Abstract
Large rivers are commonly regulated by damming, yet the effects of such disruption on bacterioplankton community structures have not been adequately studied. The aim of this study was to explore the biogeographical patterns present under dam regulation and to uncover the major drivers structuring bacterioplankton communities. Bacterioplankton assemblages in the Three Gorges Reservoir (TGR) were analyzed using Illumina Miseq sequencing by comparing seven sites located within the TGR before and after impoundment. This approach revealed ecological and spatial-temporal variations in bacterioplankton community composition along the longitudinal axis. The community was dynamic and dominated by Proteobacteria and Actinobacteria phyla, encompassing 39.26% and 37.14% of all sequences, respectively, followed by Bacteroidetes (8.67%) and Cyanobacteria (3.90%). The Shannon-Wiener index of the bacterioplankton community in the flood season (August) was generally higher than that in the impoundment season (November). Principal Component Analysis of the bacterioplankton community compositions showed separation between different seasons and sampling sites. Results of the relationship between bacterioplankton community compositions and environmental variables highlighted that ecological processes of element cycling and large dam disturbances are of prime importance in driving the assemblages of riverine bacterioplankton communities.
Similar content being viewed by others
Introduction
Prokaryotes are essential players in aquatic ecosystems, catalyzing significant biogeochemical reactions and playing central roles in aquatic food webs1,2. Natural assemblages of bacterioplankton are highly diverse and can undergo dynamics in community compositions in response to spatial-temporal environmental variation across ecosystems3. These fluctuations may result in changes in the functional roles of bacterial communities in the biogeochemical cycles4,5. There is reason to believe that bacterioplankton community indexes or indicator taxa could be developed, as a variety of studies have demonstrated that bacterioplankton have remarkable capabilities to respond to both natural and anthropogenic environmental changes6,7. However, many rivers are highly regulated for hydropower or water supply purposes, which cause critical hydrological disturbances at the spatial scale8. Reservoirs constitute a discontinuity for river systems, as they regulate water flow circulation, modify water residence time, and affect riverborne nutrients and material loads by retaining a large portion of suspended material transported by the river9,10. As a result, upstream and downstream sections of reservoirs tend to differ greatly in their physico-chemical properties11, and changes in the bacterioplankton communities are thus expected.
Seston sedimentation due to damming has been shown to cause shifts in the proportion of particle-attached bacteria12,13, which might imply changes in the composition and metabolic capabilities of the bacterial assemblages from both reaches14. Moreover, studies performed within reservoirs have reported large longitudinal shifts in bacterioplankton community structures that could ultimately be attributed to water residence time in the impoundment period15. Similarly, a clear differentiation in bacterioplankton assemblages was expected between upstream and downstream locations. However, the response of bacteria to environmental changes is not only due to replacement of the existing phylotypes but also to functional adjustments of the existing taxa15,16. Clearly, different bacterial assemblages were found in the small Sinnamary River before and after the reservoir, and large impoundments of the Danube River resulted in indiscernible effects on bacterial communities17,18. There are limited studies that compare bacterial communities before and after impoundments in reservoirs. Thus, the objective of this study was to determine the influence of damming on spatial and seasonal patterns of bacterial community compositions in a large regulated reservoir.
The Three Gorges Reservoir (TGR) was formed in 2003 after construction and impoundment of the Three Gorges Dam (TGD). The Three Gorges Reservoir is from Chongqing (west) to Yichang of Hubei province (east), with a distance of approximately 662.9 km18. The TGR, one of the largest reservoirs in the world, is comprised of a dammed river valley with a water surface area of up to 1,080 km2. According to its regulation plan, the water level in the TGR is impounded to 175 m for power generation during the winter and discharged to 145 m for flood control during the summer8. Thus, obvious aquatic ecosystem seasonal transitions are present within the TGR19, and operation of the TGD result in structurally distinctive limnological characteristics in the main stream of the Yangtze River20. The TGD along the Yangtze River causes disruptions to physicochemical variables in longitudinal profiles as well as algal biomass, species composition, diversity and richness21.
Most studies have focused on analyzing water quality variations and phytoplankton communities in TGR ecosystems22,23,24, yet have failed to analyze microbiology mechanisms within the TGR under regulation of the Three Gorges Dam. Bacterioplankton community structure compositions are likely to influence or be influenced by environmental factors (physico-chemical and hydrological) within the TGR ecosystems. Bacterioplankton community responses to environmental factors might highlight the susceptibility of reservoir microbes to environmental changes. Furthermore, the spatial-temporal variations in bacterioplankton community structures are potentially induced by the dynamic water level conditions imposed by hydropower operations of the TGD. Therefore, it is important to analyze both spatial-temporal variations of bacterioplankton community compositions in the entire TGR and to estimate whether changing environmental factors are responsible for driving bacterioplankton community structure distributions.
