Abstract
Progressive pancreatic β cell failure underlies the transition of impaired glucose tolerance to overt diabetes; endoplasmic reticulum (ER) stress expedites β cell failure in this situation. ER stress can be elicited by lipotoxicity and an increased demand for insulin in diabetes. We previously reported that the lipid droplet protein perilipin 2 (PLIN2) modulates lipid homeostasis in the liver. Here, we show that PLIN2 modulates the unfolded protein response (UPR) and ER stress in pancreatic β cells. PLIN2 expression goes up when β cells are exposed to a lipid load or to chemical ER stress inducers. Downregulation of PLIN2 ameliorates the effects of fatty acid- and chemical-induced ER stress, whereas PLIN2 overexpression exacerbates them. Diabetic Akita mice, which carry a heterozygous C96Y Ins2 mutation, exhibit elevated PLIN2 expression and ER stress in their β cells. Genetic ablation of Plin2 in Akita mice leads to mitigation of ER stress, forestalling β cell apoptosis, partially restoring β cell mass, and ameliorating diabetes. Mechanistic experiments showed that PLIN2 downregulation is associated with enhanced autophagic flux and accelerated ER stress resolution. In sum, we have identified a crucial role for PLIN2 in modulating autophagy, ER stress resolution, and β cell apoptosis and survival.
Similar content being viewed by others
Introduction
The pancreatic β cell devotes ~50% of its protein synthetic capacity towards the production of proinsulin/insulin1. In type 2 diabetes (T2D), the increased insulin production presents a challenge for the endoplasmic reticulum (ER)2, where the folding of newly synthesized proinsulin/insulin occurs. ER stress arises when misfolded proteins accumulate inside the ER lumen, triggering the unfolded protein response (UPR). The UPR preempts or alleviates ER stress by increasing chaperone protein expression to increase ER folding capacity, decreasing mRNA translation, and stimulating ER-associated degradation. When UPR fails to relieve ER stress, pancreatic β cell apoptosis ensues2,3. Elevated ER stress markers are known to occur in the pancreatic islets of patients with diabetes4,5,6.
Elevated plasma non-esterified fatty acids (NEFA) commonly occur in people with T2D7. NEFA are stored in cells as triglyceride (TG) and other specialized lipids enveloped by a monolayer of phospholipids in an organelle called lipid droplet (LD). In addition to resident lipids, numerous proteins reside on the surface of LDs to control cellular lipid homeostasis. The perilipins (PLINs) constitute the major LD proteins (LDPs) that encompass five members, PLIN1 to PLIN5. Of these, PLIN2 is a constitutively expressed LDP that occurs in essentially all cells8, including hepatocytes and pancreatic β cells9. In the liver, PLIN2 level goes up with lipid load10,11,12, and ablation of PLIN2 lowers hepatic TG content13,14. Exposure of β cells to fatty acids stimulates PLIN2 expression, whereas downregulation of PLIN2 prevents fatty acid-induced TG accumulation15. Prolonged exposure to fatty acids also induces ER stress in β cells that often culminates in their apoptosis4,16,17,18. However, the role of PLIN2 in modulating ER stress in pancreatic β cells remains unexplored.
In this study, we first examined the role of PLIN2 in the UPR of pancreatic β cells when they are exposed to NEFA. We then dissected PLIN2 function in the glucose homeostatic response of pancreatic β cells in Akita mice, which house a cysteine-to-tyrosine (C96Y) Ins2 gene mutation. An analogous INS mutation causes monogenic diabetes in humans19,20,21. This mutation precludes proper disulfide bond formation, causing proinsulin to misfold, and preventing it from maturation and secretion22,23. Akita mice display severe ER stress, failure of glucose-stimulated insulin secretion in their β cells, and diabetes. We found that PLIN2 regulates ER stress in β cells of Akita mice, and that induced downregulation of PLIN2 mitigates the ER stress response and protects β cells from apoptosis. Moreover, we showed that the protective effect of loss of PLIN2 is mediated at least in part by upregulated autophagy in the β cell.
