Abstract
Neonicotinoids are widely used insecticides, but their use is subject of debate because of their detrimental effects on pollinators. Little is known about the effect of neonicotinoids on other beneficial insects such as parasitoid wasps, which serve as natural enemies and are crucial for ecosystem functioning. Here we show that sublethal doses of the neonicotinoid imidacloprid impair sexual communication and host finding in the parasitoid wasp Nasonia vitripennis. Depending on the dose, treated females were less responsive to the male sex pheromone or unable to use it as a cue at all. Courtship behaviour of treated couples was also impeded resulting in a reduction of mating rates by up to 80%. Moreover, treated females were no longer able to locate hosts by using olfactory cues. Olfaction is crucial for the reproductive success of parasitoid wasps. Hence, sublethal doses of neonicotinoids might compromise the function of parasitoid wasps as natural enemies with potentially dire consequences for ecosystem services.
Similar content being viewed by others
Introduction
The use of insecticides is a relatively cheap and effective way to control insect pests, but it poses ecological problems by also affecting non-target organisms1. This is particularly true for chemically stable broad-spectrum insecticides that might persist in the environment and accumulate in food chains. Apart from directly causing mortality or malformation, insecticides may also harm insects at sublethal doses, for instance after the consumption of contaminated pollen and nectar2. Since many classes of insecticides are neurotoxins, they can have serious effects on the insects’ sensory system and thus impede their ability to acquire information from their environment3,4. Olfaction is of particular importance for reproduction and survival of most insect species5. Hence, the disruption of olfaction by sublethal doses of insecticides might have severe consequences for the fitness of insects and severely compromise their contribution to ecosystem services3.
Worldwide, the most applied insecticides are the neonicotinoids6,7,8. They act selectively on the insect central nervous system by binding competitively to nicotinergic acetylcholine receptors9. This causes a depolarization block of neuronal firing and inhibits nicotinergic responses even at sublethal doses, which results in an uncontrolled continuous stimulation of the respective neurons and finally the death of the animal. The economic success of neonicotinoids is due to their high effectivity and relatively low toxicity for mammals9. The development of resistances by many insect pests to other classes of insecticides additionally fostered the success of neonictinoids and resulted in a global market share of 28.5%7. Neonicotinoids are typically used as seed coatings10,11 and act systemically, i.e., after germination, the pesticides are absorbed by the roots and transported via the xylem to the aboveground parts of the plant. They can be detected not only in the plant tissue but also in guttation drops12, nectar and pollen10,13 and are thus, accessible to non-target insects14. Because of their massive use in agriculture and a relatively high stability, neonicotinoids are persistent in the environment and occur even in ground water14.
There is ample literature indicating detrimental effects of neonicotinoids on pollinators1,15. In honeybees, sublethal doses of neonicotinoids impair orientation behaviour and foraging efficiency and cause increased mortality due to homing failure16,17,18,19. Neonicotinoid treated honeybee workers show decreased sucrose responses (proboscis extension reflex) and are less active in performing “waggle dance”, an important component of forager recruitment20. Furthermore, neonicotinoids have negative effects on the immune system of honeybees21. In bumblebees, exposure to sublethal doses of neonicotinoids decrease foraging efficiency, fecundity and colony performance22,23,24. A recent field study has revealed that coating of oilseed rape seeds with a combination of neonicotinoids and pyrethroids reduces wild bee abundance, solitary bee nesting, as well as bumblebee colony growth and reproduction10. Therefore, the extensive use of neonicotinoids is discussed as an important factor contributing to the currently observed decline of wild bee diversity and abundance21,23. As a consequence, the European Union has decreed in 2013 a moratorium on the three most purchased neonicotinoids imidacloprid, chlothianidin and thiamethoxam as seed coatings for crops that attract bees25.
Besides pollinators, natural enemies of insect pests such as parasitoid wasps also perform key ecosystem services26,27. Larvae of parasitoid wasps develop in or on the body of other arthropods and kill their host at the end of development. Hence, parasitoid wasps are indispensable as natural enemies and are used for biological control of insect pests27,28,29. Many species of parasitoid wasps visit flowers and feed on nectar and pollen30. Thus parasitoid wasps are, like bees, at risk of ingesting neonicotinoids and other systemic pesticides. It has been shown that the uptake of nectar containing neonicotinoids may impair the host finding ability of females31, but little is known about the sublethal effects of neonicotinoids on the sexual communication of parasitoid wasps.
