Abstract
Oleanolic acid (3β-hydroxyolean-12-en-28-oic acid, OA) is a pentacyclic triterpenes widely distributed in food, medicinal plants and nutritional supplements. OA exhibits various pharmacological properties, such as hepatoprotective and anti-tumor effects. In this study, we analyzed the effect of OA on endothelial dysfunction induced by high glucose in human vascular endothelial cells (ECs). Western blotting showed that OA attenuated high glucose-reduced nitric production oxide (NO) as well as Akt-Ser473 and eNOS-Ser1177 phosphorylation in cultured human umbilical vein ECs (HUVECs). Next, luciferase reporter assay showed that OA activated peroxisome proliferators-activated receptor δ (PPARδ) activity. Quantitative reverse transcriptase PCR (qRT-PCR) demonstrated that OA increased the expressions of PPARδ target genes (PDK4, ADRP and ANGPTL4) in ECs. Meanwhile, the induced expressions of PDK4, ADRP and ANGPTL4 by OA were inhibited by GSK0660, a specific antagonist of PPARδ. In addition, inhibition of PPARδ abolished OA-induced the Akt-Ser473 and eNOS-Ser1177 phosphorylation, and NO production. Finally, by using Multi Myograph System, we showed that OA prevented high glucose-impaired vasodilation. This protective effect on vasodilation was inhibited in aortic rings pretreated with GSK0660. Collectively, we demonstrated that OA improved high glucose-impaired endothelial function via a PPARδ-mediated mechanism and through eNOS/Akt/NO pathway.
Similar content being viewed by others
Introduction
Oleanolic acid (OA), a pentacyclic triterpenoid compound, present in many fruits and vegetables, such as olive leaves, grape, clove and pomegranate flowers1 exhibits a wide range of pharmacological and biochemical effects2,3. OA has received much attention, and is being marketed as therapeutic drug for the treatment of liver diseases, obesity associated insulin resistance, hypertension, atherosclerosis4,5. Especially, OA has been shown to possess promising anti-diabetic effects in various in vitro and in vivo models, as well as the ability to reduce blood pressure, blood glucose levels, total cholesterol, triglyceride, low density lipoprotein, and to increase the plasma insulin and high density lipoprotein levels1,6. However, the signal pathways underlying these effects remain to be elucidated.
Substantial clinical and experimental evidence suggest that both diabetes and insulin resistance cause endothelial dysfunction, which is considered the earliest predictive factor for diabetes7,8. One of the main targets against endothelial dysfunction is to improve endothelium-dependent vasodilatation. Nitric oxide (NO) is of critical importance as a mediator of vascular tone and blood pressure. Loss of NO bioavailability is a cardinal feature of endothelial dysfunction9,10. Several factors contribute to loss of NO bioavailability in endothelial dysfunction states, including both reduced NO synthesis and NO scavenging by reactive oxygen species (ROS)11. In ECs, NO is produced by endothelial nitric oxide synthase (eNOS), which catalyzes the oxidation of L-arginine to produce NO. The activity of eNOS can be regulated by a number of post-translational modifications. Among them, protein kinase B (Akt) induces eNOS-Ser1177 phosphorylation to modulate endothelial NO production in response to a wide variety of stimuli12,13.
PPARδ is a member of ligand-activated nuclear receptor transcription factors superfamily, which is ubiquitously expressed with high levels in placenta, skeletal muscles, and adipose tissue. PPARδ is also expressed in the vascular cells including ECs, smooth muscle cells and macrophages14. PPARδ plays important roles in various physiological vascular processes such as apoptosis, survival, angiogenesis and inflammation15. PPARδ also promotes vasodilatation by stimulating NO production16. Recently, we demonstrated an endothelial-protective effect of synthetic PPARδ agonists in diabetic mice through PI3K/Akt/eNOS signaling17. In this study, we sought to investigate the effects of a natural product OA on high glucose-impaired NO production and vasorelaxation.