Methods
Animals
We purchased Akita mice in C57BL/6 background from The Jackson Laboratory22. We generated Plin2−/− mice as described previously13 and obtained Akita;Plin2−/− mice by crossing Akita mice with Plin2−/− mice. All experiments were performed in accordance with relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Metabolic measurements
We incubated isolated pancreases in acid-ethanol (0.2 N HCl in 70% ethanol) overnight at 4 °C, and homogenized them the following day. After centrifugation, we neutralized the solution to pH 7.2 with 0.4 M Tris pH 8.0 buffer. Insulin levels were then measured with an insulin ELISA kit (Mercodia). For glucose tolerance test, we injected 1.5 g of glucose/kg body weight intraperitoneally.
Cell culture and reagents
We obtained Akita β cell line and wild-type control cell line from Dr. Akio Koizumi24. Akita, control, and MIN6 β cells were cultured in DMEM (Cellgro) containing 4.5 g/L glucose, 15% FBS (Life Technologies), penicillin (100 U/ml), streptomycin (100 μg/ml), and β-mercaptoethanol (150 μM) (Sigma-Aldrich). For fatty acid treatment, we incubated cells in 1% BSA (Sigma-Aldrich) alone, BSA conjugated-oleic acid, or -palmitic acid. To induce ER stress, we treated pancreatic islets or other cells with 0.1% DMSO alone, tunicamycin, or thapsigargin (Sigma-Aldrich).
Immunoblotting
We isolated total proteins in RIPA lysis buffer supplemented with protease and phosphatase inhibitor cocktail tablets (Roche). Proteins were separated on 4–15% gradient gels (Bio-Rad) and transferred to PVDF membranes. We blocked membranes with 5% BSA-TBST and incubated them with primary antibodies overnight at 4 °C. The membranes were washed and incubated with secondary antibodies conjugated with horseradish peroxidase (Bio-Rad) on the following day, and visualized using chemiluminescence system (Millipore).
Quantitative real-time PCR
We isolated total RNA using the Trizol reagent (Life Technologies) and used iScript cDNA Synthesis Kit (Bio-Rad) for reverse-transcription. cDNA was used as a template for quantitative PCR with SYBR Green-Rox mix (Quanta BioSciences) on an Mx3000 P thermal cycler (Agilent). We calculated relative expression levels of transcripts vs. housekeeping genes using the ΔΔCT method.
Statistical analysis
Data were presented as mean ± SEM. Results were examined for statistical significance by ANOVA followed by post hoc analysis. A p-value less than 0.05 was considered significant different.
Results
PLIN2 regulates lipid homeostasis in pancreatic β cells
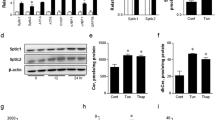
Exposure of MIN6, a mouse insulinoma cell line, to oleic acid (OA) or palmitic acid (PA) increased PLIN2 expression in these cells (Fig. 1A and B). To determine if PLIN2 expression is modulated by NEFA in vivo, we fed C57BL/6 mice a high fat diet (HFD) for 12 weeks. Compared to mice on regular chow, mice on HFD had elevated plasma NEFA (Fig. 1C) as well as increased PLIN2 protein in their pancreatic islets (Fig. 1D).
(A,B) MIN6 cells were treated with vehicle, 0.4 mM oleic acid (OA), or 0.4 mM palmitic acid (PA) for 16 hours (n = 4–6). (A) Quantitative RT-PCR. (B) Immunoblotting and quantification. GAPDH was used as a loading control. (C,D) C57BL/6 mice were fed with normal chow or 40% high fat diet (HFD) for 12 weeks (n = 6). (C) Plasma non-esterified fatty acids (NEFA). (D) Immunoblotting and quantification for PLIN2 protein in pancreatic islets. β-ACTIN was used as a loading control. (E,F) MIN6 cells were transduced with scramble-shRNA or Plin2-shRNA lentivirus. Plin2-knockdown was examined by (E) quantitative RT-PCR (n = 4) and (F) immunoblotting. β-ACTIN was used as a loading control. (G,H) MIN6 cells were treated the same as panels (A,B) (n = 5). (G) Quantification of triglycerides (TG). TG levels were normalized to total protein. *p < 0.05 in comparison with respective scramble; #p < 0.05 in comparison with vehicle. (H) Quantitative RT-PCR. All immunoblotting quantification was normalized to vehicle/control. *p < 0.05.
