Abstract
In spite of intensive investigations, the role of spontaneous breathing (SB) activity in ARDS has not been well defined yet and little has been known about the different contribution of inspiratory or expiratory muscles activities during mechanical ventilation in patients with ARDS. In present study, oleic acid-induced beagle dogs’ ARDS models were employed and ventilated with the same level of mean airway pressure. Respiratory mechanics, lung volume, gas exchange and inflammatory cytokines were measured during mechanical ventilation, and lung injury was determined histologically. As a result, for the comparable ventilator setting, preserved inspiratory muscles activity groups resulted in higher end-expiratory lung volume (EELV) and oxygenation index. In addition, less lung damage scores and lower levels of system inflammatory cytokines were revealed after 8 h of ventilation. In comparison, preserved expiratory muscles activity groups resulted in lower EELV and oxygenation index. Moreover, higher lung injury scores and inflammatory cytokines levels were observed after 8 h of ventilation. Our findings suggest that the activity of inspiratory muscles has beneficial effects, whereas that of expiratory muscles exerts adverse effects during mechanical ventilation in ARDS animal model. Therefore, for mechanically ventilated patients with ARDS, the demands for deep sedation or paralysis might be replaced by the strategy of expiratory muscles paralysis through epidural anesthesia.
Similar content being viewed by others
Introduction
The mainstream supportive measure for patients suffering from acute respiratory distress syndrome (ARDS) is mechanical ventilation1. Despite being lifesaving, mechanical ventilation itself can lead to ventilator-induced lung injury (VILI)2, contributing to a high mortality3.
Mechanical ventilation methods for ARDS patients involve preserving spontaneous breathing (SB) or complete muscles paralysis4. In spite of intensive investigations, the role of SB activity in ARDS has not been well defined yet5. Many experimental and clinical studies have also reported that SB with inspiratory muscles activity, especially the diaphragm, can produce negative pleural pressures and transpulmonary pressure, which can improve ventilation distribution6, diminish atelectasis7, and thereby reduce mechanical stress and strain of lung8. It has been proved that preserving diaphragm activity in ventilated ARDS patients is correlated to fewer complications compared with muscles paralysis. The potential benefits include increasing the aeration of dependent lung areas7,9, promoting ventilation-perfusion matching10, improving global hemodynamics and organ perfusion11, decreasing the administration of drugs such as analgesic and sedative12, preventing ventilator-induced diaphragmatic dysfunction(VIDD)13,14, decreasing ventilator-induced lung injury (VILI)15,16 and so on. Thus, some investigators have claimed that SB should be preserved even in the most severe cases of ARDS17.
Nevertheless, little has been known about the effects of expiratory muscles activities during mechanical ventilation in patients with ARDS yet. During mechanical ventilation, expiration is a passive phenomenon generated by the elastic recoil forces of respiratory system. Nonetheless, an increased respiratory drive is prevalent in patients with ARDS. In the existence of an increased respiratory drive, SB with the activity of expiratory muscles, especially abdominal muscles, theoretically can increase positive pleural pressures and intra-abdominal pressure (IAP)18, which can decrease transpulmonary pressure, reduce the end-expiratory “baby lung” volume (EELV), and thereby lead to more alveolar collapse, lung consolidation and lung injury during mechanical ventilation19. Some studies have shown that the increase of IAP, even by 10 cmH2O, may worsen lung injury and cause organs dysfunction20,21. Prasad CV et al.1 revealed that the activation of abdominal muscles can impair pressure-controlled ventilation. A recent study has also demonstrated that the shear force produced by the alveolar opening and closing of lung increases the mortality in ARDS patients22,23.
In view of the advantages and disadvantages of SB during mechanical ventilation in patients with ARDS, it was hypothesized that the activity of inspiratory muscles had beneficial effects, while that of expiratory muscles had adverse effects. Consequently, the expiratory muscle of animal model was paralyzed through epidural anesthesia, and inspiratory muscle through phrenic nerve paralysis, to establish a model maintaining diaphragm (inspiratory muscle) activity and one preserving abdominal muscles (expiratory muscle) respectively. The aim was to explore the impacts and mechanism of inspiratory and expiratory muscles activities during mechanical ventilation in ARDS animal model and test the hypothesis that the demands for deep sedation or paralysis might be replaced by the strategy of expiratory muscles paralysis through epidural anesthesia.