Results
OA improved high glucose-induced NO reduction in BAECs
Endothelial dysfunction is implicated in vascular complications of diabetic patients18. To study the effects of OA (Fig. 1a) on endothelial function in ECs, we evaluated the cytotoxicity of OA on HUVECs and BAECs firstly by using the MTT assay. Both HUVECs and BAECs were treated with the indicated concentrations (0.1–50 μM) of OA for 24 h. As shown in Fig. 1b, at a concentration up to 10 μM caused no decrease in cell viability in either cell types. Thus, this concentration was used in the following cell-based experiments. Then we examined the effect of OA on the endothelial production of NO using the NO-sensitive dye DAF-FM diacetate. As shown in Fig. 1c, treatment with high glucose (HG, 30 mM, 12 h) significantly reduced NO production compared with mannitol control. Pretreatment with OA (10 μM) effectively restored the NO production in BAECs.
(a) The chemical structure of OA. (b) HUVECs and BAECs were treated with indicated concentrations of OA for 24 h, and cell viability was measured by MTT. All data were expressed as mean ± SEM of triplicate experiments. (c) BAECs were preincubated with or without OA (10 μM) for 12 h, then, treated with high glucose (HG, 30 mM, 12 h), mannitol served as vehicle control to HG. NO was detected by using DAF-FM diacetate (40 × objective).
OA attenuated the high glucose-induced impairment of Akt-Ser473 and eNOS-Ser1177 phosphorylation
Phosphorylation of Akt at Ser473 plays a critical role in the transduction of insulin signaling and, in turn, phosphorylates its downstream substrate eNOS at Ser1177 to increase eNOS activity13,19. In HUVECs, HG treatment suppressed the phosphorylation of both Akt-Ser473 and eNOS-Ser1177. However, OA attenuated the suppression of Akt and eNOS phosphorylation by HG (Fig. 2a and b). As shown in Fig. 2c and d, OA could increase the basal levels of Akt-Ser473 and eNOS-Ser1177 phosphorylation.
(a) HUVECs were pretreated with or without OA (10 μM) for 12 h, and then exposed to normal (5 mM) or high (30 mM) glucose for 12 h. Protein levels of p-eNOS, eNOS, p-Akt and Akt were detected by using western blotting. (b) Quantification of p-eNOS/eNOS and p-Akt/Akt. (c) HUVECs were stimulated with OA (10 μM) for 24 h, cell lysates were analyzed to determine p-eNOS, eNOS, p-Akt and Akt protein levels by using western blot. (d) Quantification of p-eNOS/eNOS and p-Akt/Akt levels in HUVECs. Data were shown as mean ± SEM of independent experiments. *P < 0.05 vs. vehicle control. #P < 0.05 vs. HG.
OA activated PPARδ in ECs
We previously demonstrated a protective effect of PPARδ on endothelial function in diabetic mice through activating the Akt/eNOS signaling pathway17. Thus, we examined whether OA activated PPARδ in ECs. BAECs were transfected with the PPRE-driven luciferase reporter and PPARδ expression plasmid before exposed to OA. The reporter assay showed that OA increased the luciferase activity of PPARδ (Fig. 3a). Further, we examined the effects of OA on the expressions of the endogenous PPARδ target genes including pyruvate dehydrogenase kinase 4 (PDK4), adipose differentiation-related protein (ADRP) and angiopoietin-like protein 4 (ANGPTL4). As shown in Fig. 3b, the mRNA expressions of PDK4, ADRP and ANGPTL4, were increased by OA. In addition, pretreatment with GSK0660, a selective antagonist of PPARδ, effectively abrogated the induction of PDK4, ADRP and ANGPTL4 by OA (Fig. 3c), suggesting a PPARδ-specific mechanism.
(a) BAECs were transfected with PPRE-luc and PPARδ plasmids and then treated with OA (10 μM) for 24 h. The luciferase activities were shown as fold changes in relation to the control. (b) HUVECs were stimulated with OA (10 μM) for 24 h, PDK4, ADRP and ANGPTL4 mRNA levels were assessed by using qRT-PCR. (c) HUVECs were pretreated with GSK0660 (1 μM) for 1 h, then exposed to OA (10 μM) for 24 h, mRNA levels of PDK4, ADRP and ANGPTL4 were assessed. *P < 0.05, **P < 0.01 vs. vehicle control. #P < 0.05, ##P < 0.01 vs. OA.