In this study, 16 S rRNA gene high-throughput sequencing was conducted in different areas of the reservoir along the longitudinal axis of the TGR in order to gain in-depth insight into possible environmental factors shaping spatial-temporal variations in bacterioplankton community structures. This knowledge can also help to better understand specific bacterioplankton roles in biogeochemical cycling within the TGR and determine the potential effects of river regulations due to damming on bacterioplankton communities.
Results
Environmental conditions and physiochemical characteristics within the TGR
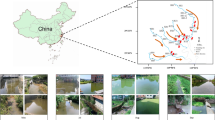
Environmental conditions and physiochemical characteristics of the samples from the seven investigated areas (Fig. 1) are shown in Table 1. The distances of the sampling sites from the TGD are shown in Table 2. Samples collected during the flood season (August 2014) were generally characterized by higher water temperature, conductivity, total phosphorus (TP) and total nitrogen (TN). TN content ranged from 1.73 to 3.23 during the flood season and from 1.44 to 1.84 during the dry season. Moreover, TN and total organic carbon (TOC) concentrations at downstream locations of sites Wanzhou (WZ), Fengjie (FJ) and Zigui (ZG) were higher those at the upstream locations of Zhutuo (ZT), Fuling (FL) and Cuntan (CT). However, dissolved oxygen (DO), total carbon (TC) and TOC concentrations in the flood season were lower than in the dry season. TC content ranged from 48.39 to 54.74 mg/L in the flood season and from 56.50 to 66.37 mg/L in the dry season. Additionally, TOC content ranged from 3.82 to 5.79 mg/L in the flood season and from 5.33 to 8.11 mg/L in the dry season. Water temperatures in downstream sections of the reservoirs were 2–5 °C higher than upstream sites.
Base map of the Yangtze river systems in the Three Gorges Reservoir and map of China were created by Adobe Phososhop CS5. Sub-graph of daily variations of reservoir in- and outflows, as well as reservoir water levels, was generated by OriginLab 9.4. Data source was from the web site of the China Three Gorges Cooporation (www.ctg.com.cn). The whole figure was combined by Adobe Phososhop CS5.
Chlorophyll a (Chl a) concentrations within the TGR
The temporal variations in Chl a concentrations at reservoir sampling sites highlighted seasonal and locational differences (Fig. 2). Chl a concentrations in the flood season (August 2014) were generally higher than concentrations in the dry season (November 2014). It is worth noting that Chl a concentrations at downstream sites (ZG and FJ) of the TGR (averaged 13.84 mg/L in flood season and 3.44 mg/L in dry season) were higher than concentrations at upstream sites (WZ, ZX, FL, CT and ZT, with an average of 0.75 mg/L and 0.95 mg/L in flood and dry seasons, respectively). Chl a concentrations at ZG, near the dam, reached 16.16 mg/L in the flood season, indicating eutrophication in the reservoir. This is in accordance with previous studies that also showed an increase in Chl a concentrations downstream from the reservoir20.
Bacterioplankton community composition within the TGR as determined by sequencing and taxa identification
Upon chimera removal, a total of 230,202 sequences were obtained from Illumina Miseq sequencing, all samples were rarefied at 9,250 sequences per sample to retain as many samples as possible. The Good’s estimator of coverage was > 98.5% for all samples. The RDP classifier25 and Greengene database26 were used to assign taxonomy to OTUs from domain to genus levels. OTUs were used to calculate Shannon-Wiener diversity and richness indexes, and these data are shown in Table 3. A total of 715 OTUs were identified and assigned into 310 genera, 190 families, 109 orders, 57 classes and 26 phyla. For whole gene sequences obtained from each sample, rarefaction curves were established at a nucleotide genetic distance of 0.03 (Figure S1). An analysis of OTUs showed variability along the longitudinal axis with water level changes within the TGR. At the phylum level, a total of 26 different bacterial phyla were identified in each sample. Across all sites, bacterioplankton communities were dominated by Proteobacteria and Actinobacteria, and these two phyla accounted for 39.26% and 37.14% of all sequences, respectively. Bacteroidetes (8.67%) and Cyanobacteria (3.90%) were also present in the remaining sequences. Only 0.15% of sequences could not be classified at the phylum level, and they were defined as bacteria-unclassified. The bacterioplankton community composition at the phylum level in different samples is shown in Fig. 3. The most common classes were Betaproteobacteria (19.09%), Acidimicrobia (11.61%) and Alphaproteobacteria (10.99%). The most abundant genera were hgcI_clade (15.50%), Limnohabitans (7.15%) and Sporichthyaceae_unclassified (4.19%).