Materials and Methods
This study was approved by the Ethics Committee of Guizhou Medical University. The treatment and care of animals were in accordance with the standards of the university.
Preparation of Animal samples
A total of 24 healthy beagle dogs (9.8–14.5 kg) were studied in the supine position. Anesthesia was completed by using Ketamine and continuous injection of Profocol. Paralysis was achieved with pancuronium. After orotracheal intubation with an 8.0-mm ID cuff tube, animals were ventilated with an EVITA 4 ventilator (Dräger Medical AG, Lübeck Germany). IPPV ventilation was set on at a VT of 10 ml/kg, FiO2 1.0, PEEP 5 cm H2O, and I: E ratio of 1:1. The respiratory rate (RR) was adjusted to keep PaCO2 within 35~45 mmHg. Lactated Ringer’s injection (6 ml/kg/h) was administered for hemodynamic stability. Catheters were inserted into the femoral artery and right jugular vein, and then connected to PiCCO system to measure mean arterial blood pressure (MPA), cardiac output and body temperature. Arterial blood samples were obtained using catheter and analyzed immediately.
Airway pressure (Paw), esophageal pressure (Peso) and intragastric pressure (Pgas) were recorded by using a multi-pair esophageal electrode-balloon combined catheter placed into the esophagus, the position of which was optimized with occlusion technique24. Airflow was measured by respiratory flow head, and integrated to obtain tidal volume. Powerlab 16/30 SP and Labchart 7.2 software on Macbook were applied to record the signals of Paw, Peso, Pgas, airflow, abdominal muscles surface electromyography (EMGab) and diaphragmatic esophageal surface electromyography (EMGdi). Animals’ body temperature was maintained at 37 °C with a heating pad, and averaged over eight breaths to calculate pressures, tidal volume, and respiratory rate.
Experimental Protocol
After 30 min of stabilization and measurements at baseline, lung injury model was achieved through intravenous injection of 0.3 ml/kg purified oleic. If needed, additional infusion oleic acid (0.2 ml each time) would be given until PaO2/FiO2 became less than 100 mmHg. When the PaO2/FiO2 ratio were consistently below 100 mmHg for 30 min, a stable model of severe ARDS was considered to be established successfully25,26,27.
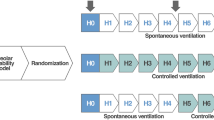
After the establishment of ARDS model and collection of data, the ventilator was switched to BIPAP mode, then the animals were randomly classified into four groups: (1) Spontaneous breathing group (BIPAPSB, n = 6), both inspiratory and expiratory muscles activities were preserved; (2) Complete muscle paralysis group (BIPAPPC, n = 6), treated with neuromuscular blocking agent (Pipecuronium bromide of 0.08 mg/kg): both inspiratory and expiratory muscles activities were absent; (3) Inspiratory muscles activity group (BIPAPAI, n = 6), treated with lumbar epidural anesthesia (ropivacaine hydrochloride at a speed of 1–2 ml/h for 8 h): inspiratory activities was preserved but expiratory muscles activities was absent; (4) Expiratory muscles activity group (BIPAPAE, n = 6), treated with phrenic nerve transection: inspiratory activities was absent but expiratory muscles activities was preserved.
For BIPAPPC group, Phigh was titrated to achieve VT ≈ 6 ml/kg. Plow was set at 10 cmH2O, FiO2 1.0, and fixed I: E = 1:1 to minimize mean Paw changes. Mandatory RR was regulated to maintain PaCO2 within 35 to 60 mmHg. For BIPAPSB group, the infusion of pancuronium was stopped to recover SB, and other ventilator settings were the same as those of BIPAPPC group. SB was confirmed by the negative deflection of Peso. For BIPAPAI group, the method of paralyzing abdominal muscles was similar to that described by Warner DO28. An epidural catheter was inserted via the second tail vertebra, and its tip was pushed forward to the position close to L4 or L5 lumbar vertebrae in the epidural space confirmed by visual observation or autopsy. 2% lidocaine was injected slowly into incremental doses of 0.5 ml via the epidural catheter until the EMGab was abolished. The subsequent continuous infusion of ropivacaine at a speed of 1–2 ml/h and other ventilator settings were the same as those of BIPAPSB group. As for BIPAPAE group, preserving expiratory muscles activity alone was achieved through phrenic nerve transection, and other ventilator settings were the same as those of BIPAPSB group.