In the present study, we investigate the effects of sublethal doses of imidacloprid on sexual communication and olfactory host finding in the jewel wasp Nasonia vitripennis, which parasitizes the pupae of several fly species. Pheromones are involved at different levels of sexual communication in N. vitripennis. Males produce in their rectum a mixture of (4 R,5 R)- and (4 R,5 S)-5-hydroxy-4-decanolides (HDL) and 4-methylquinazoline that is highly attractive to virgin females32,33. Males recognise potential mates by female-derived cuticular hydrocarbons that elicit stereotypic courtship behaviour34,35,36. After recognition of a female, the male mounts the female and performs precopulatory courtship involving a characteristic “head nodding” behaviour that serves the release of a yet unidentified aphrodisiac pheromone, which is essential for the female to signal receptivity34,37. The female signals receptivity by lowering her head and antennae and simultaneously opening her genital orifice. Subsequently, the male establishes genital contact and copulates with the female. After copulation, the male remounts the female and performs postcopulatory courtship that consists of the same elements as the precopulatory courtship34,38,39. After mating, the female is no longer attracted to the male pheromone and begins to search for a suitable host patch for oviposition32,37,40. Olfactory stimuli are also of crucial importance for host selection41,42.
In the present study, we investigate the toxic effects of externally applied imidacloprid for N. vitripennis wasps and, using standardized olfactometer bioassays, demonstrate that sublethal imidacloprid doses impede the ability of N. vitripennis females to orient toward both the male sex pheromone and host odour. Furthermore, we show that the courtship behaviour of N. vitripennis is impaired and mating rates are reduced if one or both partners are treated with sublethal doses of imidacloprid.
Results
Determination of LD50
Most previous studies on the effects of neonicotinoids on non-target insects offered the active substance with food17,18,21,22,23,24,43,44,45,46,47,48. This approach, however, does not allow for the accurate quantification of the incorporated dose, because the amount of food consumed is difficult to determine. Therefore, we applied imidacloprid as acetone solutions to the abdominal tip of cold-sedated N. vitripennis. With this technique, the dose causing 50% mortality (LD50) within a 72 h observation period was 8 ng per wasp (Fig. 1). In the bioassays, we used doses between 0.1 and 1.1 ng per wasp (1.25–13.75% of the LD50) to study the effects of sublethal imidacloprid doses on the sexual communication and host finding in N. vitripennis.
Mortality rates after 72 h of N. vitripennis treated with different doses of the neonicotinoid imidacloprid dissolved in acetone (n = 8 replicates with 8 wasps each; overlaying data points for each dose were manually shifted horizontally for clarity). The 95% confidence interval is indicated in darker grey. The dashed line indicates the LD50.
Effect of sublethal imidacloprid doses on female response to the male sex pheromone
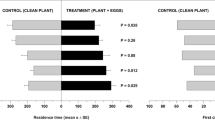
We treated virgin females with acetone (control), 0.1, 0.4 or 1.1 ng of imidacloprid and tested them 1 d after the treatment in a well-established two-choice olfactometer test49. Treated females were given the choice between synthetic male sex pheromone and pure solvent. The attraction of virgin females to the male sex pheromone decreased with increasing imidacloprid dose (Kruskal-Wallis test: H = 15.18; p < 0.001, Fig. 2A) and females treated with 1.1 ng no longer discriminated between the male pheromone and the solvent control. This effect persisted 3 d after the treatment (Kruskal-Wallis test: H = 14.71; p < 0.001, Fig. 2B).
Females were treated with solutions of imidacloprid in acetone or pure solvent (control) and tested (A) one or (B) three days after the treatment in a two-choice olfactometer. Given here are the durations females stayed in odour fields treated with the pheromone (P) or the pure solvent (C, control) during an observation time of five minutes. Box-and-whisker plots show median (horizontal line), 25–75% quartiles (box), maximum/minimum range (whiskers) and outliers (>1.5 × above box height). Statistical analysis for each treatment by Wilcoxon matched pairs test (n = 20). Comparisons between treatments by Kruskal-Wallis H-test (data given in the results) followed by multiple pairwise Mann-Whitney U-tests with sequential Bonferroni correction. Different lowercase letters within each panel indicate significant differences at p < 0.05.
Effect of sublethal imidacloprid doses on female response to host odour
In a bioassay using another type of still-air olfactometer40 mated females were given the choice between the odour of five host puparia and an unscented control. While acetone-treated control females were attracted to the host odour, females treated with 0.4 ng imidacloprid no longer preferred the volatile host cues over the unscented control (Fig. 3).