Spatial-temporal variations of bacterioplankton community composition within the TGR
The diversity and richness of each sample based on OTU analysis was assessed by calculating the Shannon-Wiener index (H), Simpson Index, Chao and ACE richness estimators (Table 3). The Shannon-Wiener indices of the bacterioplankton community in the flood season (August, 2014) were generally higher than those in the dry season (November, 2014) except at sampling sites ZX and FJ. The observed OTU numbers and Shannon-Wiener index at sampling site ZT (furthest from the TGD) in November were the lowest (238 and 3.45, respectively).
The bacterioplankton community structure was analyzed at the genus level using a heat map of the top 100 genera for the different sites and times (Fig. 4). Spatial-temporal variations of the six most abundant genera were analyzed along the longitudinal axis within the TGR. Of the six most abundant genera, three belonged to Actinobacteria, one to Proteobacteria and two to Cyanobacteria (Fig. 5). The relative abundance of the six genera were, for the most part, higher in November than in August.
Statistical analysis of the OTU distribution of bacterioplankton community composition along the longitudinal axis of the impoundment during the dry season was performed by principal coordinate analysis (PCoA). PCoA of the bacterial community compositions yielded a relatively good separation between different seasons (impoundment season and dry season) and positions (upstream and downstream), which can be seen in Fig. 6. Principal components 1 and 2 accounted for 56.19% of the total sample variability (39.47% and 20.76% for PC1 and PC2, respectively). PcoA coordinates revealed a grouping of the samples into flood season (ZT1, CT1, FL1, ZX1, WZ1, FJ1 and ZG1) and dry season clusters (ZT2, CT2, FL2, ZX2, WZ2, FJ2 and ZG2), which can be explained by their similar compositions of primary species. Moreover, PCoA plots showed comparatively high discrepancies between upstream and downstream sites, such as ZT2 grouped closer together with CT2 and FL2, suggesting a high similarity of bacterioplankton communities at these three sites.
Distribution of bacterioplankton community assemblages in relation to environmental variables within the TGR
To further determine the environmental variables associated with changes in bacterioplankton community structure, redundancy analysis (RDA) was performed with contextual parameters that significantly explained variations in bacterioplankton fingerprints. The first two axes explained up to 37.2% of RDA 1 and 33.6% of RDA 2 of the total variation in bacterial community structure (Fig. 7). The environmental variables, referenced in Table 1, were comprised of DO, TC, Chl a, pH, conductivity, phosphate phosphorus (PO4-P), nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), ammonium nitrogen (NH4-N), salinity, water pH and temperature. Of all the environmental factors investigated, Chl a appeared to have the most significant influence on bacterioplankton community assemblages. Most variances can be explained by water temperature, Chl a, PO4-P, TC and TN. There is a positive association of Axis 1 with water temperature, pH, Chl a and TN and a negative association of Axis 1 with TC, TOC, PO4-P and NO2-N. Water temperature, pH, Chl a and TN explained 13.5%, 12.8%, 11.3%, 9.8% and 7.2% of the variation in the bacterioplankton community, respectively. The partial RDA model statistically explained 38.6% of the variation (P < 0.05) in the bacterioplankton community composition (Table 4). The variation shared by significant factors was 16.0%.
Discussion
It has been well documented that river regulations due to damming would affect water physico-chemical conditions, sediment transportation27, phytoplankton compositions15,22, and bacterioplankton communities19 in the reservoirs. However, very few studies have focused on bacterioplankton community structures based on Miseq sequencing within the TGR. This study may be among the first to investigate and evaluate the potential effects of river regulations due to damming on bacterioplankton community composition along the longitudinal axis of the TGR.