OA attenuated the high glucose-induced impairment of NO production via PPARδ
In order to study whether PPARδ activity is required for the protective effect of OA on NO production against high glucose, HUVECs were pretreated with a selective PPARδ antagonist GSK0660 and then with OA (10 μM, 12 h) before the exposure to high glucose. The results demonstrated that, in the presence of GSK0660, OA failed to restore the high glucose impaired NO production (Fig. 4a). We further examined the role of PPARδ in the Akt and eNOS phosphorylation induced by OA. As shown in Fig. 4b and c, inhibition of PPARδ activity by GSK0660 significantly diminished the OA-induced eNOS-Ser1177 and Akt-Ser473 phosphorylation. In the presence of GSK0660, OA also failed to protect ECs against the high glucose-impaired phosphorylations of Akt and eNOS (Fig. 4d and e).
(a) BAECs were pretreated with GSK0660 (1 μM) for 1 h, and then stimulated with OA (10 μM) for 12 h by following normal (5 mM) or high (30 mM) glucose medium for 12 h. NO was detected by using DAF-FM probe (40 × objective). (b) HUVECs were treated as in (a). Protein levels of p-eNOS, eNOS, p-Akt and Akt levels were assessed. (c) Quantification of p-eNOS/eNOS and p-Akt/Akt levels. *P < 0.05 vs. vehicle control, #P < 0.05 vs. OA. (d) HUVECs were pretreated with GSK0660 (1 μM) for 1 h, then exposed to OA (10 μM) for 24 h, protein levels of p-eNOS, eNOS, p-Akt and Akt levels were assessed. (e) Quantification of p-eNOS/eNOS and p-Akt/Akt levels. *P < 0.05 vs. vehicle control, #P < 0.05 vs HG and ※P < 0.05 vs. OA.
OA enhanced endothelium-dependent vasodilatation via PPARδ
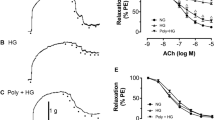
Compared with the time-matched vehicle control, OA elicited concentration-dependent relaxations pre-contracted by KCl or phenylephrine in the rat arterial segments (Fig. 5a and b). Next, aortic rings were exposed to high glucose co-incubated with or without OA for 12 h. Acetylcholine-induced endothelium-dependent vasorelaxation was impaired by high glucose (Fig. 5c). HG impaired blood vessel relaxation was attenuated by OA. However, the effect of OA was abolished when the arteries were pre-incubation with GSK0660 (Fig. 5d). These results suggest that OA improves endothelial relaxation and attenuates the high glucose impairment via the PPARδ activation.
Rat aortic arteries were pre-contracted by 60 mM K+ (a) or 10 μM Phe (b) followed by the cumulative addition of vehicle control or OA. Data were shown as mean ± SEM (n = 3. *P < 0.05, ***P < 0.001 vs. vehicle control). (c) Arterial rings were incubated in medium contained 30 mM glucose in the presence of 10 μM OA for 12 h, and ACh-induced vasodilatation was tested by using Multi Myograph System. Data are shown as mean ± SEM (n = 7). *P < 0.05, ***P < 0.001 vs. vehicle control, ###P < 0.05 vs. HG. (d) Arterial rings were incubated with GSK0660 (1 μM) for 1 h followed by the cumulative addition of OA pre-contracted by Phe (n = 5). ***P < 0.001 vs. vehicle control, ##P < 0.01, ###P < 0.001 vs. OA.
