to those of cryopreserved sperm in zebrafish (33% fertilization rate)36, goldfish (23% fertilization rate)37, loach (29% hatching rate)38, and shoveljaw carp (22% hatching rate)39, suggesting that this in vitro differentiation strategy using spermatogonia is valid. On the other hand, motility of the sperm is a major importance for fertilization and we confirmed that in vitro-produced sperm already acquired motility by activation. Although how these sperm acquired motility is not clear, we noticed that the floating sperm, which detached from the cysts, appeared to have acquired motility but not the sperm still attached to the cysts. Detailed investigation of the releasing process from the cysts might provide some information of the motility acquisition. The lower velocity and linearity of in vitro-produced sperm compared to the sperm produced in vivo, possibly declined the fertility. Further improvement will be required in culture system to differentiate sperm, which have comparable motility to the sperm produced in vivo (Supplementary movie S4, S5, Fig. S4, and Table S1).
Although DNA replication was detected in germ cells under this culture condition, the self-renewal of spermatogonia did not appear to occur dominantly: the number of spermatogonia and differentiated sperm gradually decreased with prolonged cell culture, as reported in zebrafish14. Therefore, establishment of the culture, which allows the self-renewal of undifferentiated spermatogonia or spermatogonial stem cells15, would be important for the propagation of endangered endemic fish because it allows for an unlimited supply of spermatogonia and resultant sperm. Because this in vitro spermatogenesis is simple and takes less time and effort than in vivo sperm production, it might be applicable for other endangered fish species.
We also demonstrated that zebrafish eggs can be used to validate the fertility of G. caerulescens sperm differentiated in vitro. G. caerulescens eggs are highly valuable because they are only available during the spawning season; further, it is difficult to control ovulation, and the eggs show quality variation between individuals. Zebrafish eggs, by contrast, are readily available throughout the year, and their homogeneous genetic background provides more consistent results. In addition, the transparency of zebrafish eggs allows the scoring of fertility within 2 h after fertilization, whereas G. caerulescens eggs are opaque and require 24 h (until the somite stage) to confirm fertilization.
Because fish are the most diverse group of vertebrates and show vast differences in reproduction, conservation strategies must be established using the fish of interest. In this study, we demonstrated that the combination of cryopreservation of spermatogonia and in vitro differentiation of sperm using non-spawning adults and juveniles of G. caerulescens provides not only a new strategy for the conservation of endangered endemic fish but also a simple in vitro model of vertebrate spermatogenesis. Further studies, which may allow self-renewal of spermatogonia and the production of mature eggs in vitro or by the xenogeneic transplantation of undifferentiated germ cells, will be the next step, and these key technologies will make it possible to accomplish the complete regeneration of endangered endemic fish.
Methods
Fish
Adult male honmoroko (G. caerulescens) (1 to 2 years old) were reared in outdoor ponds near Lake Biwa and were collected during the spawning period (May, spring) and during the non-spawning period (September, late summer; January, winter). Juvenile G. caerulescens males (approx. 5 months old, ~4 cm in body length) were grown from embryos and kept in indoor tanks in the laboratory under a natural light-dark cycle at 24 ± 2 °C.
To prepare unfertilized eggs for artificial insemination assays, adult female G. caerulescens (>1 year old) were collected on the day before use during the spawning period. The wild-type zebrafish strain RIKEN WT was obtained from the RIKEN Brain Science Institute (Saitama, Japan) and maintained in the laboratory under a 12 h light:12 h dark photoperiod at 27 ± 1 °C. All experiments were approved by the committee on laboratory animal care and use at Ritsumeikan University (Shiga, Japan), and performed according to the guidelines of Ritsumeikan University.
Preparation of testes
For the testicular cell culture experiments, male fish were euthanized by immersion in ethyl p-aminobenzoate (250 μg/ml; Benzocaine, Sigma-Aldrich, St. Louis, MO, USA) (for adult fish) or ice-cold water (for juvenile fish) and their surfaces were disinfected with 70% (v/v) ethanol. The testes were aseptically dissected out from the fishes and transferred into Ca2+/Mg2+-free phosphate-buffered saline (PBS) containing 0.5% (v/v) commercial bleach (Heiter, Kao, Tokyo, Japan) for sterilization. After 2-min immersion, the testes were washed three times in sterile PBS for 2 min each. All procedures were performed at room temperature unless otherwise specified.