Effects of reservoirs on water physico-chemical conditions
Reservoirs exhibit a marked degree of spatial heterogeneity in phytoplankton productivity and biomass as a result of the longitudinal gradient in the basin morphology, flow velocity, water residence time, suspended solids, and light and nutrient availability28. The TGR is a typical reservoir containing three typical zones that are distinguishable along the longitudinal axis (riverine zone, transition zone and lacustrine zone). The riverine zone consists of the ZT, CT and FL sites, the transition zone consists of the ZX and WZ sites, and the lacustrine zone consists of the FJ and ZG sites near the TGD. There are inherent differences between the upstream and downstream sites within the TGR. The upstream riverine zone (ZT, CT and FL) is a lotic environment that is characterized by lower conductivity and lower concentrations of NO3-N, TOC, particulate matter, DOC and Chl a relative to the downstream sites (Table 1, Fig. 2). These findings have been previously reported in the TGR29. The lacustrine zone (FJ and ZG) is characterized by higher Chl a (Fig. 2) that was probably due to the higher phytoplankton productivity and biomass that occurs in conjunction with the decreasing flow velocity at these two sites24. The decreasing flow velocity at these two sites increases nutrient retention downstream and homogenizes water temperatures, which favors phytoplankton reproduction30.
Anthropogenic activities might attribute to the different characteristics of the upstream and downstream sites. For example, along the longitudinal axis, sites CT, FL and WZ were near a large metropolitan area with a large population and sizeable industries, including natural gas chemical industries, petrochemical industries, new energy resources, and steel and equipment manufacturing31. These industries are the main source of new chemical materials and the heavy chemical industry in Chongqing City, which has resulted in more external nutrients flowing into the TGR31,32. As such, the NO3−-N, TP, TN, and TOC contents in these three monitoring sections were generally higher than those in other sections.
Miseq sequencing results based on 16 S rRNA indicated that there was higher α-diversity of bacterioplankton in August than in November (Table 3). This is in accordance with a previous study33. In another study, nutrient levels were considered one of the main factors in controlling seasonal variations in bacterial communities34. The Yangtze River is highly regulated for hydropower and water supply purposes. The water level in the TGR is impounded to 175 m for power generation during the winter and discharged to 145 m for flood control during the summer. These water level changes have generated significant hydrological disturbances along the longitudinal axis.
Figure 6 shows that samples were separated by site and month, suggesting that bacterioplankton exhibit complex and sensitive responses to different environments. Samples ZT2, CT2 and FL2 were grouped together, suggesting that bacterioplankton community compositions in the riverine zone of the TGR have similar characteristics in November. A succession in the composition of the bacterioplankton community along the longitudinal axis from upstream to downstream was observed (Fig. 3). It was hypothesized that the structural and functional patterns of communities was potentially impacted by TGD regulations. Water flow velocity, which is one of the most important factors affecting plankton in river systems, can be significantly decreased by damming regulation35. Seven representative sites along the TGR area represent direct dam effects at different sites.
Effects of reservoirs on bacterioplankton community compositions
The relatively higher abundance of hgcI_clade and Limnohabitans was in accordance with previous research36. The most abundant genus, the hgcI_clade (Fig. 5), is affiliated with Actinobacteria and is common in a wide range of freshwater habitats. A recent single-cell genomic study showed that this clade had a strong genetic ability to take N-rich and carbohydrate organic compounds37. The hgcI_clade also has the potential to use sunlight through the actinorhodopsin gene38, which may promote anaplerotic carbon fixation, indicating both autotrophic and heterotrophic lifestyles of this clade37. Another abundant genus, Limnohabitans belongs to the Betaproteobacteria. It has been suggested that Limnohabitans plays an important role in water bacterioplankton communities because of its high rates of substrate uptake and growth on algae-derived substrates39. Previous studies have shown that Polynucleobacter and CL500-29_marine_group were driven by NO3−/NO2−40,41. However, the nitrification capability of the hypoxia-adapted Marine Group is unknown and is worth further investigation. It is interesting that Pseudarcicella and Flavobacterium within the Bacteroidetes phylum were predominantly found at site ZT in November (Fig. 4). The high turbidity in the bottom water would prevent the penetration of light to the bottom and thus inhibit the growth of Synechococcus and Cyanobacteria_norank (both belong to Cyanobacteria phylum) at sites ZT and CT. As mentioned above, high nutrient loads might favor the growth of these specific groups, and this suggests that there may be anthropogenic activity resulting in higher nutrient zones at these sites. High nutrient concentrations resulted in high Chl a concentrations of X at sites FJ and ZG, and this is consistent with the higher Cyanobacterial biomass at these two sites42.
Bacterioplankton community compositions in relation to environmental variables
To determine whether water hydrography and environmental factors have on impact on bacterioplankton community composition, redundancy analysis was performed on X and Y datasets (Fig. 7). Water temperature, Chl a, PO4-P, TC and TN best explained the observed variability in bacterioplankton community composition. The result was in accordance with several studies that confirmed that the phytoplankton community and water temperature were influential factors on the seasonal variation of bacterioplankton community composition43,44. Water temperature is always a potentially limiting factor because microbes have optimum temperature characteristics45. Thus, bacterioplankton community composition changes as the water temperature fluctuates. In this study, Chl a was also a significant factor on bacterioplankton community composition. Concentration of Chl a was mainly associated with the biomass of phytoplankton46. It has been reported that phytoplankton plays an important role in regulating bacterioplankton community composition in natural systems47,48. The reason for this phenomenon was probably that the dissolved organic matter produced by the phytoplankton was considered an important carbon source for the bacteria49,50.