Discussion
In this study, we have demonstrated for the first time that OA, as a natural product, can ameliorate the high glucose-triggered endothelial function by activating the nuclear receptor PPARδ. Previous studies showed that pentacyclic triterpenes from fruits and plants could increase estrogen receptor activities20 and synthetic triterpenes has been identified as ligands for the PPARγ21. In addition, reporter gene assays showed that oleanolic acid increased PPARα activity in spontaneously transformed keratinocyte cell line HaCaT and CV-1 cells22. Punica granatum flower (PGF) and its component oleanolic acid enhanced the PPARα reporter activity in human embryonic kidney 293 cells, and this effect was completely suppressed by a selective PPARα antagonist23,24. We and others have previously demonstrated that synthetic PPARδ agonists such as GW501516 and GW0742 could ameliorate a number of pathological features associated with diabetes and metabolic syndrome. In ECs, these agonists inhibited the expression of pro-inflammatory genes14. In mouse models for atherosclerosis, synthetic agonists of PPARδ reduced atherosclerotic lesions25. In obese diabetic mice, the PPARδ agonists prevented fatty liver by repressing sterol regulatory element binding protein-1 (SREBP1) and improved vascular function17,26. These results clearly indicate that PPARδ is a promising target for treating metabolic syndrome and related cardiovascular diseases. However, clinical uses of such potent synthetic agonists have been seriously hindered largely due to the claimed cardiovascular adverse effects of PPARγ27. Therefore, searching natural compounds with PPARδ modulating properties and, in particular, vascular protective activity, represent a promising approach for metabolic syndrome and related vascular disorder, also in view of their advantageous effects in endothelial system and obesity prevention28.
Emerging evidence revealed the beneficial effects of OA on endothelial function in vascular disorders associated to metabolic diseases. OA induced vasodilatation in isolated aortas from both normotensive and spontaneously hypertensive rats5,29. NO was involved in the vasodilator responses of OA, and in ECs, OA induced phosphorylation of eNOS-Ser1177 to increase NO production30. Further studies have shown that OA increased the phosphorylation of Akt-Ser473 and led to eNOS activation31. These observations were largely consistent with our results. However, the mechanisms by which OA regulates Akt/eNOS pathway were poorly understood. Herein, we demonstrated that OA exerts the endothelial protective effects mainly by PPARδ activation. This notion was supported by its capacity of activating the PPAR-reporter (Fig. 3a) and the induction of multiple endogenous PPARδ target genes, PDK4, ANGPL4 and ADRP (Fig. 3b). More importantly, the effects of OA were attenuated by a selective PPARδ antagonist GSK0660 (Fig. 3c). It remains to be examined whether this pentacyclic triterpenoid serves as a bona fide ligand for PPARδ or enhances the transcriptional activity of PPARδ by a post-translational modification.
It is well known that exposure to high glucose impairs endothelial functions and decreases the NO production. These deleterious effects are attributed to the inhibition of expression and/or activity of eNOS32,33. PPARδ agonists could ameliorate the endothelium-dependent vasodilatation impaired by HG via activating PI3K/Akt/eNOS pathway in MAECs17. In the present study, we found that OA protected endothelial cells from high glucose injury (Fig. 1). This effect could also be inhibited by blocking PPARδ (Fig. 2). In endothelium-specific PPARδ deficient mice, both endothelium-dependent relaxations to ACh and endothelium independent relaxations to the NO donor were significantly impaired in the arteries, accompanied by decreased eNOS-Ser1177 phosphorylation34. It is suggested that OA restores HG-impaired endothelial function by a PPARδ-mediated activation of Akt/eNOS signaling pathways. It is worth noting that OA and PPARδ both possess an anti-oxidative property35. Under oxidative stress, eNOS undergoes uncoupling and produces superoxide rather than NO. GTP cyclohydrolase I (GTPCH I), a rate-limiting enzyme responsible for tetrahydrobiopterin (BH4) de novo synthesis, switches eNOS from uncoupled to coupled state to balance the NO and reactive oxygen species (ROS) generations36. GTPCH I protein level was up-regulated by GW501516, as well as the production of BH4 in endothelial progenitor cells37. Thus, the effects of OA on GTPCH I expression and eNOS coupling remain to be investigated.
In summary, this study provided evidence that OA, a component of pentacyclic triterpenes, is a natural modulator of PPARδ and ameliorates the high glucose impaired endothelial dysfunction. It is suggested that OA has a potential application in the treatment of diabetes related vascular diseases.
Materials and Methods
Ethics statement
The animal experiment was approved by the institutional review board of Xi’an Jiaotong University and performed in accordance with the institutional and national guidelines for the care and use of animals.