Histological analysis of testes
Testicular fragments of randomly selected ten individual fish per group were fixed with 4% (w/v) paraformaldehyde in PBS supplemented with 10% (v/v) acetic acid at 4 °C overnight. The fixed tissues were washed with PBS, dehydrated in ethanol and xylene, embedded in paraffin, and cut into 5-μm-thick sections.
After deparaffinization, some of the sections were stained with haematoxylin and eosin (HE). Adjacent sections were immunohistochemically stained. Briefly, deparaffinized sections were incubated in 0.05% (v/v) citraconic anhydride solution (pH 7.4; Immunosaver, Nissin EM, Tokyo, Japan) for 45 min at 98 °C for antigen retrieval. Following the permeabilization with 0.2% (v/v) Triton X-100 for 10 min, the sections were blocked in PBS containing 4% (v/v) normal goat serum for 30 min. Thereafter, tissue sections were incubated overnight at 4 °C with diluted antibodies in PBS containing 4% normal goat serum. The following primary antibodies were used: rabbit anti-zebrafish Vasa (GTX128306: GeneTex, Irvine, CA, USA; diluted 1:1000), rat anti-Drosophila Vasa (Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA, USA; diluted 1:200), mouse anti-rat PCNA (P8825: Sigma-Aldrich, St. Louis, MO, USA; diluted 1:2000), and rabbit anti-human Sox9 (AB5535: Millipore, Temecula, CA, USA; diluted 1:500). Next, they were incubated with the appropriate secondary antibodies diluted in PBS for 45 min. The following secondary antibodies were used: Alexa Fluor 488-conjugated goat anti-rabbit IgG (A-11008: Invitrogen, Carlsbad, CA, USA; diluted 1:500), Alexa Fluor 568-conjugated goat anti-mouse IgG (A-11004: Invitrogen; diluted 1:500), and Alexa Fluor 568-conjugated goat anti-rat IgG (ab175476: Abcam, Cambridge, MA, USA; diluted 1:100). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI, Dojindo Laboratory) for 20 min. Negative control samples received identical staining preparations, except the primary antibody. Images were obtained at ×400 magnification using an inverted fluorescence microscope (IX70: Olympus, Tokyo, Japan) equipped with a CCD camera (VB-7000, Keyence, Osaka, Japan). Developmental stages of spermatogenetic cells were identified according to the classification of closely related cyprinid zebrafish40.
Culture of testicular cells
Primary cell culture was performed as described for male germ cell cultures of zebrafish18 with several modifications. Freshly dissected testicular fragments were minced and dissociated in Leibovitz’s L-15 medium (L5520: Sigma-Aldrich) containing 500 U/ml collagenase type IV (17104-019: Life Technologies, Gaithersburg, MD, USA) for 2 h at 28 °C under constant shaking (140 rpm) with gentle pipetting every 20 min. The cell suspension was diluted ten times with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered Leibovitz’s L-15 medium containing 1% (w/v) bovine serum albumin fraction V (BSA, Sigma-Aldrich) and filtered through a 100-μm pore-size nylon mesh (Cell strainer, Corning, Corning, NY, USA). After centrifugation at 120 × g for 7 min, the cell pellet was resuspended in testicular cell culture medium (TCCM)18 supplemented with 10% (v/v) foetal bovine serum (FBS, Invitrogen), 3% (v/v) common carp (Cyprinus carpio) serum, 100 ng/ml epidermal growth factor (EGF, Peprotech, Rocky Hill, NJ, USA), 100 ng/ml basic fibroblast growth factor (bFGF, Peprotech), 100 ng/ml insulin-like growth factor-I (IGF-I, Peprotech), 10 μM forskolin (Sigma-Aldrich), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), 10 IU/ml human chorionic gonadotropin (HCG, ASKA Pharmaceutical, Tokyo, Japan), 10 IU/ml pregnant mare’s serum gonadotropin (PMSG, ASKA Pharmaceutical), 50 ng/ml 11-ketotestosterone (11-KT, Cosmo Bio, Tokyo, Japan), 100 ng/ml 17β-estradiol (E2, Wako Pure Chemicals, Osaka, Japan), and 10 ng/ml 17α,20β-dihydroxy-4-pregnen-3-one (DHP, Toronto Research Chemicals, North York, NO, Canada). The cells originated from one testis of spawning adult were then seeded in three 35-mm plastic dishes containing 1.5 ml of the medium. In the case of non-spawning adult and juvenile fish, the cells isolated from one testis and five paired testes (in total ten testes) were seeded in one dish, respectively. The cells were then incubated at 18 °C in humidified air, and the medium was changed every 3 or 4 days. For adherent culture, 0.1% (w/v) gelatine-coated tissue culture dishes (3000-035: AGC Techno Glass, Shizuoka, Japan) were used. Cell culture images under adhesion and suspension conditions were obtained at ×200 and ×100 magnification, respectively, using an inverted microscope.