In conclusion, this study investigated bacterioplankton community assemblages and specific taxa in the large-scale Three Gorges Reservoir ecosystem using high-throughput sequencing. There were differences in bacterioplankton community structures along the longitudinal axis of the TGR and between the two operational periods (August and November) that show clear shifts in bacterial clades within the same taxa. Our results showed that bacterioplankton community assemblages in the TGR were mostly related to spatial parameters rather than physical-chemical variables. This suggests that ecological processes pertaining to river network length are of prime importance.
Methods
Study sites and sample collection
Seven sampling locations along the longitudinal axis within the TGR were selected from upstream to downstream: Zhutuo (ZT), Cutan (CT), Fuling (FL), Zhongxian (ZX), Wanzhou (WZ), Fengjie (FJ) and Zigui (ZG, near the TGD) (Fig. 1). Sampling sites were selected to span the entirety of the TGR within the Yangtze River. According to the reservoir’s operation strategy, the water level in the TGR is impounded to 175 m for power generation at the end of October. Water level is maintained during the whole dry season mainly for hydropower production. In February of the coming year, water level in the TGR is gradually decreases for maintaining downstream flow and preparing reservoir capacity for the incoming floods until the end of May. The water level is maintained at 145 m before the flood season19,51. The frequency distribution of water residence time of the TGR in 2014 (Figure S2), calculated based on daily data of reservoir capacity and reservoir inflow, showed that there were generally two to three peaks of counts in daily water residence time in the reservoir. Water residence time in August and November covers two of them, representing the two states of the reservoir, i.e., the lotic and lentic, throughout the year. In this case, samples were collected in August and November (2014) to capture the variations of bacterioplankton community potentially resulting from the impoundment and discharge stages of the TGD.
At each site, water samples (5 L) were collected from 0.5, 5 and 10 m of the middle point of the river and mixed fully in a bucket. The water samples were then subsampled for DNA-based analysis and physiochemical analyses. One liter of sample water was subsequently filtered on board through a 0.22-μm filter paper (Millipore, MA, USA) for bacterial DNA. Upon return, the filters were stored at −86 °C until DNA extraction. Water samples were also pre-processed based on standard methods for physicochemical analysis.
Physicochemical analyses
Water stage data was collected from the monitoring department of the China Three Gorges Corporation (http://www.ctgpc.com.cn/). Temperature, dissolved oxygen (DO), pH and conductivity were measured using a YSI Pro2030 (YSI Incorporated, Ohio, USA) in situ. TC, TOC and TN were measured by a Vario TOC Cube analyzer (Elemen-tar, Hanau, Germany). NH4-N, NO3-N, NO2-N, TP, and PO4-P were measured with a UV-2700 spectrophotometer (Shimadzu, Kyoto, Japan) using a colorimetric method as described previously29. Chl a was extracted from a Whatman GF/C filter for 24 h with 90% acetone, centrifuged at 3,000 rpm for 10 min and then spectrophotometrically quantified.
DNA extraction and Polymerase Chain Reaction (PCR)
Total DNA was extracted from the 0.22-μm filters using a PowerWater DNA Extraction kit (Mo Bio, CA, USA) according to the manufacturer’s instructions. The V3-V4 region of bacterial 16 S rRNA gene was amplified using primer set 338 F/806R52. All PCR amplifications were conducted in triplicate for each sample.
Illumina MiSeq sequencing and analysis
Amplicons were gel purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, USA) according to the manufacturer’s instructions and quantified using QuantiFluor™-ST (Promega, USA). Purified amplicons were pooled in equal amounts and paired-end sequenced on the Illumina MiSeq platform at a read length of 2 × 300 bp (Majorbio, Shanghai)
Trimmed sequences were paired-end aligned using fastq-join and screened for quality using the following parameters: quality score ≥ 35 over a 50 nt window, no ambiguous bases, homopolymers ≤ 8 nt, and primer and barcode matching with 100% identity53. Sequences were chimera checked using UCHIME software54. The operational taxonomic units (OTUs) were picked at 97% sequence similarity and identified using the Ribosomal Database Project (RDP) classifier retrained with Greengenes (http://greengenes.secondgenomes). The NAST algorithm was used for alignment and Greengenes. Sequences were quality filtered and rarefied to 9,725 sequences per sample. OTU based analyses [sequencing coverage estimation, the Shannon-Wiener index (H), Chao and ACE richness estimators] were performed using the Quantitative Insights Into Microbial Ecology (QIIME) program55.