Females were treated with a solution of imidacloprid in acetone or the pure solvent (control) and tested in a two-choice-olfactometer. Given here are the durations females stayed in one of two odour fields scented with either five pupae of the green bottle fly, Lucilia caesar, or left unscented (control) during an observation time of five minutes. Box-and-whisker plots show median (horizontal line), 25–75% quartiles (box), maximum/minimum range (whiskers) and outliers (>1.5 × above box height). Statistical analysis by Wilcoxon matched pairs test (n = 20).
Effect of sublethal imidacloprid doses on courtship behaviour
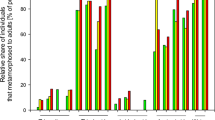
To study the sublethal effects of imidacloprid on the courtship behaviour of N. vitripennis, we observed couples in which the male, the female or both partners had been treated with acetone (control), 0.1, 0.4 or 1.1 ng imidacloprid. If only the male was treated, the mating rate was significantly lower at the medium and highest dose when compared to solvent-treated controls (Freeman-Halton test across treatments: p < 0.001, Fig. 4A). Copulations were significantly delayed at all of the tested doses (Kruskal-Wallis test: H = 12.99, p = 0.005, Fig. 5A). Additionally, several typical courtship elements performed by imidacloprid-treated males were delayed in a dose-dependent manner (Fig. S1a–d). If only the female was treated, the mating rate was decreased only at the highest dose (Freeman-Halton test across treatments: p < 0.001, Fig. 4B). However, when copulations did occur under these conditions, already 0.4 ng imidacloprid delayed receptivity signalling by females (Kruskal-Wallis test: H = 9.77 p = 0.021, Fig. 5B) suggesting that the perception of the male aphrodisiac pheromone was impaired in these females. In couples with both partners treated, mating rates decreased at all tested imidacloprid doses by up to 80% (Freeman-Halton test across treatments: p < 0.001, Fig. 4C).
Mating rates of N. vitripennis couples with (A) the male, (B) the female or (C) both partners treated with a solution of imidacloprid in acetone or pure solvent (control). P-values refer to comparisons between different imidacloprid doses and the control mating rate (=100%; statistical analysis by Fisher’s exact test, n = 20 for each experiment; Freeman-Halton test across treatments significant for all panels at p < 0.001).
(A) Time between start of the observation and copulation in couples with treated males. (B) Time between ‘male mounts the female’ and ‘female shows receptivity signal’ in couples with treated females. Wasps were treated with a solution of imidacloprid in acetone or the pure solvent (Con). Numbers of replicates (given in parentheses) differ because only those couples that started courtship within 5 min were included. Box-and-whisker plots show median (horizontal line), 25–75% quartiles (box), maximum/minimum range (whiskers) and outliers (>1.5 × above box height). Different lowercase letters indicate significant differences at p < 0.05; statistical analysis by Kruskal-Wallis test (data given in the results) followed by multiple pairwise Mann-Whitney U-tests with sequential Bonferroni correction.
Discussion
The present study sheds light on a hitherto neglected non-target effect of neonicotinoids by showing that sublethal doses of imidacloprid disrupt the sexual communication in a parasitoid wasp. Virgin N. vitripennis females treated with imidacloprid were hampered in their ability to locate the male sex pheromone. Their delayed receptivity signaling during courtship furthermore indicates that they were also less responsive to the male aphrodisiac. Likewise, imidacloprid-treated males were less effective in recognising females and performing courtship suggesting that they were impaired in perceiving the female-derived cuticular hydrocarbons. This might explain why the mating rates of imidacloprid-treated couples decreased.
The use of host-associated chemical cues for host finding was impeded in imidacloprid-treated N. vitripennis females. This result and previous findings in a braconid wasp parasitizing lepidopteran larvae31 suggest that sublethal neonicotinoid doses affect the host finding ability of parasitic wasps adversely. Mate and host finding are fitness-relevant competencies for parasitoid wasps. Therefore, the effects observed here as well as the decreased fecundity and survival found in other species after exposure to neonicotinoids2,50, likely compromise the key function of parasitoid wasps as natural enemies in terrestrial ecosystems. More subtle fitness-relevant effects caused by neonicotinoids such as a hampered ability to adjust the optimal offspring sex ratio45,51 might additionally decrease the reproductive success of parasitoid wasps in the field.
The estimated economic value attributed to biological control by predators and parasitoids in the USA amounts to a total of 4.5 billion US$ per year26. Hence, a lower effectiveness of parasitoid wasps caused by sublethal effects of neonicotinoids may not only have severe ecological but also economic consequences. There is no reason to assume that the reported effects on sexual communication and foraging behaviour are restricted to parasitoid wasps. Given that similar effects on the chemical communication will likely be found in other insect taxa, the ecological and economic consequences might be even more severe because of the key role insects play as prey for animals from higher trophic levels26. A recently shown correlation between imidacloprid concentrations in the ground water and the decline of insectivorous birds in the Netherlands corroborate these concerns52.