The composition of the TCCM was Leibovitz’s L-15 medium supplemented with 2 mM L-glutamine (Sigma-Aldrich), 50 U/ml penicillin–50 μg/ml streptomycin (Nacalai Tesque, Kyoto, Japan), 100 μg/ml Kanamycin sulfate (Nacalai Tesque), 800 μM CaCl2 (Wako Pure Chemicals), 200 μg/ml L-arginine (Sigma-Aldrich), 20 μg/ml L-aspartic acid (Sigma-Aldrich), 15 μg/ml L-histidine–HCl (Sigma-Aldrich), 72.5 μg/ml L-lysine–HCl (Sigma-Aldrich), 20 μg/ml L-proline (ICN Biomed, Milano, Italy), 0.5% BSA, 10 mM HEPES (Nacalai Tesque), 2 embryos per ml of common carp embryo extract, and 20% Milli-Q water (Millipore)18. Common carp embryo extract was prepared according to the procedure described previously41. Briefly, 2-day-old larvae obtained through artificial insemination42 were sterilized with Ringer’s solution for fresh water fish (116 mM NaCl, 2.9 mM KCl, 1.8 mM CaCl2, and 5 mM HEPES in water) containing 0.5% commercial bleach and washed twice with the Ringer’s solution. The washed embryos were then suspended in the same volume of Leibovitz’s L-15 medium and homogenized twenty times on ice with a Dounce tissue grinder. The resulting homogenate was diluted with Leibovitz’s L-15 medium to 200 embryos/ml, centrifuged at 6500 × g for 10 min at 4 °C, and the supernatant was collected. The supernatant was sterilized through a 0.2-μm pore filter and stored at −80 °C.
Immunochemical staining of cultured cells
Adherent cells were washed twice with PBS and fixed with 4% paraformaldehyde in PBS for 15 min. Following two washes with PBS, the cells were permeabilized by 0.2% Triton X-100 for 10 min, blocked with 4% normal goat serum, and stained with rabbit anti-Vasa antibody and Cy3-conjugated goat anti-rabbit IgG (AP132C: Millipore; diluted 1:500) or Alexa Fluor 488-conjugated goat anti-rabbit IgG. For the suspension culture, cell aggregates were harvested by centrifugation at 10 × g for 1 min and embedded in iPGell (GenoStaff, Tokyo, Japan), followed by fixation in 4% paraformaldehyde at 4 °C overnight. The fixed cell aggregates were then embedded in paraffin, cut into 5-μm-thick sections, and stained with antibodies against Vasa, PCNA, and Sox9 as described. Nuclei were counterstained with DAPI. Fluorescence images of cultured cells under adhesion and suspension conditions were obtained at ×400 and ×40 magnification using an inverted fluorescence microscope.
DNA replication of spermatogenic cells in vitro
To trace the progress of spermatogenesis in vitro, the Click-iT EdU cell proliferation assay kit (C10337, Invitrogen) was used for labelling and the detection of DNA replication, according to the manufacturer’s instructions. In short, EdU was added to the culture medium at a final concentration of 10 μM on the second day of adherent testicular cell culture prepared from juvenile fish. After 48 h of culture, the medium was replaced by fresh medium without EdU. At the time of detecting the DNA replication, adherent cells were washed twice with PBS and fixed with 4% PFA on the culture dish. Following two washes with 3% BSA in PBS, the cells were permeabilized by 0.5% Triton X-100 for 20 min and EdU-labeled DNA was conjugated to Alexa Fluor 488-N3 by CuSO4-catalyzed click chemistry43. For suspended cells including sperm, cells were collected by centrifugation of the culture medium at 800 × g for 5 min. The cells in the pellet was fixed, permeabilized, stained for EdU in the centrifugation tubes, and the stained cells were spread onto hydrophilic glass slides. Fluorescent images were obtained at ×400 magnification using an inverted microscope.