All measurements were performed every 2 hours. PL were calculated by the difference between Paw and Peso. During BIPAP ventilation mode, mean Paw can be calculated as follows29,30: (Phigh × Thigh + Plow × Tlow)/(Thigh + Tlow), where Thigh is the length of time for Phigh, and Tlow is that for Plow. When RR was adjusted to fix Thigh: Tlow ratio at 1:1, mean Paw could keep constant at (Phigh + Plow)/2. With the above method, the mean Paw of all experimental groups was maintained the same, regardless of the existence of SB. A simplified closed-circuit helium dilution method was utilized to measure EELV at Plow10 cmH2O during an end-expiratory pause31. Dead space/tidal volume ratio (VD/VT) was calculated by using Enghoff equation32. Samples of IL-6 and IL-8 in plasma were collected before and after the induction of lung injury at the end of the 8 h of MV. Supernatant aliquots were frozen at −80 °C for analysis after being centrifuged at 3,000 rpm for 15 min. An ELISA kit for dogs was employed to measure the Plasma levels of IL-6 and IL-830. After eight hours of ventilation, the animals were euthanized with 20 ml of intravenous 10% potassium chloride. Five sections in the right upper, middle and lower lobes were stained with hematoxylin and eosin for pathological analysis. Lung tissue was examined by a pathologist blinded to the group allocations. Based on combined pathomorphological changes criteria, lung injury severity was rated on a five-point scale, involving alveolar congestion, alveolar edema and interstitial edema, lymphocytes infiltration, erythrocytes infiltration and granulocytes infiltration, micro thrombi as well as fibrinous exudates. Each sample was graded as follows15,33: minimal changes: 0; mild: 1, moderate: 2; severe: 3; maximal changes: 4. The sum of graded scores was the total histopathological lung injury score.
Statistical Analysis
All data are represented as means ± SD. Kolmogorov–Smirnov test was adopted to assess normal distribution. Paired t-test was utilized to compare the continuous data of the same group before and after the interventions. Multiple-group comparisons were made through ANOVA or Kruskal-Wallis test as appropriate. Repeated measures ANOVA were applied to test respiratory variables changes between different time points and groups, and a post hoc analysis was performed following LSD-t procedure as appropriate. IBM SPSS Statistics 21 was used for statistical analyses, and P < 0.05 was considered to be statistical significance of difference.
Results
In fact, a total of 27 beagle dogs were employed, and 24 of them finished the experiment. At baseline, no significant differences were observed in HR, MPA, OI and respiratory mechanics parameters. After inducing injury, the gas exchange worsened, and the values of OI decreased below100 mmHg. Besides, significant differences were observed compared with the values of OI at baseline in all experimental groups.
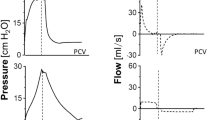
Figure 1 shows the tracing records of Paw, Pes, Pgas, PL, Airflow, EMGab and EMGdi in four groups of representative animals. The mean Paws were comparable for all groups during the entire experiment. SB occurred rarely at Phigh in all groups. Due to its preserving of both inspiratory and expiratory muscles activities, BIPAPSB group presented larger fluctuations of Pes, Pgas and peak PL compared with other groups, and kept the ratio of SB to total MV above 60%. In BIPAPPC group, no inspiratory nor expiratory muscles activity was observed, so its Peso showed a positive change in the inspiratory phase. In BIPAPAI group, which showed only inspiratory muscles activity, presented lower ΔPeso, Pgas, peak PL, more even PL and longer time at Phigh compared with BIPAPSB group, and the ratio of SB to total MV decreased from 60%~100% to 10%~50%. In BIPAPAE group, Peso showed no negative swing and was kept in positive range in the inspiratory phase.