An important question is whether the tested doses (0.1–1.1 ng/wasp) are realistic under field conditions. Parasitic wasps may come into contact with sublethal doses of systemic neonicotinoids by feeding on contaminated floral and extrafloral nectar or guttation drops of treated plants. Guttation drops of plants grown from imidacloprid-treated seeds may contain very high levels of the active substance (10–200 ppm12). The consumption of a few nanolitres of those guttation drops would result in doses comparable to the ones used here. It is unknown, however, whether parasitoid wasps or other insects consume guttation drops7. The neonicotinoid concentrations in nectar from treated plants are highly variable7,14. An average maximum level in nectar of 1.9 ppb (based on 20 published studies) has been reported7 suggesting that this is an unlikely option for parasitoid wasps to ingest the amounts tested here. However, a recent study on Eucalyptus trees reported much higher levels (660 ppb), which were shown to be sufficient to cause decreased survival and fitness in four parasitoid species53. Furthermore, our results suggest that there is a long-term effect of imidacloprid (Fig. 2B), indicating that detrimental effects may accumulate during long-term exposure to very low doses54. Finally, orally consumed neonicotinoids might have even stronger effects than the externally applied doses used here. Exposure of N. vitripennis females to 200 ppb imidacloprid (dissolved in sucrose solution), for instance, resulted in 50% mortality after 72 h and impeded optimal sex allocation by the females45. The reported average maximum level of neonicotinoids in nectar (1.9 ppb7) is approximately 1% of this concentration. In our study, we observed significant effects at 1–5% of the LD50 determined for our topical application method. This suggests that exposure of females to contaminated nectar might have similar effects on the chemical orientation of N. vitripennis under natural conditions. An alternative way by which parasitoid wasps may take up neonicotinoids is via the host. Juvenile stages of parasitoid wasps may ingest the toxins when feeding on a contaminated host and the same is true for adult females when performing host feeding prior to oviposition2. Also, the mere contact of parasitoid wasps with plant leaves that had been treated with systemic neonicotinoids has been shown to cause negative effects on survival of the wasps indicating that a transfer of sublethal doses might be possible by that way50.
Many insecticides are neurotoxins that impede the ability of insects to acquire and process information from their environment. Consequently, insecticides other than neonicotinoids, such as organophosphates and pyrethroids, have also been shown to interfere at sublethal doses with the sexual communication and foraging success of insects including parasitoid wasps55,56,57,58,59,60. It therefore seems reasonable to include the study of sublethal effects of insecticides and other anthropogenic substances on the chemical communication of beneficial insects during the approval process for novel substances. The Nasonia system is ideal to develop the standardized methods for this pupose, as the pheromones are well-characterised, effective behavioural bioassays are available, and the insects are easily reared in quantity. Thus, the present study not only provides decision makers with important knowledge on hitherto neglected non-target effects of neonicotinoids but also offers tools to study these effects in candidate pesticides to be tested for approval in the future.
Methods
Insects
The N. vitripennis wasps used in this study originated from the inbred strain Phero01 and were reared on freeze-killed puparia of the green bottle fly Lucilia caesar as described previously35. To obtain virgin wasps of defined age, parasitoid pupae were excised from host puparia 1–2 days prior to eclosion and kept singly in 1.5 ml microcentrifuge tubes. Each wasp was used only once in the experiments. Treatment with imidacloprid or solvent (control) was performed 0–12 h after eclosion.
Determination of LD50
Acetone solutions (210 nl) containing 0.0, 0.4, 1.1, 2.7, 5.7, 11.8, and 24.0 ng (n = 4 replicates with eight wasps per dose, sex and replicate) imidacloprid were applied to the abdominal tip of cold sedated wasps using a Nanoliter 2010 microinjector mounted to a micromanipulator (World Precision Instruments). Because of the quantitative absorption of the solution through the anal orifice, this approach allowed control of the exact dose taken up by the insects and determination of the LD50. Previous studies revealed that low volumes of acetone applied by this method have no visible toxic effects on N. vitripennis wasps61,62,63. Mortality was determined after 72 h and the LD50 was interpreted from the dose-mortality curve calculated using the polynomial regression of the R package ggplot264.