We next examined the role of PLIN2 in NEFA-induced ER stress. We generated stable Plin2-knockdown MIN6 cells using shRNA-lentivirus and confirmed the knockdown efficiency by quantitative RT-PCR and immunoblotting (Fig. 1E and F). The Plin2-knockdown cells displayed markedly lower TG on exposure to OA and PA, which raised the cellular TG ~8 and ~6 folds, respectively, in control cells (Fig. 1G). It was previously reported that saturated NEFA were more potent than unsaturated NEFA in inducing ER stress17,18; we therefore examined whether PA induces ER stress in MIN6 cells. We found that PA increased Atf4 and Chop, the downstream targets of UPR transducer PERK (Fig. 1H). Notably, the PA-stimulated Atf4 and Chop upregulation was blunted in Plin2-knockdown cells (Fig. 1H), indicating that reduced PLIN2 expression ameliorates NEFA-induced ER stress in pancreatic β cells.
Chemical-induced ER stress upregulates PLIN2 expression in pancreatic β cells
We treated MIN6 cells with ER stress inducer tunicamycin (TM) and found that the treatment increased PLIN2 expression at both the mRNA and protein levels (Fig. 2A and B). The same treatment also stimulated PLIN2 protein expression (and a trend towards increased Plin2 mRNA expression) in isolated mouse islets (Fig. 2C and D). In addition to TM, thapsigargin (THAPS), an ER stress inducer that works via different mechanisms25, also stimulated PLIN2 expression in MIN6 cells (Fig. 2E and F). The results suggest that increased ER stress per se is associated with upregulated PLIN2 expression.
(A,B) MIN6 cells and (C,D) isolated C57BL/6 mouse pancreatic islets were treated with vehicle or 5 μg/ml tunicamycin (TM) for 6 hours (n = 4–6). (A,C) Quantitative RT-PCR and (B,D) immunoblotting and quantification for PLIN2 expression. GAPDH was used as a loading control. (E,F) MIN6 cells treated with vehicle or 5 μM thapsigargin (THAPS) for 6 hours (n = 4–6). (E) Quantitative RT-PCR and (F) immunoblotting and quantification for PLIN2 expression. GAPDH was used as a loading control. All immunoblotting quantification was normalized to vehicle. *p < 0.05.
Downregulation of PLIN2 protects against chemical-induced ER stress in β cells
We hypothesized that reduced PLIN2 level attenuates ER stress and its downstream effects. We first treated control and Plin2-knockdown MIN6 cells with TM and assayed for molecules that inform all three branches of the UPR. We found that TM stimulated the levels of p-PERK, p-eIF2α (PERK pathway), and nuclear ATF6 protein (ATF6 pathway) (Fig. 3A) and upregulated the ratio of sXbp1 to uXbp1 mRNA (IRE1α pathway) (Fig. 3B) in control MIN6 cells. In contrast, TM-induced activation of the PERK pathway was significantly blunted in Plin2-knockdown MIN6 cells (Fig. 3A). The ratio of sXbp1 to uXbp1 mRNA and the nuclear ATF6 protein levels were also attenuated in Plin2-knockdown MIN6 cells (Fig. 3A and B).
(A,B) MIN6 cells and (C,D) pancreatic islets of wild-type (WT) and Plin2−/− mice were treated with vehicle or 5 μg/ml tunicamycin (TM) for 6 hours. (A,C) Immunoblotting and quantification for p-PERK, PERK, p-eIF2α, eIF2α, and nuclear ATF6 protein. β-ACTIN and P84 were used as loading controls for total and nuclear proteins respectively. (B,D) Semi-quantitative RT-PCR and quantification results for spliced Xbp1 (sXbp1) and unspliced Xbp1 (uXbp1) mRNA. Rn18s was used as a loading control. Quantification was normalized to scramble-shRNA vehicle or WT vehicle. *p < 0.05.
We next isolated pancreatic islets from Plin2−/− and wild-type mice and treated them with TM. Corroborating the results using MIN6 cells, we found that the levels of p-PERK, p-eIF2α, nuclear ATF6 proteins (Fig. 3C), and of sXbp1 mRNA were significantly attenuated in islets isolated from Plin2−/− compared with wild-type mice (Fig. 3D). Taken together, the results indicate that partial or complete deficiency of PLIN2 attenuates TM-induced UPR signaling pathways in β cells.