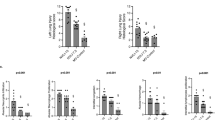
As shown in Table 1, MPAs were similar among the groups during the entire experiment. The levels of PaCO2 were below 60 mmHg in all animals. However, BIPAPAI and BIPAPSB groups, which preserved inspiratory muscles activity presented with higher EELV than BIPAPPC and BIPAPAE groups respectively (p < 0.05) (Fig. 2). In addition, BIPAPAI group resulted in a lower VD/VT compared with the other three groups after 2 h of ventilation (p < 0.05). The VD/VT in BIPAPSB group tended to be lower than those in BIPAPPC and BIPAPAE groups after 2 h of ventilation, and reached significant differences after 6 h of ventilation (p < 0.05) (Fig. 3). BIPAPAI group resulted in a higher OI compared with the other three groups after 2 h of mechanical ventilation. BIPAPSB group presented a higher OI than BIPAPPC and BIPAPAE groups did after 6 h of ventilation (p < 0.05).
BIPAPSB = biphasic positive airway pressure with SB; BIPAPPC = biphasic positive airway pressure with muscles paralysis; BIPAPAI = biphasic positive airway pressure with inspiratory muscles activity; BIPAPAE = biphasic positive airway pressure with expiratory muscles activity. SB = spontaneous breathing; *P < 0.05, vs. other groups.
BIPAPSB = biphasic positive airway pressure with SB; BIPAPPC = biphasic positive airway pressure with muscles paralysis; BIPAPAI = biphasic positive airway pressure with inspiratory muscles activity; BIPAPAE = biphasic positive airway pressure with expiratory muscles activity. SB = spontaneous breathing; *P < 0.05, vs. other groups.
As indicated in Fig. 4: Plasma levels of IL-6 and IL-8 were comparable among groups before and after the induction of lung injury. After 8 h of MV, the lowest IL-8 levels was observed in BIPAPAI group, and the highest IL-6 and IL-8 levels in plasma was observed in BIPAPAE group when compared with other groups (p < 0.05).
BIPAPSB = biphasic positive airway pressure with SB; BIPAPPC = biphasic positive airway pressure with muscles paralysis; BIPAPAI = biphasic positive airway pressure with inspiratory muscles activity; BIPAPAE = biphasic positive airway pressure with expiratory muscles activity. SB = spontaneous breathing; NS = no significantly difference. *P < 0.05, vs. other groups.
As displayed in Table 2, BIPAPAI and BIPAPSB groups, which preserved inspiratory muscles activity, resulted in a lower sum of lung injury scores and wet/dry weight ratio (Fig. 5) in lung tissues compared with BIPAPPC and BIPAPAE groups (p < 0.05). BIPAPAI group presented less lung congestion, alveolar edema, alveolar infiltration of neutrophils and interstitial infiltration of lymphocyte. BIPAPAE group showed more alveolar collapse, inflammatory cell infiltration, alveolar congestion, greater thickness of alveolar wall, and interstitial edema with hyaline membrane formation (Fig. 6).
BIPAPSB = biphasic positive airway pressure with SB; BIPAPPC = biphasic positive airway pressure with muscles paralysis; BIPAPAI = biphasic positive airway pressure with inspiratory muscles activity; BIPAPAE = biphasic positive airway pressure with expiratory muscles activity. SB = spontaneous breathing; NS = no significantly difference, *P < 0.05 vs. BIPAPSB and BIPAPAI groups.
BIPAPSB = biphasic positive airway pressure with SB; BIPAPPC = biphasic positive airway pressure with muscles paralysis; BIPAPAI = biphasic positive airway pressure with inspiratory muscles activity; BIPAPAE = biphasic positive airway pressure with expiratory muscles activity. The BIPAPAI group had minimal alveolar congestion, and inflammatory cell infiltration. The BIPAPAI group showed mild thickening of the alveolar walls, alveolar congestion, and hemorrhage. In the BIPAPAE group, inflammatory cell infiltration, thickening of the alveolar walls, alveolar congestion, and more prominent hemorrhagic areas were observed.