Reagents
OA (93.4% in purity) was from ChromaDex (Irvine, CA, USA). The antibodies against phosphorylation eNOS (Ser1177), eNOS, phosphorylation Akt (Ser473), Akt were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against β-actin and horseradish peroxidase (HRP)-conjugated secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fetal bovine serum (FBS) was from HyClone (Longan, UT, USA). Thiazolyl blue tetrazolium bromide (MTT) was from Sigma-Aldrich (St. Louis., MO, USA). 4-amino-5-methylamino-2′, 7′-difluorofluorescein (DAF-FM) diacetate was from Life Science Ltd. (Oregon, CA, USA). Polyvinylidene difluoride (PVDF) membranes for western blot analysis was purchased from Roche Diagnostics (Mannheim, Germany).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured in medium 199 containing heparin (0.1 mg/ml), acidic fibroblast growth factor (FGF) (10 ng/ml), L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 U/ml) and 20% FBS. Bovine aortic endothelial cells (BAECs) were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS, penicillin (100 U/ml), streptomycin (100 U/ml).
MTT Assay for Cell Viability
Cell viability was evaluated by MTT assay. HUVECs and BAECs were seeded in 96-well plates and cultured until 80% confluence. Then the cells were treated with the indicated concentrations of OA for 24 h before incubation with 5 mg/ml MTT at 37 °C in 5% CO2 atmosphere for 4 h. Next, the culture medium was removed and the formazan formed in the reaction was dissolved in 150 μl DMSO. Cell viability was presented as a percentage of the vehicle.
Quantitative Reverse Transcriptase-PCR (qRT-PCR)
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), converted to cDNA by iScript cDNA synthesis kit (Bio-rad, Hercules, CA). Real-time PCR was performed by using SYBR Green Supermixes (Bio-rad) and a 7500 Real-time PCR machine (Applied Biosystems, Foster City, CA). The reaction conditions consisted of: stage 1, 95 °C for 10 min; stage 2, 40 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, which were concluded by the melting curve analysis process. Fold changes of gene expression were calculated using the 2−ΔΔCt method. The primer sequences are listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Western blotting
Cellular proteins were extracted with lysis buffer (50 mM Tris-HCl, pH 7.5, 15 mM EGTA, 100 mM NaCl, 0.1% Triton X-100 and the protease inhibitors). Protein samples were separated on 10% SDS-PAGE gels, transferred to PVDF membranes, and incubated overnight at 4 °C with primary antibodies directed against phosphorylation eNOS (Ser1177) (1:1000), eNOS (1:1000), phosphorylation Akt (Ser473) (1:2000), Akt (Ser473) (1:2000) or β-actin (1:5000). After washing blots to remove excessive primary antibody binding, the membranes were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody (1:3000) at room temperature, then visualized by the ECL chemoluminescence system.
Plasmids, Transfection and Reporter Assay
The plasmids expressing PPARδ and PPRE-TK-luciferase reporter contains 3 copies of PPAR-response elements (PPRE) from the acyl-coenzyme A (CoA) oxidase gene were transfected into subconfluent BAECs together with pRSV-gal, a plasmid expressing β-galactosidase by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). 24 hours later, the cells were treated with or without OA. Cell lysates were harvested to measure the luciferase and β-gal activities.
Detection of NO
NO release was measured with using DAF-FM diacetate. Briefly, BAECs seeded on glass coverslips and treated with different stimuli. By the end of treatment, the cells were incubated with DMEM containing DAF-FM (5 μM) for 30 min in the dark at 37 °C and then washed with PBS. Images were obtained using fluorescence microscopy (Olympus America Inc., NY, USA).
Vascular reactivity
Aortic rings (3 mm) were dissected from Sprague-Dawley rats and incubated with different treatments at 37 °C in 5% CO2, then mounted in Multi Myograph System (Danish Myo Technology A/S, Denmark) to measure the isometric force. During the whole experiment, the solution was continuously oxygenated with a gas mixture of 95% O2 plus 5% CO2. To determine the vasodilatory effect of OA, KCl (60 mM) or phenylephrine (Phe, 10 μM) was used to constrict arterial rings in advance. After sustained contraction was obtained, the concentration-dependent responses of OA (0.01–100 μM) were examined. In order to evaluate the role of high glucose, arterial rings were incubated in medium containing 30 mM glucose in the presence or absence of 10 μM OA to examine the acetylcholine-induced vasodilation.