Statistical analyses and graphical outputs
The three datasets generated by Chl a concentration were performed using SPSS Statistics software v. 19.0 (IBM, Armonk, NY) by One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test56.
To give further insight into bacterioplankton community composition and environmental variable associations, RDA was used by means of the varpart function in the VEGAN R package57. Forward-selected environmental variables were confirmed as P < 0.05 in the selection procedure by Monte Carlo permutation test. Moreover, to differentiate the sole influence of each significant parameter, partial RDA was performed following the forward selection.
Nucleotide sequence accession numbers
The sequences obtained from Miseq sequencing were submitted to the National Center for Biotechnology Information Sequence Read Archive database (http://www.ncbi.nlm.nih.gov/sra/) under the BioProject accession number PRJNA305461.
Additional Information
How to cite this article: Li, Z. et al. Responses of spatial-temporal dynamics of bacterioplankton community to large-scale reservoir operation: a case study in the Three Georges Reservoir, China. Sci. Rep. 7, 42469; doi: 10.1038/srep42469 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Pernthaler, J. Predation on prokaryotes in the water column and its ecological implications. Nature Reviews Microbiology 3, 537–546 (2005).
Crump, B. C. et al. Circumpolar synchrony in big river bacterioplankton. Proceedings of the National Academy of Sciences of the United States of America 106, 21208–21212 (2009).
Kirchman, D., Ai, D., Findlay, S., Fischer, D., Kirchman, D. L., Dittel, A. I., Findlay, S. E. G. & Fischer, D. Changes in bacterial activity and community structure in response to dissolved organic matter in the Hudson River, New York. Aquat Microb Ecol 35: 243-257. Aquatic Microbial Ecology 35, 243–257 (2004).
Lu, L. et al. Diversity of two-domain laccase-like multicopper oxidase genes in Streptomyces spp.: identification of genes potentially involved in extracellular activities and lignocellulose degradation during composting of agricultural waste. Applied and environmental microbiology 80, 3305–3314 (2014).
Comte, J. & Giorgio, P. A. D. Linking the patterns of change in composition and function in bacterioplankton successions along environmental gradients. Ecology 91, 1466–1476 (2010).
Simek, K. et al. Spatio-temporal patterns of bacterioplankton production and community composition related to phytoplankton composition and protistan bacterivory in a dam reservoir. Aquatic microbial ecology 51, 249–262 (2008).
Kirchman, D. L., Dittel, A. I., Malmstrom, R. R. & Cottrell, M. T. Biogeography of major bacterial groups in the Delaware Estuary. Limnology & Oceanography 50, 1697–1706 (2005).
Li, Z. et al. Soil–air greenhouse gas fluxes influenced by farming practices in reservoir drawdown area: A case at the Three Gorges Reservoir in China. Journal of Environmental Management 181, 64–73 (2016).
Chen, M. et al. Global Landscape of Total Organic Carbon, Nitrogen and Phosphorus in Lake Water. Scientific reports 5 (2015).
Crump, B. C. & Baross, J. A. Characterization of the bacterially-active particle fraction in the Columbia River estuary. Marine Ecology Progress 206, 13–22 (2000).
Sabater, S. Encrusting algal assemblages in a Mediterranean river basin. Archiv für Hydrobiologie 114, 555–573 (1989).
Kondratieff, P. F. & Simmons, G. M. Jr. Microbial colonization of seston and free bacteria in an impounded river. Hydrobiologia 128, 127–133 (1985).
Crump, B. C., Baross, J. A. & Simenstad, C. A. Dominance of particle-attached bacteria in the Columbia River estuary, USA. Aquatic Microbial Ecology 14, 7–18 (1998).
Besemer, K., Moeseneder, M. M., Arrieta, J. M., Herndl, G. J. & Peduzzi, P. Complexity of bacterial communities in a river-floodplain system (Danube, Austria). Applied and environmental microbiology 71, 609–620 (2005).
Ruiz-González, C., Proia, L., Ferrera, I., Gasol, J. M. & Sabater, S. Effects of large river dam regulation on bacterioplankton community structure. FEMS microbiology ecology 84, 316–331 (2013).