Discussion
On the basis of the ARDS animal model, the research findings indicate that the activation of inspiratory muscles increased EELV, improved oxygenation and decreased lung injury scores. On the contrary, the activation of expiratory muscles decreased EELV, worsened oxygenation and increased lung injury scores. That is to say, inspiratory and expiratory muscles had different impacts on ARDS animal model during mechanical ventilation. The activation of inspiratory muscles (diaphragm) had beneficial effects, while that of expiratory muscles (abdominal muscle) exerted adverse effects. Before discussing the results of this experiment, the following items need to be explained. An oleic acid-induced ARDS model with many basic features of ARDS was utilized in this study27. Treatment with a same dose of oleic acid in the same way can produce a reasonable reproducibility of lung damage34. Studies have confirmed an inverse correlation between injurious ventilation and IL-6, IL-8 levels. Hence, we selected IL-6 and IL-8, the most significant inflammatory factors during the mechanical ventilation in ARDS35. A static pressure–volume curve obtained through super syringe method showed that the lower inflection points were around 8–9 cm H2O for injury lungs. In consequence, Plow (PEEP) was set at 10 cm H2O for all experimental animals during mechanical ventilation.
To our knowledge, none of the previous studies has tried to separate the activities of inspiratory and expiratory muscles activities and explored the impacts of inspiratory and expiratory muscles activity during mechanical ventilation in ARDS. With a comparable ventilator setting, this study has proved that the activation of inspiratory muscles could lead to better oxygenation. This outcome can be easily explained. Firstly, inspiratory muscles activity increased EELV in this experiment. It has been proved that an increase in EELV is equivalent to the increase in oxygenation; secondly, it was also observed that inspiratory muscles activity reduced the VD/VT, which has a positive impact on oxygenation. Finally, inspiratory muscles activity improved oxygenation by promoting dorsal-caudal distribution of ventilation, and improving dead space ventilation and ventilation-perfusion matching.
Based on the findings of this research, the total lung injury score, wet/dry weight ratio in lung tissues as well as IL-6 and IL-8 levels in plasma were lower in BIPAPAI groups. This outcome is similar to those of other studies with mild or moderate ARDS models15,16. From the represented tracing in the experiment, it could be observed that inspiratory muscles activity resulted in increased transpulmonary pressure at Plow (PEEP) without increasing transpulmonary pressure at Phigh. Increased transpulmonary pressure at Plow recruited collapsed lung units and favored more aeration into dependent regions, while increased EELV improved lung mechanical stress distribution, and reduced stress and strain (VT/EELV), as well as the major determinant of VILI. Furthermore, more aeration into dependent regions attenuated lung tissue recruitment and decruitment cycling, decreased hyperinflation in non-dependent lung zones, and thereby reduced lung injury.
In contrary to inspiratory muscles, the findings suggest that the activation of expiratory muscles worsen oxygenation. Douglas et al. have proved that EELV is parallel to oxygenation36. In this experiment, lower oxygenation was observed in BIPAPAE groups as expiratory muscles activity decreased the EELV. It was also observed that expiratory muscles activity resulted in an increase of PTP, which means the work of breathing and oxygen consumption increased. In addition, the activation of expiratory muscles elevated IAP, decreased PL, reduced lung volume and increased compression atelectasis or consolidation37. The above factors led to a greater dead space and a higher heterogeneity of ventilation-perfusion ratio38, and thereby worsen gas exchange.
In patients with ARDS, the relationship between expiratory muscles activity and VILI is not clear. Henzler D et al.20 have proven that respiratory muscles activity during mechanical ventilation would cause greater lung damage in the presence of IAP. This study has also demonstrated that expiratory muscles activity would increase the W/D ratio, total lung injury scores and system inflammation. The potential mechanisms are as followings: Firstly, expiratory muscles activity could increase the value of ΔPes, which can promote the formation of pulmonary edema and aggravate lung injury37. Secondly, the activation of expiratory muscles could significantly increase Pgas, a surrogate of IAP. The activation of expiratory muscles, particularly abdominal muscles, can raise IAP even higher than 20 cm H2O39. Hence, the unopposed increase of IAP can cause greater lung injury by reducing PL in dependent zones20. Thirdly, the activation of expiratory muscles could counteract the effect of PEEP of recruiting the collapsed lung, which would result in atelectrauma. Moreover, it was observed that the inactivation of expiratory muscles resulted in more even PL and prolonged Thigh which was presumed to achieve the aim of therapy for alveolar recruitment and attenuate lung injury; Reducing the high ratio of SB to total MV to 10~30% as clinically recommended during BIPAP mode of ventilation could decrease peak PL and attenuate lung injury7. Finally, it was also observed the activation of expiratory muscles resulted in the reduction of EELV, so atelectrauma, lung strain, and main determinants of VILI may be further increased.