Statistical analysis
Quantitative data are expressed as mean ± SEM. Student’s t test and ANOVA were used to analyze the statistical significance for the differences between two or among more groups, respectively. The dose response curves were analyzed by using two-way ANOVA followed by Bonferroni post-tests. P < 0.05 was considered significant. Non-quantitative results were representative of at least three independent experiments.
Additional Information
How to cite this article: Zhang, Z. et al. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPARδ activation. Sci. Rep. 7, 40237; doi: 10.1038/srep40237 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
de Melo, C. L. et al. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chemico-biological interactions 185, 59–65, doi: 10.1016/j.cbi.2010.02.028 (2010).
Lee, W., Yang, E. J., Ku, S. K., Song, K. S. & Bae, J. S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo . Inflammation 36, 94–102, doi: 10.1007/s10753-012-9523-9 (2013).
Jager, S., Trojan, H., Kopp, T., Laszczyk, M. N. & Scheffler, A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules 14, 2016–2031, doi: 10.3390/molecules14062016 (2009).
Yunoki, K. et al. Effect of dietary wine pomace extract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. Journal of agricultural and food chemistry 56, 12052–12058, doi: 10.1021/jf8026217 (2008).
Madlala, H. P. et al. Vascular Endothelium-Dependent and Independent Actions of Oleanolic Acid and Its Synthetic Oleanane Derivatives as Possible Mechanisms for Hypotensive Effects. PloS one 11, e0147395, doi: 10.1371/journal.pone.0147395 (2016).
Castellano, J. M., Guinda, A., Delgado, T., Rada, M. & Cayuela, J. A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 62, 1791–1799, doi: 10.2337/db12-1215 (2013).
Mudau, M., Genis, A., Lochner, A. & Strijdom, H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovascular journal of Africa 23, 222–231, doi: 10.5830/CVJA-2011-068 (2012).
Hadi, H. A. & Suwaidi, J. A. Endothelial dysfunction in diabetes mellitus. Vascular health and risk management 3, 853–876 (2007).
Salimi, S. et al. Association of plasma nitric oxide concentration and endothelial nitric oxide synthase T-786C gene polymorphism in coronary artery disease. Pathophysiology: the official journal of the International Society for Pathophysiology/ISP 19, 157–162, doi: 10.1016/j.pathophys.2012.04.003 (2012).
Bonetti, P. O., Lerman, L. O. & Lerman, A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, thrombosis, and vascular biology 23, 168–175 (2003).
Salisbury, D. & Bronas, U. Reactive oxygen and nitrogen species: impact on endothelial dysfunction. Nursing research 64, 53–66, doi: 10.1097/NNR.0000000000000068 (2015).
Siragusa, M. & Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Archiv: European journal of physiology 468, 1125–1137, doi: 10.1007/s00424-016-1839-0 (2016).
Heiss, E. H. & Dirsch, V. M. Regulation of eNOS enzyme activity by posttranslational modification. Current pharmaceutical design 20, 3503–3513 (2014).
Neels, J. G. & Grimaldi, P. A. Physiological functions of peroxisome proliferator-activated receptor beta. Physiological reviews 94, 795–858, doi: 10.1152/physrev.00027.2013 (2014).
Cheang, W. S., Tian, X. Y., Wong, W. T. & Huang, Y. The peroxisome proliferator-activated receptors in cardiovascular diseases: experimental benefits and clinical challenges. British journal of pharmacology 172, 5512–5522, doi: 10.1111/bph.13029 (2015).
Quintela, A. M. et al. PPARbeta activation restores the high glucose-induced impairment of insulin signalling in endothelial cells. British journal of pharmacology 171, 3089–3102, doi: 10.1111/bph.12646 (2014).
Tian, X. Y. et al. PPARdelta activation protects endothelial function in diabetic mice. Diabetes 61, 3285–3293, doi: 10.2337/db12-0117 (2012).
Howangyin, K. Y. & Silvestre, J. S. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arteriosclerosis, thrombosis, and vascular biology 34, 1126–1135, doi: 10.1161/ATVBAHA.114.303090 (2014).
Kolluru, G. K., Siamwala, J. H. & Chatterjee, S. eNOS phosphorylation in health and disease. Biochimie 92, 1186–1198, doi: 10.1016/j.biochi.2010.03.020 (2010).