Flow cytometric analyses
For the flow cytometric analyses of cell composition in testes, dissociated testicular cells were washed by centrifugation at 800 × g for 7 min with PBS and fixed with 70% (v/v) ice-cold ethanol for 30 min. After centrifugation, the pelleted cells were washed by centrifugation with PBS containing 0.1% BSA and 0.1% saponin. The cells were then re-suspended in 4% normal goat serum for blocking. After 30 min incubation, rabbit anti-Vasa antibody was added (diluted 1:100) and incubated one hour at room temperature. Following two washes by centrifugation with PBS containing BSA and saponin, Alexa Fluor 488-conjugated goat anti-rabbit IgG was added (diluted 1:500) and incubated for 45 min. The cells were then washed by centrifugation with PBS containing BSA and saponin and treated with 1 mg/ml RNase A (Nacalai Tesque) for 30 min at 37 °C. After centrifugation, the cell nuclei were stained with 50 μg/ml of PI (Wako Pure Chemicals) for 15 min in the dark. These testicular cells were filtered through a 40-μm nylon mesh (N-N0330T: NBC Meshtec, Tokyo, Japan), diluted with PBS containing 1% BSA, and subjected to flow cytometry.
For the analyses of cells grown in adherent culture, germ cells and somatic cells were prepared separately. Briefly, the germ cell colonies were detached from the adherent somatic cells by using PBS for 20 min at room temperature. The detached germ cells and the supernatant medium containing sperm were combined to prepare the germ cell fraction, which was centrifuged at 800 × g for 7 min and washed twice with PBS. The pelleted cells were dissociated using 0.06% (w/v) trypsin (27250-042: Gibco, Grand Island, NY, USA) containing 1.32 mM ethylene diamine tetraacetic acid for 10 min. The remaining somatic cells were harvested by trypsinization to obtain the somatic cell fraction. The trypsin was neutralized by adding Leibovitz’s L-15 medium containing 10% FBS and the cells in the suspension was washed again by centrifugation with PBS. These cells were fixed and the nuclei were stained with PI as described above. These germ or somatic cell fractions were filtered through a 40-μm nylon mesh, and subjected to flow cytometry. For the cultured cells in suspension, the whole culture medium containing cell aggregates and detached sperm was centrifuged at 800 × g for 7 min, and the pelleted cells were treated as described for the germ cell above. For pre-culture controls, dissociated cells from adult and juvenile testes were prepared and analysed as described in testicular cells.
To determine the ploidy of hybrid embryos produced by G. caerulescens sperm and zebrafish eggs described below, hatched hybrid and zebrafish larvae were dissociated in Leibovitz’s L-15 medium containing 500 U/ml collagenase for 1 h at 28 °C with gentle pipetting every 10 min. The dissociated cells were washed twice by centrifugation at 120 × g for 5 min with PBS, and the pelleted cells were fixed and stained with PI as described. Squeezed sperm from mature male G. caerulescens and zebrafish was also fixed, stained, and analysed as haploid controls.
Flow cytometry was performed on a FACSCalibur (BD Biosciences, San Jose, CA, USA) equipped with an argon laser (488-nm). The fluorescence signals of the Vasa (FL1-H, 530/30 nm bandpass) and PI (FL2-A, 585/42 bandpass) were collected in the logarithmic and linear mode, respectively. Forward scatter (FSC-H) and side scatter (SSC-H) signals were collected using linear and logarithmic mode, respectively. The spectral overlap between Alexa Fluor 488 and PI was corrected by hardware compensation. To identify spermatogenic cells of various developmental stage and somatic cells in testes, cells were separated using SSC and FL1. In the case of ploidy analysis only PI staining was performed and analysed using SSC and FL2. A minimum of 3,000 events were collected for each sample with “LO” (12 μl/min) flow rate. Data were analysed with CellQuest Pro software (ver. 6.0, BD Biosciences).
Cryopreservation of testicular cells
To preserve testicular cells, dissociated testicular cells were cryopreserved using rapid (vitrification) cooling method. Briefly, the dissociated testicular cells were washed with ten volumes of HEPES-buffered Leibovitz’s L-15 medium containing 1% BSA. Aliquots containing dissociated cells of two pairs of testes (for non-spawning adult fish) or five pairs of testes (for juvenile fish) were separated and then centrifuged at 120 × g for 7 min. The pelleted cells were re-suspended in 200 μl of DAP213 solution (2 M DMSO, 1 M acetamide, and 3 M propylene glycol) (RCHEFM001: ReproCELL, Kanagawa, Japan)44, transferred into CryoTube Vial (377267: Thermo Scientific, Waltham, MA, USA), and cooled rapidly by plunging it into liquid nitrogen within 15-sec. For warming, the cryopreserved vial was removed from the liquid nitrogen tank and the cells were warmed quickly by adding the 1 ml pre-warmed (37 °C) Leibovitz’s L-15 medium supplemented with 10% FBS (thawing solution) within 15-sec. The cell suspension was diluted with 9 ml thawing solution and washed by centrifugation at 120 × g for 3 min. Following the removal of the supernatant, the cell pellet was re-suspended with the cell culture medium and cultured as described above.