PLIN2 accumulation enhances ER stress of pancreatic β cells
To determine if PLIN2 overproduction per se affects the TM-stimulated UPR in pancreatic β cells, we generated MIN6 cells that stably overexpress PLIN2 by retrovirus transduction. The upregulated PLIN2 expression was confirmed by quantitative RT-PCR and immunoblotting (Fig. 4A and B). Interestingly, gene transduction-induced PLIN2 overexpression further augmented the TM-stimulated Atf4 and Chop responses in these cells (Fig. 4C), indicating that PLIN2 overexpression per se leads to an exaggerated UPR in pancreatic β cells.
(A,B) MIN6 cells were transduced with control or Plin2-retrovirus. PLIN2 overexpression was examined by (A) quantitative RT-PCR (n = 4) and (B) immunoblotting and quantification. GAPDH was used as a loading control. (C) Quantitative RT-PCR. Control and Plin2-overexpression MIN6 cells treated with vehicle or 5 μg/ml tunicamycin (TM) for 6 hours (n = 4–6). (D,E) Total RNA and protein were collected from control wild-type (WT) β cells and Akita β cells (n = 5–6). PLIN2 expression was examined by (D) quantitative RT-PCR and (E) immunoblotting and quantification. GAPDH was used as a loading control. (F,G) Control and Plin2-knockdown Akita β cells were treated with vehicle or 5 μg/ml TM for 6 hours (n = 4–6). (F) Immunoblotting and quantification. GAPDH was used as loading control. (G) Quantitative RT-PCR. All immunoblotting quantification was normalized to vehicle/control. *p < 0.05.
Effect of alteration of PLIN2 expression on the UPR
We next sought to determine if the increased ER stress in the pancreatic β cells of Akita mice is also associated with changes in PLIN2 expression. We found that, indeed, PLIN2 expression was upregulated in Akita β cells compared to controls (Fig. 4D and E). Plin2-knockdown Akita cells displayed attenuated p-PERK and p-eIF2α expression compared with control Akita cells under basal conditions and after TM (Fig. 4F). There was also a significant attenuation of the mRNA expression of Atf4 and Chop in Plin2-knockdown Akita β cells with and without TM treatment (Fig. 4G). Thus, induced PLIN2 deficiency significantly downregulates the exaggerated UPR in Akita β cells.
PLIN2 ablation alleviates hyperglycemia in Akita mice
We bred Akita mice into Plin2−/− mice and compared the glucose homeostasis of wild-type, Plin2−/−, Akita, and Akita;Plin2−/− mice. Glucose tolerance test (GTT) in 14-week-old Plin2−/− and wild-type mice revealed no significant difference in plasma glucose or insulin between the two genotypes (Fig. 5A and B). We next measured random morning blood glucose levels and found no difference between Plin2−/− and wild-type mice (Fig. 5C). In agreement with previous studies22,26, we observed that Akita mice developed hyperglycemia at ~8 weeks of age. In contrast, the hyperglycemia was significantly attenuated in Akita;Plin2−/− mice (Fig. 5D). There was no difference in the body weights of wild-type and Plin2−/− mice (Fig. 5E). Akita mice showed considerable lag in weight gain compared with wild-type mice. Loss of PLIN2 expression partially rescued the failure to gain weight in Akita mice (Fig. 5E and F). Akita mice also displayed significantly lower random morning plasma insulin than wild-type. Notably, the plasma insulin of Akita;Plin2−/− mice was consistently higher than that of Akita mice (Fig. 5H). There was no difference in plasma insulin concentrations between wild-type and Plin2−/− mice during the same time period (Fig. 5G). Moreover, ablation of Plin2 improved the GTT response in Akita mice (Fig. S1A). Collectively, these data indicate that the loss of PLIN2 partially rescues the insulin deficiency and hyperglycemia of Akita mice.
(A) Blood glucose and (B) plasma insulin levels at designated time points during intraperitoneal glucose tolerance test of 14-week-old male mice (n = 7–10). Mice were fasted for 4 hours in the morning prior to injection with 1.5 g glucose/kg body weight. (C,D) Random morning blood glucose levels, (E,F) body weight, and (G,H) random morning plasma insulin levels at indicated age (n = 6–10). *p < 0.05.