Effect of sublethal imidacloprid doses on the female response to the male sex pheromone
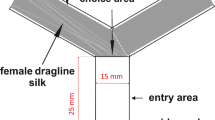
The effect of imidacloprid (0.0, 0.1, 0.4 and 1.1 ng/wasp 1 d after treatment; 0.0, 0.4 and 1.1 ng/wasp 3 d after treatment) on the response of females to the male-produced sex pheromone (n = 20 for each treatment) was tested in our well-established two-choice olfactometer (for details see ref. 49). Briefly, 1 μl of the synthetic sex pheromone dissolved in dichloromethane (200 ng/μl RS, 100 ng/μl RR, and 3 ng 4-MQ, synthesized as described in refs 33 and 63) were applied to a disk of filter paper. The ratio of the pheromone components was consistent with the previously reported composition32,33. Control paper disks were treated with the same amount of pure solvent. After evaporation of the solvent, test and control disks were put into the test and control cavity of the olfactometer and females were released individually into its centre. The time females spent in the test and control cavity was recorded for five minutes using The Observer XT observational software (Noldus, Wageningen, The Netherlands). The olfactometer was turned by 90° after every observation to avoid biased results due to side preferences.
Effect of sublethal imidacloprid doses on the female response to host odour
The response to host odour by females treated with 0.4 ng imidacloprid and solvent treated control wasps (n = 20) was tested 1 d after treatment using a linear still-air olfactometer described in detail elsewhere40. Briefly, the olfactometer consisted of an angular acrylic tube (14 cm × 1 cm × 1 cm) that was divided into two 4-cm test zones at either end and a 5-cm neutral zone in the middle. Five hosts were presented at the end of the test zone and visual contact was prevented by a thin sheet of non-transparent gauze. The control zone was designed identically but left empty. Prior to the test, host volatiles were allowed to diffuse into the olfactometer tube for 5 min. Subsequently, N. vitripennis females were released individually into the neutral zone and the time they spent in the test and control zone was recorded for 5 min. The olfactometer was rotated by 180° after every observation to compensate for any unforeseen asymmetry of the set-up.
Effect of sublethal imidacloprid doses on the courtship behaviour
The courtship behaviour of N. vitripennis couples treated with three different sublethal doses of imidacloprid (0.1, 0.4, 1.1 ng per wasp; either males, females or both were treated) and solvent treated control wasps (n = 20 for each treatment) was videotaped 1d after the treatment. Observations lasted until the end of postcopulatory courtship but were terminated after 5 min if couples had not started courtship by that time. Video recordings were analysed using the video module of The Observer XT software. For all observations, the act of copulation was documented and the mating rate for all treatments was calculated. Depending on the treated sex, different elements of the courtship behaviour were also recorded for those couples that did mate. (a) Couples with treated males: (i) time between contact of a male with the female and mounting; (ii) time between receptivity signalling (withdrawing of the antennae by the female) and copulation; (iii) duration of copulation; (iv) time between copulation and remounting the female (after re-mounting, postcopulatory behaviour is shown); (v) total time between start of the observation and copulation. (b) Couples with treated females: (i) time between a male mounting the female and receptivity signalling (lowering of the antennae by the female). This time is correlated to male head nodding duration, a behaviour that is shown by males when applying the aphrodisiac pheromone to the female antennae to elicit receptivity34,37; (ii) total time spent by a male mounting the female. For couples with both partners treated, only the mating rate was recorded.
Statistical analyses
Because not all data met the assumptions for parametric statistical analyses, we used non-parametric tests throughout. The sample size for the experiments (n = 20 for all experiments) was calculated using G-power 3.1.9.2 scientific software65 considering an α-error of 0.05, a minimum statistical power of 0.8 and effect sizes obtained in similar experiments in previous studies (Cohen’s d ≥0.8). Couples that did not copulate within the observation period of five minutes were excluded from the statistical analysis of all courtship parameters related to copulation (e.g. “time until copulation”). Residence times of female wasps in test and control fields of the olfactometers were compared by Wilcoxon matched-pairs tests. The residence times in the pheromone treated odour field of female wasps treated with different imidacloprid doses were compared by Kruskal-Wallis H-tests followed by multiple pairwise Mann-Whitney U-tests with sequential Bonferroni correction. Mating rates of imidacloprid-treated wasps were compared by Freeman-Halton tests followed by Fisher’s exact tests with sequential Bonferroni correction for individual comparisons between treated and control wasps. Durations of individual courtship elements in couples with imidacloprid-treated wasps and untreated control couples were compared by Kruskal-Wallis H-tests followed by multiple pairwise Mann-Whitney U-tests with sequential Bonferroni correction. All statistical analyses were done using Past 3.0 statistical software.
Additional Information
How to cite this article: Tappert, L. et al. Sublethal doses of imidacloprid disrupt sexual communication and host finding in a parasitoid wasp. Sci. Rep. 7, 42756; doi: 10.1038/srep42756 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Pisa, L. W. et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 22, 68–102, doi: 10.1007/s11356-014-3471-x (2015).