Viabilities of cryopreserved testicular cells were assessed on the bases of the intactness of the plasma membrane using the trypan blue exclusion test just after the dilution with the thawing solution. In short, 10 μl of 0.5% (w/v) trypan blue solution (Kanto Chemical, Tokyo, Japan) was added to a same volume of the diluted testicular cell suspension and incubated for 3 min. The number of trypan blue-negative and -positive cells were counted using a hematocytometer, and the percentages of membrane intact cells were calculated according to the following formula: percentages of membrane intact cells = number of trypan blue-negative cells/number of total cells.
Time-lapse imaging of cultured cells
Cryopreserved testicular cells of non-spawning adult were counted using a hematocytometer soon after thawing and seeded in a gelatin-coated tissue culture dish (35-mm) containing 4.5 ml of the medium at a very low cell density (approx. 1,000 cells per dish). The culture medium was sealed with 2 ml of mineral oil (M8410, Sigma-Aldrich) to prevent evaporation. The cell culture dish was then placed on the stage of an inverted microscope (PrimoVert HDcam, Carl Zeiss, Oberkochen, Germany) mounted in an incubator (MB-6110C, Mitsuboshi Boeki, Hyogo, Japan) set to 18 °C. Images were captured once every 100 sec at ×100 magnification and played back at 5 frames per sec (fps). The time-lapse video generated using Labscope software (ver. 2.0, Carl Zeiss) on an iPad (Apple, Cupertino, CA, USA) was then converted to mp4 format at 40 fps using every third frame, giving a final playback speed of 12,000 × real speed; video conversion was performed using AviUtl (ver. 1.00, http://spring-fragrance.mints.ne.jp/aviutl/).
Analysis of sperm motility
Sperm motility was analysed according to the previous study45 with slight modifications. Briefly, culture medium containing sperm differentiated from non-spawning adult testes was centrifuged at 800 × g for 7 min to concentrate the sperm. The majority of the supernatant was discarded, and the cell pellet was resuspended in the remaining medium (<100 μl). A 10-μl of concentrated sperm suspension was placed on a self-made glass chamber (approximately 150-μm deep), cover slipped, and then the sperm were activated by adding a 100 μl deionized water. Images were recorded at ×100 magnification over 2 min from 5 sec before the sperm activation using an inverted microscope. Since motility of G. caerulescens sperm decreases very rapidly after mixing with water, sperm motility was analysed using the first 10 sec movie from the water addition. In each sample, the swimming path of 50 individual motile sperm was manually tracked using ImageJ (ver. 1.51 h; National Institutes of Health, Bethesda, MD, USA) plugin MTrackJ (ver. 1.5.1; Erasmus University, Rotterdam, Netherlands)46. Each track was followed for 1 sec divided into seven steps. The kinematic parameters that define sperm motility, including curvilinear velocity (VCL, the actual velocity along the trajectory), straight line velocity (VSL, the straight line distance between the start and end points of the track divided by the time of the track), and linearity (LIN, the ratio of net distance moved to total path distance (VSL/VCL × 100))47, were measured. Freshly squeezed sperm from mature male G. caerulescens was also analysed after 2 × 104 times dilution with culture medium.
Fertilization assay
Unfertilized eggs were collected from mature female G. caerulescens and zebrafish as reported48,49. A batch of eggs collected from one female was fertilized with the in vitro-differentiated sperm recovered from one 35-mm dish. Number of sperm was counted using hemocytometer after the sperm collection. The sperm suspension was concentrated by the centrifugation at 800 × g for 7 min, added to the unfertilized eggs in the dish, and the dish was swirled for 1 min to mix the sperm and eggs well. Then, dechlorinated tap water was added gradually with shaking, and the eggs were incubated at 23 °C for G. caerulescens eggs or at 28 °C for zebrafish eggs. We checked the embryonic development at the somite stage (for G. caerulescens eggs, approximately 24 h post-insemination) or the four-cell stage (for zebrafish eggs, approximately 3 h post-insemination) and at the hatching stage (for G. caerulescens and zebrafish eggs, 7 and 3 days post-insemination, respectively). Somite stage was chosen for checking the fertilization of G. caerulescens eggs, because the cleavage could not be seen through their opaque chorion. At somite stage, however, the fertilized eggs became distinguishable from unfertilized eggs as unfertilized eggs ruptured in the chorion and darkened. While in zebrafish egg, four-cell stage was chosen for checking the fertilization, because unfertilized zebrafish eggs have occasionally undergone a single, asymmetric cleavage. Freshly squeezed sperm from mature male G. caerulescens was used as a positive control. Squeezed sperm cultured for 27 days in the same culture conditions as the testicular cells was used as a negative control. Both control sperm samples were used at the sperm-egg ratio of the sperm recovered from one male per batch of eggs from one female.