Deletion of PLIN2 partially rescues β cell mass in Akita mice
Histological examination of tissue sections showed that presence or absence of PLIN2 did not affect the size of pancreatic islets in mice with wild-type background (Fig. 6A). In confirmation of previous studies26, Akita mice had much smaller islets than wild-type. In comparison, the islets of Akita;Plin2−/− mice were significantly larger than those in Akita mice (Fig. 6A). Quantification of the ratio of islet to total pancreas area from pancreas sections revealed that Plin2−/− and wild-type mice displayed similar total islet area. The area in Akita mice was ~25% of wild-type; loss of PLIN2 in Akita;Plin2−/− mice led to a doubling in islet area (Fig. 6B). Immunofluorescence staining showed morphologically indistinguishable islets from wild-type and Plin2−/− mice, whereas Akita mice had much smaller islets that stained poorly with insulin antibody. Loss of PLIN2 in Akita mice partially rescued these defects. Quantification of the ratio of insulin-positive islet area to total pancreas area showed that ablation of Plin2 in Akita;Plin2−/− mice partially rescued the β cell mass (Fig. S1B). Quantification of the insulin content in pancreas extracts of mice of different genotypes revealed that the presence or absence of PLIN2 had no effect in wild-type mice (Fig. 6D). In contrast, pancreatic insulin content was markedly reduced in Akita mice, and loss of PLIN2 partially reversed the insulin deficiency (Fig. 6D).
(A) Hematoxylin and eosin staining of pancreas sections from 14-week-old mice. (B) Quantification of the islet area relative to the pancreas area. Total islet and pancreas area were counted from 6 sections per mouse (n = 5). (C) Insulin (green) and glucagon (red) immunofluorescence staining of pancreas sections from 14-week-old mice. Nuclei were stained with DAPI (blue). (D) Total pancreas insulin content of 14-week-old mice (n = 7). (E) Quantification of BrdU-positive β cells and (F) TUNEL-positive β cells. Percent BrdU-positive and TUNEL-positive β cells were calculated from 6 sections per mouse (n = 5). *p < 0.05. Scale bar = 50 μm.
We quantified the BrdU-positive cells in the islets of mice and found that the four genotypes exhibit similar proportions of BrdU-positive cells (Fig. 6E). We then examined the proportion of apoptotic cells and found a very low level of TUNEL-positive cells in wild-type and Plin2−/− islets (Fig. 6F). Whilst Akita β cells displayed an increased number of TUNEL-positive cells than wild-type β cells, loss of PLIN2 in Akita;Plin2−/− mice reduced the proportion of apoptotic cells in Akita mice (Fig. 6F). Therefore, the relative preservation of β cell mass and insulin content in Akita;Plin2−/− mice appears to be mainly a result of downregulation of apoptosis.
PLIN2 modulates autophagic flux in pancreatic β cells
Activated autophagy has been found to ameliorate ER stress27,28. We found that knockdown of Plin2 in MIN6 cells raised the level of LC3II/LC3I and decreased the level of sequestosome 1 (SQSTM1/P62) (Fig. 7A), indicating that enhanced autophagy may underlie the improved ER stress in Plin2-deficient β cells. We transfected MIN6 cells with LC3-GFP and found an increased number of LC3 punctae in Plin2-knockdown compared to control cells. Addition of chloroquine (CQ), which inhibits the fusion of autophagosomes with lysosomes, led to an increased number of punctae, which remained significantly higher in Plin2-knockdown compared with control cells (Fig. 7B). These data support the interpretation that downregulation of PLIN2 stimulates autophagic flux in pancreatic β cells.
(A) Immunoblotting and quantification. Control and Plin2-knockdown MIN6 cells were treated with vehicle or 5 μg/ml tunicamycin (TM) for 6 hours. GAPDH was used as loading control. (B) Control and Plin2-knockdown MIN6 cells were transfected with LC3-GFP construct and subquently treated with vehicle or 0.1 mM chloroquine (CQ) for 6 hours. Nuclei were stained with DAPI (blue). The number of LC3-GFP punctae (green dots) was quantified. Total of 50 GFP-positive cells were calculated each group. (C) Immunoblotting and quantification. Wild-type control and Akita β cells were treated with vehicle or 5 μg/ml TM for 6 hours. GAPDH was used as loading control. (D) Immunoblotting and quantification. Cells were treated with vehicle or 0.1 mM CQ for 6 hours. GAPDH was used as loading control. All immunoblotting quantification was normalized to scramble-shRNA vehicle. *p < 0.05. Scale bar = 5 μm.