Comte, J. & Del Giorgio, P. A. Composition influences the pathway but not the outcome of the metabolic response of bacterioplankton to resource shifts. PLoS One 6, e25266 (2011).
Winter, C., Hein, T., Kavka, G., Mach, R. L. & Farnleitner, A. H. Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Applied and environmental microbiology 73, 421–431 (2007).
Ye, L., Han, X., Xu, Y. & Cai, Q. Spatial analysis for spring bloom and nutrient limitation in Xiangxi Bay of Three Gorges Reservoir. Environmental monitoring and assessment 127, 135–145 (2007).
Yan, Q. et al. Impacts of the Three Gorges Dam on microbial structure and potential function. Scientific reports 5, 8605 (2015).
Liu, L., Liu, D., Johnson, D. M., Yi, Z. & Huang, Y. Effects of vertical mixing on phytoplankton blooms in Xiangxi Bay of Three Gorges Reservoir: Implications for management. Water research 46, 2121–2130 (2012).
Wu, J. et al. The three gorges dam: an ecological perspective. Frontiers in Ecology and the Environment 2, 241–248 (2004).
Qingyun, Y., Yuhe, Y., Weisong, F., Zhigang, Y. & Hongtao, C. Plankton community composition in the Three Gorges Reservoir Region revealed by PCR-DGGE and its relationships with environmental factors. Journal of Environmental Sciences 20, 732–738 (2008).
Kuang, Q., Bi, Y., Zhou, G., Cai, Q. & Hu, Z. Study on the phytoplankton in the Three Gorges Reservoir before and after sluice and the protection of water quality. Acta hydrobiologica sinica 29, 358 (2005).
Xiao, Y., Li, Z., Guo, J., Fang, F. & Smith, V. H. Succession of phytoplankton assemblages in response to large-scale reservoir operation: a case study in a tributary of the Three Gorges Reservoir, China. Environmental monitoring and assessment 188, 1–20 (2016).
Cole, J. R. et al. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic acids research 35, D169–D172 (2007).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and environmental microbiology 72, 5069–5072 (2006).
Dang, T. H. et al. Long-term monitoring (1960–2008) of the river-sediment transport in the Red River Watershed (Vietnam): temporal variability and dam-reservoir impact. Science of the Total Environment 408, 4654–4664 (2010).
Thornton, K. W., Kimmel, B. L. & Payne, F. E. Reservoir limnology: ecological perspectives (John Wiley & Sons, 1990).
Li, Z. et al. Spatio-temporal variations of carbon dioxide and its gross emission regulated by artificial operation in a typical hydropower reservoir in China. Environ Monit Assess 186, 3023–3039, doi: 10.1007/s10661-013-3598-0 (2014).
Prats, J., Armengol, J., Marcé, R., Sánchez-Juny, M. & Dolz, J. In The Ebro River Basin 77–95 (Springer, 2011).
Jiang, D., Hao, G., Huang, L. & Zhang, D. Use of Cusp Catastrophe for Risk Analysis of Navigational Environment: A Case Study of Three Gorges Reservoir Area. Plos One 11 (2016).
He, K., Deng, X. & Management, S. O. Measures for Developing Waste Material Logistics in Chongqing Industrial Park. Logistics Technology (2014).
Wang, S. et al. Diversity of microbial plankton across the Three Gorges Dam of the Yangtze River, China. Geoscience Frontiers 3, 335–349 (2012).
Tirodimos, I., Haidich, A. B., Dardavessis, T. & Arvanitidou, M. Diversity and seasonal variability of bacterial community structure in the river Aliakmon, Greece: analysis by the molecular technique FISH. River research and applications 26, 887–893 (2010).
Bier, R. L., Voss, K. A. & Bernhardt, E. S. Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams. The ISME journal 9, 1378–1390 (2015).
Sun, Z. et al. Community dynamics of prokaryotic and eukaryotic microbes in an estuary reservoir. Scientific reports 4, 6966 (2014).
Ghylin, T. W. et al. Comparative single-cell genomics reveals potential ecological niches for the freshwater acI Actinobacteria lineage. The ISME journal 8, 2503–2516 (2014).
Sharma, A. K. et al. Actinorhodopsin genes discovered in diverse freshwater habitats and among cultivated freshwater Actinobacteria. Isme Journal 3, 726–737 (2009).
Kasalický, V., Jezbera, J., Hahn, M. W. & Šimek, K. The diversity of the Limnohabitans genus, an important group of freshwater bacterioplankton, by characterization of 35 isolated strains. PloS one 8, e58209 (2013).