Genotyping
Genomic DNA was prepared from hatched larvae of G. caerulescence, hybrid produced by G. caerulescence sperm and zebrafish egg, and zebrafish using PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, USA). C-terminal region of Sox9b orthologs were amplified using primers 5′-cagatcaagacggagcagctgag-3′ and 5′-gggtctggacagctgtgtgtagac-3′, which locate in exon 3 of zebrafish sequence.
The PCR reactions were performed using Ex Taq DNA polymerase (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions with 10 ng of genomic DNA as a template. Amplification program was initial denaturation at 98 °C for 1 min, and 35 cycles of denaturation at 98 °C for 10 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 30 sec, followed by a final extension at 72 °C for 5 min. The PCR products were separated on 3% (w/v) agarose gel electrophoresis.
Statistical analyses
Prior to statistical analyses, the percentage data (LIN for sperm motility and fertilization and hatching rates for fertilization assays) were transformed into arcsine values through arcsine square root transformation. Differences between the values of sperm kinematic parameters (VCL, VSL, and LIN) of in vitro and in vivo differentiated sperm were analysed using Student’s T-test. The numbers of differentiated sperm from cryopreserved non-spawning adult fish under adhesion and suspension conditions were also compared by Student’s T-test. In the sperm produced from cryopreserved cells, the effects of culture methods (adhesion vs. suspension) and origin of testicular cells (non-spawning adult vs. juvenile fish) on the fertilization and hatching rates were analysed by two-way analysis of variance (ANOVA) followed by Sidak’s post hoc test. A computer program (SPSS for Windows, Version 12.0, SPSS Inc., IL, USA) was used for statistical analyses.
Additional Information
How to cite this article: Higaki, S. et al. In vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens). Sci. Rep. 7, 42852; doi: 10.1038/srep42852 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Cardinale, B. J. et al. Biodiversity loss and its impact on humanity. Nature 486, 59–67, doi: 10.1038/nature11148 (2012).
Pimm, S. L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752, doi: 10.1126/science.1246752 (2014).
Yang, H. & Tiersch, T. R. Current status of sperm cryopreservation in biomedical research fish models: zebrafish, medaka, and Xiphophorus. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 149, 224–232 (2009).
Chao, N.-H. & Liao, I. C. Cryopreservation of finfish and shellfish gametes and embryos. Aquaculture 197, 161–189 (2001).
Tiersch, T. R., Yang, H., Jenkins, J. A. & Dong, Q. Sperm cryopreservation in fish and shellfish. Society of Reproduction and Fertility supplement, 493–508 (2007).
Robles, V., Cabrita, E., Acker, J. & Herráez, P. In Methods in reproductive aquaculture: marine and freshwater species (eds E. Cabrita, V. Robles, & P. Herráez ) Ch. 9 - Embryo cryopreservation: what we know until now-, 265–294 (CRC Press, 2009).
Zhang, T. In Life in the frozen state (eds Barry J. Fuller, Nick Lane, & Erica E. Benson ) Ch. 14 - Cryopreservation of gametes and embryos of aquatic species-, 415–435 (CRC Press, 2004).
Saito, T., Goto-Kazeto, R., Arai, K. & Yamaha, E. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biology of Reproduction 78, 159–166 (2008).
Okutsu, T., Shikina, S., Kanno, M., Takeuchi, Y. & Yoshizaki, G. Production of trout offspring from triploid salmon parents. Science 317, 1517–1517 (2007).
Yoshizaki, G. et al. Sexual plasticity of ovarian germ cells in rainbow trout. Development 137, 1227–1230 (2010).
Lee, S., Iwasaki, Y., Shikina, S. & Yoshizaki, G. Generation of functional eggs and sperm from cryopreserved whole testes. Proceedings of the National Academy of Sciences 110, 1640–1645 (2013).