Under basal conditions, Akita cells displayed higher LC3II/LC3I ratio and lower P62 compared to wild-type cells (Fig. 7C). Compared with wild-type β cells, both chemical (TM) and pathophysiological (Akita) ER stress inducer led to a marked increase in LC3II/LC3I ratio and a decrease in P62, suggesting that increased ER stress leads to increased autophagic flux. We also noticed that TM treatment did not further boost the LC3II/LC3I ratio in Akita β cells, presumably because the increased autophagic flux was already at a saturated level under basal conditions (Fig. 7C).
We next examined the autophagic flux in the WT and Akita β cells with or without Plin2 knockdown. Plin2-knockdown in wild-type or Akita β cells raised the level of LC3II/LC3I and decreased the level of P62 (Fig. 7D). Addition of CQ increased the LC3II/LC3I ratio and P62 levels in both scramble-shRNA and Plin2-knockdown wild-type and Akita cells (Fig. 7D). It is noteworthy that the LC3II/LC3I ratio was significantly higher in Plin2-knockdown than in scramble-shRNA Akita cells after CQ treatment (Fig. 7D).
Discussion
In this study, we sought to determine the role of PLIN2 in modulating β cell function under ER stress conditions. We used mouse pancreatic islets, β cell lines and Akita mice to investigate the effects of PLIN2 under artificial and pathophysiological ER stress conditions and found that NEFA- and chemical-induced ER stress stimulated PLIN2 expression in β cells, and downregulation of PLIN2 ameliorated the deleterious effects of ER stress. Importantly, PLIN2 level was upregulated in β cells isolated from Akita mice, whose β cells exhibited severe ER stress because of failure of proinsulin processing. Genetic ablation of Plin2 in Akita mice led to significant amelioration of hyperglycemia, decreased β cell apoptosis, and partially restored β cell mass and pancreatic insulin content. Finally, we found that the Plin2-deficient β cells display enhanced autophagic flux and accelerated ER stress resolution.
Many reports have shown that the PLIN2 expression level is correlated with intracellular lipid accumulation10,11,12,13. In fact, PLIN2 that is not associated with LD is reported to be rapidly degraded through the ubiquitin/proteasome pathway29. Interestingly, TM was found to stimulate intracellular lipid accumulation in vitro and in vivo30,31. Here, we also found that the intracellular TG levels in Akita and TM-treated cells are elevated compared with wild-type and vehicle controls, respectively. The increased TG levels may underlie the upregulated PLIN2 levels observed in Akita and TM-treated β cells. However, whether mechanisms independent of lipid accumulation, including mutant insulin and UPR-related activation, lead to increased PLIN2 remain to be explored.
Using MIN6 cells, antisense oligonucleotide, and human islets, Faleck et al. reported that PLIN2 is important in lipid metabolism and PA-stimulated insulin secretion in β cells15. Our study agrees with the findings that downregulation of PLIN2 decreases TG content in β cells. However, the effect of PA reported by Faleck et al. was acute (~1 h). It is in contrast to the effect of prolonged PA exposure which leads to ER stress17,18. Here, we show that downregulation of PLIN2 protects cells from prolonged NEFA exposure-induced ER stress. Further studies are required to investigate the complexity of PLIN2 function under different pathophysiological conditions.
Interestingly, using INS-1 β cells, Borg et al.32 reported that overexpression of PLIN1, a PLIN2-related protein, protects against β cell dysfunction after prolonged exposure to PA. PLIN1 is known to protect LDs from lipolysis by neutral lipases8; overexpressing PLIN1 may reduce lipolysis and hence prevent oxidative stress. Thus, there are different ways to overcome lipotoxicity. Furthermore, although PLIN1 and PLIN2 are LDPs that share limited structural similarities, their regulation, function and tissue distribution are very different8,33, and the two proteins may play very different roles in pancreatic β cells.