Mußmann, M. et al. Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proceedings of the National Academy of Sciences 108, 16771–16776 (2011).
Liu, J. et al. Phylogenetic shifts of bacterioplankton community composition along the Pearl Estuary: the potential impact of hypoxia and nutrients. Frontiers in microbiology 6 (2015).
Lindström, E. S., Vrede, K. & Leskinen, E. Response of a member of the Verrucomicrobia, among the dominating bacteria in a hypolimnion, to increased phosphorus availability. Journal of Plankton Research 26, 241–246 (2004).
Niu, Y. et al. Phytoplankton community succession shaping bacterioplankton community composition in Lake Taihu, China. Water Research 45, 4169–4182 (2011).
Shade, A. et al. Interannual dynamics and phenology of bacterial communities in a eutrophic lake. Limnology & Oceanography 52, 487–494 (2007).
Pomeroy, L. R., Wiebe, W. J., Pomeroy, L. R. & Wiebe, W. J. Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Fundamental & Clinical Pharmacology 28, 257–267 (2014).
Jacox, M. G., Hazen, E. L. & Bograd, S. J. Optimal Environmental Conditions and Anomalous Ecosystem Responses: Constraining Bottom-up Controls of Phytoplankton Biomass in the California Current System. Scientific Reports 6 (2016).
Silke, L. & Klaus, J. Regulation of bacterial biomass and community structure by metazoan and protozoan predation. Limnology & Oceanography 46, 121–134 (2001).
Höfle, M. G., Haas, H. & Dominik, K. Seasonal dynamics of bacterioplankton community structure in a eutrophic lake as determined by 5S rRNA analysis. Applied & Environmental Microbiology 65, 3164–3174 (1999).
Cole, J. J., Likens, G. E. & Strayer, D. L. Photosynthetically produced dissolved organic carbon: An important carbon source for planktonic bacteria1. Limnology & Oceanography 27, 1080–1090 (1982).
Jones, S. E., Newton, R. J. & Mcmahon, K. D. Evidence for structuring of bacterial community composition by organic carbon source in temperate lakes. Environmental Microbiology 11, 2463–2472 (2009).
Li, Z. et al. Spatio-temporal variations of carbon dioxide and its gross emission regulated by artificial operation in a typical hydropower reservoir in China. Environmental Monitoring & Assessment 186, 3023–3039 (2014).
Lee, C. K., Barbier, B. A., Bottos, E. M., McDonald, I. R. & Cary, S. C. The inter-valley soil comparative survey: the ecology of Dry Valley edaphic microbial communities. The ISME journal 6, 1046–1057 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. (2014).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods 7, 335–336 (2010).
Norusis, M. & Spss, I. IBM SPSS Statistics 19 Guide to Data Analysis: International Edition. (2011).
Oksanen, J. et al. The vegan package. Community ecology package, 631–637 (2007).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51509233 and 51679226), National Science and Technology Pillar Program (2015BAD13B03), the Western Action Program founded by the Chinese Academy of Sciences (KZCX2-XB3-14), and the National Key Science and Technology Project for Water Environmental Pollution Control (2014ZX07104-006-02). The study was also supported by the China Three Gorges Corporation. We also thank Ling Wang, Cheng Zhang, Zhiwei Sun and Hongtao Gao, who participated in field sampling and lab analysis.
Author information
Authors and Affiliations
Contributions
Z.L., L.L. and J.G. designed the study and contributed to writing the manuscript. J.Y., J.Z., B.H. and L.X. analyzed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Z., Lu, L., Guo, J. et al. Responses of spatial-temporal dynamics of bacterioplankton community to large-scale reservoir operation: a case study in the Three Gorges Reservoir, China. Sci Rep 7, 42469 (2017). https://doi.org/10.1038/srep42469
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42469
This article is cited by
-
The effect of alfalfa cultivation on improving physicochemical properties soil microorganisms community structure of grey desert soil
Scientific Reports (2023)
-
Sedimentary geochemistry mediated by a specific hydrological regime in the water level fluctuation zone of the Three Gorges Reservoir, China
Environmental Science and Pollution Research (2023)
-
Community structure and network interaction of aerobic methane-oxidizing bacteria in Chongqing’s central urban area in the Three Gorges Reservoir, China
Environmental Science and Pollution Research (2023)
-
Flow regulation controls sediment, carbon, and nutrient dynamics across the elevation gradient in the water level fluctuation zone of the Three Gorges Reservoir, China
Journal of Soils and Sediments (2023)
-
Bacterioplankton community indicators for seasonal variation in a fragmented subtropical river
Environmental Monitoring and Assessment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.