Miura, C., Miura, T., Yamashita, M., Yamauchi, K. & Nagahama, Y. Hormonal induction of all stages of spermatogenesis in germ-somatic cell coculture from immature Japanese eel testis. Development, Growth & Differentiation 38, 257–262 (1996).
Hong, Y. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro . Proceedings of the National Academy of Sciences 101, 8011–8016 (2004).
Sakai, N. Transmeiotic differentiation of zebrafish germ cells into functional sperm in culture. Development 129, 3359–3365 (2002).
Kawasaki, T., Siegfried, K. R. & Sakai, N. Differentiation of zebrafish spermatogonial stem cells to functional sperm in culture. Development 143, 566–574, doi: 10.1242/dev.129643 (2016).
Braat, A. K., Speksnijder, J. E. & Zivkovic, D. Germ line development in fishes. Int J Dev Biol 43, 745–760 (1999).
Schulz, R. W. et al. Spermatogenesis in fish. Gen Comp Endocrinol 165, 390–411, doi: 10.1016/j.ygcen.2009.02.013 (2010).
Sakai, N. In vitro male germ cell cultures of zebrafish. Methods 39, 239–245 (2006).
Schulz, R. et al. Spermatogenesis in fish. General and Comparative Endocrinology 165, 390–411 (2010).
Chiang, E. F. L. et al. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Developmental Biology 231, 149–163 (2001).
Kobayashi, T., Kajiura-Kobayashi, H., Guan, G. & Nagahama, Y. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Developmental Dynamics 237, 297–306 (2008).
Yoshida, S., Sukeno, M. & Nabeshima, Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726, doi: 10.1126/science.1144885 (2007).
Higaki, S. et al. Establishment of testicular and ovarian cell lines from Honmoroko (Gnathopogon caerulescens). Fish Physiology and biochemistry 39, 701–711 (2013).
Fujioka, Y. Induction of gynogenetic diploids and cytological studies in honmoroko Gnathopogon caurulescens . Bulletin of the Japanese Society of Scientific Fisheries (Japan) 59, 493–500 (1993).
Schreeb, K., Groth, G., Sachsse, W. & Freundt, K. The karyotype of the zebrafish (Brachydanio rerio). Journal of Experimental Animal Science 36, 27–31 (1993).
Ueno, K., Ye, Y. & Umeoka, T. A comparative study of chromosomes in the cyprinid fish genera Gnathopogon and Squalidus of Japan. Nippon Suisan Gakkaishi 58, 1273–1277 (1992).
Walker, C. In Methods in Cell Biology - The Zebrafish: Genetics and Genomics - (eds H. W., Detrich, M., Westerfield & L. I., Zon ) Ch. 3 - Haploid screens and gamma-ray mutagenesis -, 43–70 (Elsevier, 1998).
Ministry of the Environment Government of Japan, Red list of Japanese brackish and freshwater fishes 2015, (Date of access: 29/12/2016) http://www.env.go.jp/press/files/jp/28060.pdf (2015).
Tanaka, M. Vertebrate female germline-the acquisition of femaleness. Wires Dev Biol 3, 231–238, doi: 10.1002/wdev.131 (2014).
Maki, I. Studies on the population dynamics of Gnathopogon caerulescens Sauvage (Pisces), in Lake Biwa, Japan V. Relation between the annual fluctuation of growth of the under-yearling fish and that of the population abundance in winter. Ecological Research 18, 158–166 (1968).
Nakamura, M. In Cyprinid fishes of Japan. Studies on the life history of cyprinid fishes of Japan (ed M. Nakamura ) 117–125 (Research Institute of Natural Resources Tokyo, 1969).
Okuzawa, K., Furukawa, K., Aida, K. & Hanyu, I. Annual reproductive cycle of the honmoroko Gnathopogon elongatus caerulescens . Bulletin of the Japanese Society of Scientific Fisheries 52, 1957–1960 (1986).
Shikina, S., Ihara, S. & Yoshizaki, G. Culture conditions for maintaining the survival and mitotic activity of rainbow trout transplantable type A spermatogonia. Molecular Reproduction and Development 75, 529–537 (2008).
Kurita, K. & Sakai, N. Functionally distinctive testicular cell lines of zebrafish to support male germ cell development. Molecular Reproduction and Development 67, 430–438 (2004).