Desneux, N., Decourtye, A. & Delpuech, J. M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106, doi: 10.1146/annurev.ento.52.110405.091440 (2007).
Lurling, M. & Scheffer, M. Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol. Evol. 22, 374–379, doi: 10.1016/j.tree.2007.04.002 (2007).
Tricoire-Leignel, H., Thany, S. H., Gadenne, C. & Anton, S. Pest insect olfaction in an insecticide-contaminated environment: info-disruption or hormesis effect. Front. Physiol. 3, 6, doi: 10.3389/fphys.2012.00058 (2012).
Wyatt, T. D. Pheromones and Animal Behaviour. Communication by Smell and Taste. (Cambridge University Press, 2014).
Jeschke, P., Nauen, R., Schindler, M. & Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908, doi: 10.1021/jf101303g (2011).
Godfray, H. C. J. et al. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B-Biol. Sci. 281, 9, doi: 10.1098/rspb.2014.0558 (2014).
Simon-Delso, N. et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34, doi: 10.1007/s11356-014-3470-y (2015).
Matsuda, K. et al. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22, 573–580, doi: 10.1016/s0165-6147(00)01820-4 (2001).
Rundlof, M. et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80, doi: 10.1038/nature14420 (2015).
Alsayeda, H., Pascal-Lorber, S., Nallanthigal, C., Debrauwer, L. & Laurent, F. Transfer of the insecticide C14 imidacloprid from soil to tomato plants. Environ. Chem. Lett. 6, 229–234, doi: 10.1007/s10311-007-0121-2 (2008).
Girolami, V. et al. Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. J. Econ. Entomol. 102, 1808–1815, doi: 10.1603/029.102.0511 (2009).
Dively, G. P. & Kamel, A. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 60, 4449–4456, doi: 10.1021/jf205393x (2012).
Bonmatin, J. M. et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67, doi: 10.1007/s11356-014-3332-7 (2015).
Cresswell, J. E. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20, 149–157, doi: 10.1007/s10646-010-0566-0 (2011).
Yang, E. C., Chuang, Y. C., Chen, Y. L. & Chang, L. H. Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 101, 1743–1748, doi: 10.1603/0022-0493-101.6.1743 (2008).
Fischer, J. et al. Neonicotinoids interfere with specific components of navigation in honeybees. Plos One 9, 10, doi: 10.1371/journal.pone.0091364 (2014).
Henry, M. et al. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350, doi: 10.1126/science.1215039 (2012).
Schneider, C. W., Tautz, J., Grünewald, B. & Fuchs, S. RFID Tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera . PLoS ONE 7, e30023, doi: 10.1371/journal.pone.0030023 (2012).
Eiri, D. M. & Nieh, J. C. A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J. Exp. Biol. 215, 2022–2029, doi: 10.1242/jeb.068718 (2012).
Brandt, A., Gorenflo, A., Siede, R., Meixner, M. & Buchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Ins. Physiol. 86, 40–47, doi: 10.1016/j.jinsphys.2016.01.001 (2016).
Gill, R. J. & Raine, N. E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Func. Ecol. 28, 1459–1471, doi: 10.1111/1365-2435.12292 (2014).
Gill, R. J., Ramos-Rodriguez, O. & Raine, N. E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–U119, doi: 10.1038/nature11585 (2012).
Laycock, I., Lenthall, K. M., Barratt, A. T. & Cresswell, J. E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21, 1937–1945, doi: 10.1007/s10646-012-0927-y (2012).
Commission, E. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxamand imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Union 139, 12–26 (2013).
Losey, J. E. & Vaughan, M. The economic value of ecological services provided by insects. Bioscience 56, 311–323, doi: 10.1641/0006-3568(2006)56[311:tevoes]2.0.co;2 (2006).
LaSalle, J. & Gauld, I. D. Parasitic Hymenoptera and the biodiversity crisis. Redia 74, 315–334 (1991).
Quicke, D. L. J. Parasitic Wasps. (Chapman & Hall, 1997).
Godfray, H. C. J. Parasitoids (Princeton University Press, 1994).
Jervis, M. A., Kidd, N. A. C., Fitton, M. G., Huddleston, T. & Dawah, H. A. Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 27, 67–105, doi: 10.1080/00222939300770051 (1993).
Stapel, J. O., Cortesero, A. M. & Lewis, W. J. Disruptive sublethal effects of insecticides on biological control: Altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biol. Control 17, 243–249, doi: 10.1006/bcon.1999.0795 (2000).