The current study has several major limitations. Firstly, BIPAP ventilated mode was used in this study. Therefore, we are not sure whether these results can be extended to other modes. Secondly due to protective strategy with a LTV used in this experiment, we cannot preclude the opposite effects of inspiratory or expiratory muscles activities on a high tidal volume injurious ventilation; Thirdly, the RR and nervous distribution of canine may not be the same as those of human beings. In view of this, it cannot be guaranteed that the the results of this study would be applicable to human patients and further studies are needed. Fourthly, an oleic acid-induced ARDS model was applied in this study, and its findings cannot be extrapolated to other ARDS models. Fifthly, since the long duration of ventilation time may influence the accuracy of the experiment, such as hypercapnia, influence of experimental procedure, and excessive use of drugs, observation of 8 hours of ventilation was used in this study. Indeed, a more prolonged study period might generate greater physiologic and morphologic difference between the experimental groups. Sixthly, in allusion to BIPAPAP and BIPAPPC groups, ropivacaine hydrochloride was adopted for paralysis, and propofol for anesthesia. Given this, the possibility that these drugs could affect pulmonary inflammatory response cannot be ruled out.
In conclusion, inspiratory and expiratory muscles in this animal model of ARDS have different impacts during mechanical ventilation. The activity of inspiratory muscles has beneficial effects, whilst that of expiratory muscles exerts adverse effects. As a result, the demands for deep sedation or paralysis might be replaced by the strategy of expiratory muscles paralysis through epidural anesthesia in mechanically ventilated patients with ARDS, which could preserve the advantages and avoid the disadvantages of SB. Nonetheless, changes in the management of mechanical ventilation in patients with ARDS require more evidence and a further research is necessary to confirm these results.
Additional Information
How to cite this article: Zhang, X. et al. An experimental study on the impacts of inspiratory and expiratory muscles activities during mechanical ventilation in ARDS animal model. Sci. Rep. 7, 42785; doi: 10.1038/srep42785 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Prasad, C. V. & Drummond, G. B. Abdominal muscle action during expiration can impair pressure controlled ventilation. Anaesthesia 59, 715–718 (2004).
Shekar, K. & Fraser, J. F. Ventilator-induced lung injury. N. Engl. J. Med. 370, 979 (2014).
Roy, S. et al. Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock 39, 28–38 (2013).
Papazian, L. et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 363, 1107–1116 (2010).
Guldner, A. et al. Spontaneous breathing in mild and moderate versus severe acute respiratory distress syndrome. Curr Opin Crit Care 20, 69–76 (2014).
Putensen, C. et al. Interfacing between spontaneous breathing and mechanical ventilation affects ventilation-perfusion distributions in experimental bronchoconstriction. Am. J. Respir. Crit. Care Med. 151, 993–999 (1995).
Wrigge, H. et al. Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care 9, R780–789 (2005).
Guldner, A. et al. Higher levels of spontaneous breathing induce lung recruitment and reduce global stress/strain in experimental lung injury. Anesthesiology 120, 673–682 (2014).
Wrigge, H. et al. Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology 99, 376–384 (2003).
Yoshida, T. et al. The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: airway pressure release ventilation versus pressure support ventilation. Anesth. Analg. 109, 1892–1900 (2009).
Slutsky, A. S. & Tremblay, L. N. Multiple system organ failure. Is mechanical ventilation a contributing factor. Am. J. Respir. Crit. Care Med. 157, 1721–1725 (1998).
Schmidt, U. H. & Hess, D. R. Does spontaneous breathing produce harm in patients with the acute respiratory distress syndrome. Respir Care 55, 784–786 (2010).
Vassilakopoulos, T. & Petrof, B. J. Ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 169, 336–341 (2004).
McCool, F. D. & Tzelepis, G. E. Dysfunction of the diaphragm. N. Engl. J. Med. 366, 932–942 (2012).
Xia, J. et al. Spontaneous Breathing with Biphasic Positive Airway Pressure Attenuates Lung Injury in Hydrochloric Acid-induced Acute Respiratory Distress Syndrome. Anesthesiology 120, 1441–1449 (2014).
Carvalho, N. C. et al. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Crit. Care Med. 42, e702–715 (2014).
Putensen, C. et al. The impact of spontaneous breathing during mechanical ventilation. Curr Opin Crit Care 12, 13–18 (2006).