Saiki, A. et al. Establishment of in vitro spermatogenesis from spermatocytes in the medaka, Oryzias latipes . Development, Growth & Differentiation 39, 337–344 (1997).
Yang, H., Carmichael, C., Varga, Z. M. & Tiersch, T. R. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio . Theriogenology 68, 128–136 (2007).
Bercsényi, M., Magyary, I., Urbányi, B., Orbán, L. & Horváth, L. Hatching out goldfish from common carp eggs: interspecific androgenesis between two cyprinid species. Genome 41, 573–579 (1998).
Yasui, G. S., Arias-Rodriguez, L., Fujimoto, T. & Arai, K. A sperm cryopreservation protocol for the loach Misgurnus anguillicaudatus and its applicability for other related species. Animal Reproduction Science 116, 335–345 (2009).
Tsai, S., Spikings, E., Hwang, C.-C. & Lin, C. Effects of the slow cooling during cryopreservation on the survival and morphology of Taiwan shoveljaw carp (Varicorhinus barbatulus) spermatozoa. Aquatic Living Resources 23, 119–124 (2010).
Leal, M. C. et al. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biology of Reproduction 81, 177–187 (2009).
Westerfield, M. In The zebrafish book (ed M. Westerfield ) Ch. 10 - Recipes -, 10.14 (University Oregon Press, 2007).
Billard, R., Cosson, J., Perchec, G. & Linhart, O. Biology of sperm and artificial reproduction in carp. Aquaculture 129, 95–112 (1995).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo . Proceedings of the National Academy of Sciences 105, 2415–2420 (2008).
Fujioka, T., Yasuchika, K., Nakamura, Y., Nakatsuji, N. & Suemori, H. A simple and efficient cryopreservation method for primate embryonic stem cells. International Journal of Developmental Biology 48, 1149–1154 (2004).
Guyón, N. F. et al. Impairments in aromatase expression, reproductive behavior, and sperm quality of male fish exposed to 17β-estradiol. Environmental Toxicology and Chemistry 31, 935–940 (2012).
Meijering, E., Dzyubachyk, O. & Smal, I. Methods for cell and particle tracking. Methods Enzymol 504, 183–200 (2012).
Rurangwa, E., Kime, D., Ollevier, F. & Nash, J. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture 234, 1–28 (2004).
Fujioka, Y. Thermolabile sex determination in honmoroko. Journal of Fish Biology 59, 851–861 (2001).
Westerfield, M. In The zebrafish book (ed M. Westerfield ) Ch. 2 - Breeding -, 2.12–12.21 (University Oregon Press, 2007).
Acknowledgements
This work was funded in part by Grants-in-Aid for Challenging Exploratory Research (23651248 and 15K14440 to T.T.) and Scientific Research on Priority Areas (21028021 to T.T.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. This study was also supported by the NIG Collaborative Research Program (2010-A41, 2011-A36, 2012-A28, 2013-A47, 2014-A45, 2016-A36 to T.T.), the Ritsumeikan Global Innovation Research Organization (R-GIRO) program (to T.T.), a Center of Innovation Trial Program from Japan Science and Technology Agency, JST (to T.T.), and the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan (to S.H.). The authors thank Syuichi Shimomura, Tetsuo Yamamoto, Taemon Yamamoto and personnel of the Shiga Prefectural Fisheries Experimental Station for providing Gnathopogon caerulescens (Honmoroko) and carp serum. We also thank Takefumi Yamamoto and Yasuhiro Mori, Shiga University of Medical Science, for their excellent technical support.
Author information
Authors and Affiliations
Contributions
T.T. conceived the research. S.H., N.S., and T.T. designed the experiments. S.H., M.S., K.K., T.To., T.K., and T.T. performed the research. S.H., T.K., I.T., Y.F., N.S., and T.T. analysed the data. S.H. and T.T. wrote the manuscript with suggestions from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Higaki, S., Shimada, M., Kawamoto, K. et al. In vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens). Sci Rep 7, 42852 (2017). https://doi.org/10.1038/srep42852
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42852
This article is cited by
-
Engineered reproductive tissues
Nature Biomedical Engineering (2020)
-
Cryopreservation of testicular tissue from Murray River Rainbowfish, Melanotaenia fluviatilis
Scientific Reports (2020)
-
Nutrition and metabolism of glutamate and glutamine in fish
Amino Acids (2020)
-
Cryopreservation of male and female gonial cells by vitrification in the critically endangered cyprinid honmoroko Gnathopogon caerulescens
Fish Physiology and Biochemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.