In our study, the ablation of Plin2 in Akita mice partially reverses hyperglycemia and insulin deficiency. Interestingly, the partial preservation of β cell mass and improvement of hyperglycemia were also observed in different reports when Cebpb, Chop, or Pik3r1 was deleted to prevent ER stress-induced β cell apoptosis26,34,35. Importantly, treatment of Akita mice with rapamycin, which induces autophagy, also led to a partial restoration of pancreatic insulin content compared with vehicle-treated Akita mice36. Our study and other reports suggest that induced autophagy and reduced β cell apoptosis partially ameliorate, but do not completely reverse, the severe phenotype in Akita mice.
Basal autophagy is important for the maintenance of normal pancreatic islet architecture and function37,38,39. Many studies have found that properly activated autophagy helps maintain ER homeostasis and protects against excessive ER stress27,28,38,40. Especially relevant to this study is the fact that chemical-mediated stimulation of autophagy was found to improve ER stress-induced diabetes in Akita mice36. Importantly, we showed that pancreatic β cells isolated from Akita mice display increased basal autophagy compared to wild-type control cells, which may function as a protective response to the persistent ER stress in Akita β cells. Unlike wild-type cells, which respond to TM by increased autophagy, the pancreatic β cells in Akita mice in the basal state appeared to already exhibit maximal autophagic flux, which failed to be further augmented by TM treatment (Fig. 7C). Interestingly, however, the autophagy capacity could be further enhanced when we knocked down Plin2 in both wild-type and Akita β cells (Fig. 7D).
The persistent basal ER stress in Akita β cells causes cellular lipids as well as PLIN2 to accumulate, as observed in other types of cells41. The observation that Akita β cells exhibit increased autophagy in the backdrop of elevated PLIN2 may seem paradoxical. It is noteworthy, however, that there are multiple pathways by which a cell can activate autophagy, e.g., the UPR signaling pathways IRE1-JNK and PERK-eIF2α have both been shown to mediate LC3 conversion independently and play a pivotal role in ER stress-induced autophagy42,43. In this study, we found that induced PLIN2 downregulation stimulates autophagic flux, whereby protecting the Akita;Plin2−/− β cells from the deleterious effects of inducer-activated ER stress in Akita mice. Herein, we have uncovered a previously unappreciated role of PLIN2 in modulating autophagy, ER stress resolution, and β cell survival.
Additional Information
How to cite this article: Chen, E. et al. PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells. Sci. Rep. 7, 40855; doi: 10.1038/srep40855 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Schuit, F. C., Kiekens, R. & Pipeleers, D. G. Measuring the balance between insulin synthesis and insulin release. Biochem. Biophys. Res. Commun. 178, 1182–1187 (1991).
Harding, H. P. & Ron, D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51, Suppl 3, S455–61 (2002).
Back, S. H. & Kaufman, R. J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793 (2012).
Laybutt, D. R. et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50, 752–763 (2007).
Huang, C. J. et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56, 2016–2027 (2007).
Marchetti, P. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50, 2486–2494 (2007).
Boden, G. & Shulman, G. I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32 Suppl 3, 14–23 (2002).
Bickel, P. E., Tansey, J. T. & Welte, M. A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta 1791, 419–440 (2009).
Larsson, S., Resjo, S., Gomez, M. F., James, P. & Holm, C. Characterization of the lipid droplet proteome of a clonal insulin-producing beta-cell line (INS-1 832/13). J. Proteome Res. 11, 1264–1273 (2012).
Gao, J. & Serrero, G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J. Biol. Chem. 274, 16825–16830 (1999).
Imamura, M. et al. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol. Endocrinol. Metab. 283, E775–83 (2002).
Dalen, K. T., Ulven, S. M., Arntsen, B. M., Solaas, K. & Nebb, H. I. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J. Lipid Res. 47, 931–943 (2006).
Chang, B. H. et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 26, 1063–1076 (2006).
Chang, B. H., Li, L., Saha, P. & Chan, L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 51, 2132–2142 (2010).
Faleck, D. M. et al. Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 299, E249–57 (2010).
Akerfeldt, M. C. et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 57, 3034–3044 (2008).