Iscoe, S. Control of abdominal muscles. Prog. Neurobiol. 56, 433–506 (1998).
Zhang, X. et al. Correction: Abdominal Muscle Activity during Mechanical Ventilation Increases Lung Injury in Severe Acute Respiratory Distress Syndrome. PLoS ONE 11, e0149325 (2016).
Henzler, D. et al. Effects of preserved spontaneous breathing activity during mechanical ventilation in experimental intra-abdominal hypertension. Intensive Care Med 36, 1427–1435 (2010).
Malbrain, M. L. et al. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care 11, 156–171 (2005).
Caironi, P. et al. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 181, 578–586 (2010).
Pelosi, P. et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am. J. Respir. Crit. Care Med. 164, 122–130 (2001).
Baydur, A. et al. A simple method for assessing the validity of the esophageal balloon technique. The American review of respiratory disease 126, 788–791 (1982).
Suh, G. Y. et al. Partial liquid ventilation with perfluorocarbon improves gas exchange and decreases inflammatory response in oleic acid-induced lung injury in beagles. J. Korean Med. Sci. 14, 613–622 (1999).
Schweiger, J. W. et al. Chest wall disruption with and without acute lung injury: effects of continuous positive airway pressure therapy on ventilation and perfusion relationships. Crit. Care Med. 31, 2364–2370 (2003).
Wang, H. M. et al. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res 40, 305–316 (2008).
Warner, D. O. et al. Chest wall motion during epidural anesthesia in dogs. J. Appl. Physiol. 70, 539–547 (1991).
Frawley, P. M. & Habashi, N. M. Airway pressure release ventilation: theory and practice. AACN Clin Issues 12, 234–246; quiz 328–329 (2001).
Xia, J. et al. Effect of spontaneous breathing on ventilator-induced lung injury in mechanically ventilated healthy rabbits: a randomized, controlled, experimental study. Crit Care 15, R244 (2011).
Patroniti, N. et al. Lung volume in mechanically ventilated patients: measurement by simplified helium dilution compared to quantitative CT scan. Intensive Care Med 30, 282–289 (2004).
Hardman, J. G. & Aitkenhead, A. R. Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesth. Analg. 97, 1846–1851 (2003).
Dembinski, R. et al. Pumpless extracorporeal lung assist for protective mechanical ventilation in experimental lung injury. Crit. Care Med. 35, 2359–2366 (2007).
Matute-Bello, G. et al. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L379–399 (2008).
Binnie, A. et al. Biomarkers in acute respiratory distress syndrome. Curr Opin Crit Care 20, 47–55 (2014).
Douglas, W. W. et al. Improved oxygenation in patients with acute respiratory failure: the prone position. The American review of respiratory disease 115, 559–566 (1977).
Daoud, E. G. et al. Airway pressure release ventilation: what do we know. Respir Care 57, 282–292 (2012).
Esquer, C. et al. Mechanisms of hypoxemia episodes in spontaneously breathing preterm infants after mechanical ventilation. Neonatology 94, 100–104 (2008).
Duggan, J. E. & Drummond, G. B. Abdominal muscle activity and intraabdominal pressure after upper abdominal surgery. Anesth. Analg. 69, 598–603 (1989).
Acknowledgements
This study was supported by National Nature Science Foundation of China (No. 81361128003), National Nature Science Foundation of China (No. 81490534), National Nature Science Foundation of China (No. 81660018), and the Health and Family Planning Commission Foundation of Guizhou Province (Grant No. gzwjkj2015-1-034034).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: R.C., X.Z., Y.Z. and J.D. Performed the experiments: X.Z., W.W., Y.Z., Y.J. and R.C. Analyzed the data: R.C., X.Z., W.W. and Y.Z. Contributed reagents/materials/analysis tools: R.C., X.Z. W.W. and Y.Z. Wrote the paper: X.Z. and R.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, X., Du, J., Wu, W. et al. An experimental study on the impacts of inspiratory and expiratory muscles activities during mechanical ventilation in ARDS animal model. Sci Rep 7, 42785 (2017). https://doi.org/10.1038/srep42785
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42785
This article is cited by
-
Effect and mechanical mechanism of spontaneous breathing on oxygenation and lung injury in mild or moderate animal ARDS
BMC Pulmonary Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.