Mallavadhani, U. V. et al. Three new pentacyclic triterpenes and some flavonoids from the fruits of an Indian Ayurvedic plant Dendrophthoe falcata and their estrogen receptor binding activity. Chemical & pharmaceutical bulletin 54, 740–744 (2006).
Wang, Y. et al. A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor gamma. Molecular endocrinology 14, 1550–1556, doi: 10.1210/mend.14.10.0545 (2000).
Lee, H. K., Nam, G. W., Kim, S. H. & Lee, S. H. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-alpha pathway. Experimental dermatology 15, 66–73, doi: 10.1111/j.0906-6705.2005.00386.x (2006).
Huang, T. H. et al. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: role of lowering circulating lipids. British journal of pharmacology 145, 767–774, doi: 10.1038/sj.bjp.0706245 (2005).
Li, Y., Qi, Y., Huang, T. H., Yamahara, J. & Roufogalis, B. D. Pomegranate flower: a unique traditional antidiabetic medicine with dual PPAR-alpha/-gamma activator properties. Diabetes, obesity & metabolism 10, 10–17, doi: 10.1111/j.1463-1326.2007.00708.x (2008).
Ding, Y., Yang, K. D. & Yang, Q. The role of PPARdelta signaling in the cardiovascular system. Progress in molecular biology and translational science 121, 451–473, doi: 10.1016/B978-0-12-800101-1.00014-4 (2014).
Qin, X. et al. Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology 48, 432–441, doi: 10.1002/hep.22334 (2008).
Saag, K. G. et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357, 2028–2039, doi: 10.1056/NEJMoa071408 (2007).
Ehrenborg, E. & Skogsberg, J. Peroxisome proliferator-activated receptor delta and cardiovascular disease. Atherosclerosis 231, 95–106, doi: 10.1016/j.atherosclerosis.2013.08.027 (2013).
Rodriguez-Rodriguez, R., Herrera, M. D., Perona, J. S. & Ruiz-Gutierrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. The British journal of nutrition 92, 635–642 (2004).
Rodriguez-Rodriguez, R. et al. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. British journal of pharmacology 155, 535–546, doi: 10.1038/bjp.2008.289 (2008).
Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: molecular mechanisms and therapeutic perspectives. Curr Med Chem 22, 1414–1425 (2015).
Ding, Y., Vaziri, N. D., Coulson, R., Kamanna, V. S. & Roh, D. D. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. American journal of physiology. Endocrinology and metabolism 279, E11–17 (2000).
Du, X. L. et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. The Journal of clinical investigation 108, 1341–1348, doi: 10.1172/JCI11235 (2001).
d’Uscio, L. V. et al. Mechanisms of vascular dysfunction in mice with endothelium-specific deletion of the PPAR-delta gene. Am J Physiol Heart Circ Physiol 306, H1001–1010, doi: 10.1152/ajpheart.00761.2013 (2014).
Fan, Y. et al. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 28, 315–321, doi: 10.1161/ATVBAHA.107.149815 (2008).
Wang, S. et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension 52, 484–490, doi: 10.1161/HYPERTENSIONAHA.108.112094 (2008).
He, T., Smith, L. A., Lu, T., Joyner, M. J. & Katusic, Z. S. Activation of peroxisome proliferator-activated receptor-{delta} enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension 58, 287–294, doi: 10.1161/HYPERTENSIONAHA.111.172189 (2011).
Acknowledgements
This study was supported by grants from the National Science Foundation of China (31430045, 81300242, 30881220108005, and 81470373).
Author information
Authors and Affiliations
Contributions
Zhang, Z. and Jiang, M. performed most of experiments, analyzed the data and wrote the draft of the manuscript. Xie, X. and Wang, X. performed a part of experiments. Yang, H. analyzed the chemical structure and revised the manuscript. Xiao, L. and Wang, N. designed the experiments, provided funding, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Z., Jiang, M., Xie, X. et al. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPARδ activation. Sci Rep 7, 40237 (2017). https://doi.org/10.1038/srep40237
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40237
This article is cited by
-
Cucurbitacins extracted from Cucumis melo L. (CuEC) exert a hypotensive effect via regulating vascular tone
Hypertension Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.