Ruther, J., Stahl, L. M., Steiner, S., Garbe, L. A. & Tolasch, T. A male sex pheromone in a parasitic wasp and control of the behavioral response by the female’s mating status. J. Exp. Biol. 210, 2163–2169, doi: 10.1242/jeb.02789 (2007).
Ruther, J., Steiner, S. & Garbe, L. A. 4-methylquinazoline is a minor component of the male sex pheromone in Nasonia vitripennis . J. Chem. Ecol. 34, 99–102, doi: 10.1007/s10886-007-9411-1 (2008).
van den Assem, J., Jachmann, F. & Simbolotti, P. Courtship behavior of Nasonia vitripennis (Hym., Pteromalidae): some qualitative, experimental evidence for the role of pheromones. Behaviour 75, 301–307, doi: 10.1163/156853980X00456 (1980).
Steiner, S., Hermann, N. & Ruther, J. Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis . J. Chem. Ecol. 32, 1687–1702, doi: 10.1007/s10886-006-9102-3 (2006).
Buellesbach, J., Greim, C., Raychoudhury, R. & Schmitt, T. Asymmetric assortative mating behaviour reflects incomplete pre-zygotic isolation in the Nasonia species complex. Ethology 120, 834–843, doi: 10.1111/eth.12250 (2014).
Ruther, J., Thal, K., Blaul, B. & Steiner, S. Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signalling. Anim. Behav. 80, 1035–1040, doi: 10.1016/j.anbehav.2010.09.008 (2010).
van den Assem, J. Insect Parasitoids, 13th Symposium of the Royal Entomological Society of London (eds Waage, J. & Greathead, D. ) Ch. 5, 137–167 (Academic Press, 1989).
van den Assem, J. & Visser, J. Aspects of sexual receptivity in female Nasonia vitripennis (Hym., Pteromalidae). Biol. Behav. 1, 37–56 (1976).
Steiner, S. & Ruther, J. How important is sex for females of a haplodiploid species under local mate competition? Behav. Ecol. 20, 570–574, doi: 10.1093/beheco/arp033 (2009).
Frederickx, C., Dekeirsschieter, J., Verheggen, F. J. & Haubruge, E. Host-habitat location by the parasitoid, Nasonia vitripennis Walker (Hymenoptera: Pteromalidae). J. Forens. Sci. 59, 242–249, doi: 10.1111/1556-4029.12267 (2014).
Whiting, A. R. Biology of parasitic wasp Mormoniella vitripennis = Nasonia brevicornis (Walker). Quart. Rev. Biol. 42, 333–406, doi: 10.1086/405402 (1967).
Rabhi, K. K. et al. Low doses of a neonicotinoid insecticide modify pheromone response thresholds of central but not peripheral olfactory neurons in a pest insect. Proc. R. Soc. B-Biol. Sci. 283, 7, doi: 10.1098/rspb.2015.2987 (2016).
Thiel, S. & Kohler, H. R. A sublethal imidacloprid concentration alters foraging and competition behaviour of ants. Ecotoxicology 25, 814–823, doi: 10.1007/s10646-016-1638-6 (2016).
Whitehorn, P. R. et al. Sex allocation theory reveals a hidden cost of neonicotinoid exposure in a parasitoid wasp. Proc. R. Soc. B-Biol. Sci. 282, 6, doi: 10.1098/rspb.2015.0389 (2015).
Mommaerts, V. et al. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 19, 207–215, doi: 10.1007/s10646-009-0406-2 (2010).
Moffat, C. et al. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 6, doi: 10.1038/srep24764 (2016).
Peng, Y. C. & Yang, E. C. Sublethal dosage of imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci. Rep. 6, 19298, doi: 10.1038/srep19298 (2016).
Ruther, J., McCaw, J., Böcher, L., Pothmann, D. & Putz, I. Pheromone diversification and age-dependent behavioural plasticity decrease interspecific mating costs in Nasonia . PLoS ONE 9, e89214, doi: 10.1371/journal.pone.0089214 (2014).
Prabhaker, N., Castle, S. J., Naranjo, S. E., Toscano, N. C. & Morse, J. G. Compatibility of two systemic neonicotinoids, imidacloprid and thiamethoxam, with various natural enemies of agricultural pests. J. Econ. Entomol. 104, 773–781, doi: 10.1603/EC10362 (2011).
Cook, N., Green, J., Shuker, D. M. & Whitehorn, P. R. Exposure to the neonicotinoid imidacloprid disrupts sex allocation cue use during superparasitism in the parasitoid wasp Nasonia vitripennis . Ecol. Entomol. 41, 693–697, doi: 10.1111/een.12344 (2016).