Karaskov, E. et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147, 3398–3407 (2006).
Kharroubi, I. et al. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145, 5087–5096 (2004).
Stoy, J. et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 104, 15040–15044 (2007).
Ahamed, A. et al. Permanent neonatal diabetes mellitus due to a C96Y heterozygous mutation in the insulin gene. A case report. JOP 9, 715–718 (2008).
Yang, Y. & Chan, L. Monogenic Diabetes: What It Teaches Us on the Common Forms of Type 1 and Type 2 Diabetes. Endocr. Rev. 37, 190–222 (2016).
Yoshioka, M., Kayo, T., Ikeda, T. & Koizumi, A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46, 887–894 (1997).
Wang, J. et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 103, 27–37 (1999).
Nozaki, J. et al. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 9, 261–270 (2004).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103 (2000).
Matsuda, T. et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J. Clin. Invest. 120, 115–126 (2010).
Bernales, S., McDonald, K. L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4, e423 (2006).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Xu, G. et al. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280, 42841–42847 (2005).
Rutkowski, D. T. et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell. 15, 829–840 (2008).
Kim, A. J., Shi, Y., Austin, R. C. & Werstuck, G. H. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell. Sci. 118, 89–99 (2005).
Borg, J. et al. Perilipin is present in islets of Langerhans and protects against lipotoxicity when overexpressed in the beta-cell line INS-1. Endocrinology 150, 3049–3057 (2009).
Londos, C., Sztalryd, C., Tansey, J. T. & Kimmel, A. R. Role of PAT proteins in lipid metabolism. Biochimie 87, 45–49 (2005).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Winnay, J. N., Dirice, E., Liew, C. W., Kulkarni, R. N. & Kahn, C. R. P85alpha Deficiency Protects Beta-Cells from Endoplasmic Reticulum Stress-Induced Apoptosis. Proc. Natl. Acad. Sci. USA 111, 1192–1197 (2014).
Bachar-Wikstrom, E. et al. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes 62, 1227–1237 (2013).
Jung, H. S. et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell. Metab. 8, 318–324 (2008).
Ebato, C. et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell. Metab. 8, 325–332 (2008).
Riahi, Y. et al. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia 59, 1480–1491 (2016).
Choi, S. E. et al. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology 150, 126–134 (2009).
Basseri, S. & Austin, R. C. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem. Res. Int. 2012, 841362 (2012).
Kouroku, Y. et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 14, 230–239 (2007).
Ding, W. X. et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 171, 513–524 (2007).
Acknowledgements
The authors thank Jongoh Kim (Baylor College of Medicine) for suggestions and Sand Ku (Baylor College of Medicine) for administrative assistance. This work was supported by National Institutes of Health Grant P30-DK-079638 (to L.C.), American Diabetes Association Grant 1-14-MN-01, the Cunningham (Chris, Casey, and Jennifer) Family Foundation, the Frank and Cindy Liu Family Foundation, and the T.T. & W.F. Chao Global Foundation (all to L.C.) and NIH-DK-084495 and American Heart Association Grant 16GRNT30990040 to B.H.-J.C. L.C. was supported by the Betty Rutherford Chair in Diabetes Research from the Baylor St. Luke’s Medical Center (Houston, TX).
Author information
Authors and Affiliations
Contributions
E.C. designed and conducted the studies, analyzed the data, wrote and edited the manuscript. T.H.T. contributed to the data and editing of the manuscript. L.L. contributed to the data. P.S. helped the design of the experiments. B.H.-J.C. and L.C. conceived and designed the study, analyzed the data, wrote and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, E., Tsai, T., Li, L. et al. PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells. Sci Rep 7, 40855 (2017). https://doi.org/10.1038/srep40855
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40855
This article is cited by
-
ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma
Cancer & Metabolism (2022)
-
Early overnutrition reduces Pdx1 expression and induces β cell failure in Swiss Webster mice
Scientific Reports (2019)
-
Genomics of lipid-laden human hepatocyte cultures enables drug target screening for the treatment of non-alcoholic fatty liver disease
BMC Medical Genomics (2018)
-
Metabolic pathways at the crossroads of diabetes and inborn errors
Journal of Inherited Metabolic Disease (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.