Hallmann, C. A., Foppen, R. P. B., van Turnhout, C. A. M., de Kroon, H. & Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343, doi: 10.1038/nature13531 (2014).
Paine, T. D., Hanlon, C. C. & Byrne, F. J. Potential risks of systemic imidacloprid to parasitoid natural enemies of a cerambycid attacking Eucalyptus. Biol. Control 56, 175–178, doi: 10.1016/j.biocontrol.2010.08.007 (2011).
van der Sluijs, J. P. et al. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sust. 5, 293–305, doi: 10.1016/j.cosust.2013.05.007 (2013).
Zhou, H. C., Du, J. W. & Huang, Y. P. Effects of sublethal doses of malathion on responses to sex pheromones by male Asian corn borer moths, Ostrinia furnacalis (Guenee). J. Chem. Ecol. 31, 1645–1656, doi: 10.1007/s10886-005-5804-1 (2005).
Wei, H. & Du, J. Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer, Ostrinia furnacalis. Pest. Biochem. Physiol. 80, 12–20, doi: 10.1016/j.pestbp.2004.05.001 (2004).
Delpuech, J. M., Dupont, C. & Allemand, R. Effects of deltamethrin on the specific discrimination of sex pheromones in two sympatric Trichogramma species. Ecotoxicol. Environ. Saf. 84, 32–38, doi: 10.1016/j.ecoenv.2012.06.007 (2012).
Delpuech, J. M. et al. Inhibition of sex pheromone communications of Trichogramma brassicae (Hymenoptera) by the insecticide chlorpyrifos. Environ. Toxicol. Chem. 17, 1107–1113, doi: 10.1897/1551-5028(1998)017<1107:iospco>2.3.co;2 (1998).
Delpuech, J. M., Gareau, E., Terrier, O. & Fouillet, P. Sublethal effects of the insecticide chlorpyrifos on the sex pheromonal communication of Trichogramma brassicae . Chemosphere 36, 1775–1785, doi: 10.1016/s0045-6535(97)10071-6 (1998).
Delpuech, J. M., Legallet, B., Terrier, O. & Fouillet, P. Modifications of the sex pheromonal communication of Trichogramma brassicae by a sublethal dose of deltamethrin. Chemosphere 38, 729–739, doi: 10.1016/s0045-6535(98)00310-5 (1999).
Blaul, B. & Ruther, J. How parasitoid females produce sexy sons: a causal link between oviposition preference, dietary lipids and mate choice in Nasonia. Proc. R. Soc. B-Biol. Sci. 278, 3286–3293, doi: 10.1098/rspb.2011.0001 (2011).
Blaul, B. et al. Oleic acid is a precursor of linoleic acid and the male sex pheromone in Nasonia vitripennis . Ins. Biochem. Mol. Biol. 51, 33–40, doi: 10.1016/j.ibmb.2014.05.007 (2014).
Ruther, J. et al. Epimerisation of chiral hydroxylactones by short-chain dehydrogenases/reductases accounts for sex pheromone evolution in Nasonia . Sci. Rep. 6, 34697, doi: 10.1038/srep34697 (2016).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer, 2016).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Meth. 39, 175–191, doi: 10.3758/bf03193146 (2007).
Acknowledgements
The authors thank Melanie Schlossberger for rearing the insects.
Author information
Authors and Affiliations
Contributions
L.T. and J.R. conceived the study. L.T. and T.P. collected and analysed the data. J.H. provided the synthetic (4 R, 5 S)-5-hydroxy-4-decanolide. J.R. wrote the manuscript. L.T., T.P. and J.H. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tappert, L., Pokorny, T., Hofferberth, J. et al. Sublethal doses of imidacloprid disrupt sexual communication and host finding in a parasitoid wasp. Sci Rep 7, 42756 (2017). https://doi.org/10.1038/srep42756
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42756
This article is cited by
-
Persistence of imidacloprid in trunk injected horse chestnut and its impact on Cameraria ohridella (Lepidoptera: Gracillariidae)
Applied Entomology and Zoology (2024)
-
Larval exposure to azadirachtin induced locomotor deficits, and impairs olfactory and gustatory preference in adults of Drosophila melanogaster (Diptera: Drosophilidae)
International Journal of Tropical Insect Science (2022)
-
Neonicotinoids suppress contact chemoreception in a common farmland spider
Scientific Reports (2020)
-
Transgenerational effects from single larval exposure to azadirachtin on life history and behavior traits of Drosophila melanogaster
Scientific Reports (2019)
-
Extremely low neonicotinoid doses alter navigation of pest insects along pheromone plumes
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.