Abstract
The C-repeat binding factor (CBF) is crucial for regulation of cold response in higher plants. In Arabidopsis, the mechanism of CBF3-caused growth retardation is still unclear. Our present work shows that CBF3 shares the similar repression of bioactive gibberellin (GA) as well as upregulation of DELLA proteins with CBF1 and -2. Genetic analysis reveals that DELLAs play an essential role in growth reduction mediated by CBF1, -2, -3 genes. The in vivo and in vitro evidences demonstrate that GA2-oxidase 7 gene is a novel CBF3 regulon. Meanwhile, DELLAs contribute to cold induction of CBF1, -2, -3 genes through interaction with jasmonate (JA) signaling. We conclude that CBF3 promotes DELLAs accumulation through repressing GA biosynthesis and DELLAs positively regulate CBF3 involving JA signaling. CBFs and DELLAs collaborate to retard plant growth in response to low temperature.
Similar content being viewed by others
Introduction
Temperature is one of the major environmental factors limiting plant growth. In particular, cold stress is a serious threat to the sustainability of crop yields. While cold extremes during the winter may affect survival, reduced growth at low temperature during the growing season is a key factor limiting plant distribution globally. The changes of ambient temperature affect plant development at multiple points during the lifecycle - from seed germination, plant architecture to flowering and reproductive development. It is crucial that we learn to understand how plants regulate growth in low temperature; this may lead to strategies of manipulating the threshold levels to switch from growth arrest to maintenance of growth. Flowering plants possess a large regulatory network for low temperature responses1. In this network, a group of AP2 domain-containing proteins, known as C-repeat (CRT)/Dehydration Responsive Element (DRE) Binding factors (CBF/DREB), plays a crucial role in cold acclimation, an adaptive response that many plant species use to enhance their freezing resistance after an initial exposure to a nonfreezing low temperature2.
In Arabidopsis thaliana, there are three linearly clustered CBF1, -2, -3 genes3,4, also known as DREB1b, DREB1c and DREB1a, respectively, which are identified as key regulators of cold response5. Besides, there are three other highly similar genes, CBF4, DWARF AND DELAYED FLOWERING (DDF)1 and DDF26. CBF4 is a shared component in both temperature and drought responses7 and DDF1 and DDF2 are involved in response to high salinity8. In addition to freezing response, CBF1, -2 or -3 can be rapidly induced by nonfreezing cold stress such as 4 °C or 10 °C and their protein products activate downstream genes known as the CBF regulons, leading to protection of plant cells from low temperature injury9. Constitutive expression of CBF1, -2 or -3 in A. thaliana results in similar effects of increased cold tolerance as well as altered biochemical composition such as proline, glucose, fructose, sucrose and raffinose10,11,12,13. At the same time, transgenic plants constitutively overexpressing either CBF1, -2 or -3 exhibit similar morphological and developmental phenotypes including stunted growth and delayed flowering, even under non-stressful growth conditions4,14. The phenomenon of growth retardation caused by the overexpression of CBF genes or their homologs has been reported in multiple plant species including those with agricultural importance, such as tomato (Solanum lycopersicum)15, rice (Oryza sativa)16, tobacco (Nicotiana tabacum)17, poplar (Populus balsamifera)18, potato (S. tuberosum)19 and peanut (Arachis hypogaean)20. Although it is clear that the CBF pathway has a role in affecting plant growth and development, the regulatory mechanism of CBF-caused growth reduction involving downstream genes is uncertain. The fact that both homologous and heterologous expression of CBF genes can elicit plant growth repression prevents the effective use of CBF genes in molecular breeding. Therefore, the requirement of uncovering how CBF genes modulate plant growth under cold stress has been raised.
Previous studies have shown that plant growth regulation during environmental changes is related to phytohormones14,21. It has been reported that the dwarfism in CBF1 overexpressing plants including S. lycopersicum, N. tabacum and A. thaliana can be rescued by exogenous Gibberellin (GA) treatment but not by application of other phytohormones15,17,22. Our previous work also showed that bioactive GA levels were reduced in young leaves of CbCBF-ox tobacco and the growth inhibition of CbCBF-ox plants was partially due to GA deficiency23. These results provide indirect evidence that GA metabolism and signal transduction has a role in CBF-induced plant growth reduction. However, it was also reported that GA treatment could not reverse the growth repression in CBF3-ox tobacco (N. tabacum)17, and effects of GA on CBF2-ox plants are still not known. Thus, it is unclear that whether GA has similar interactions with CBF1, -2, -3 transcription factors. In particular, regulatory nodes in this network have yet to be identified, indicating that detailed regulation of GA and CBF genes still need to be deeply investigated.
Bioactive GAs promote plant cell elongation24,25 and are synthesized with the activities of GA20-oxidases26 and GA3-oxidases27,28, but reduced by GA2-oxidases29. Bioactive GAs can bind to the Gibberellin Insensitive Dwarf1 (GID1) receptor, and the GA-GID1 complex together with the SCFSLY1 E3 ligase facilitate ubiquitination of DELLA proteins and their subsequent degradation by the 26S proteasome30,31. DELLAs are the master negative regulators of the GA signaling and their abundance will lead to severe growth restriction32,33,34,35,36. There are five DELLAs [REPRESSOR of gal-3 (RGA), GA INSENSITIVE (GAI), RGA-LIKE1 (RGL1), RGL2, and RGL3] in A. thaliana, which display overlapping but non-identical functions in repressing GA responses37. Although it is known that both the cold-induced CBFs and GA signaling pathways regulate plant growth and stress tolerance, it is unclear whether and how these pathways directly interact with each other.
The goals of this study were to better understand the crosstalk between GA signaling and CBF3. We have tested the bioactive GA levels and DELLA accumulation in cbf3 knock-out mutant and CBF3-ox plants, uncovering the positive role of CBF3 in DELLA modulation. Meanwhile, we have also shown the contribution of DELLAs in cold induction of CBF3 through interaction with jasmonate (JA) signaling. Our results clarify the role of CBF3 in the interplay with GA signaling and identify GA2ox7 as a novel CBF3 regulon.
Results
CBF3 mediates cold induced reduction of gibberellin level and plant growth retardation
In A. thaliana, dwarfism of GA-deficient mutant ga1-3 can be reversed by the treatment of GA338, while GA-insensitive mutant gai cannot39. To determine whether the growth retardation phenotypes caused by increased CBF3 resemble GA-deficient or GA-insensitive mutants, we investigated the GA3 response of CBF1-ox, CBF2-ox and CBF3-ox plants. We treated CBF1-ox, CBF2-ox and CBF3-ox seedlings with GA3 both in MS plates and in soil. Interestingly, CBF1-ox, CBF2-ox and CBF3-ox plants exhibited similar phenotypes under low concentration of GA3 treatments (Fig. 1a; Fig. S1). Growth retardation caused by CBF3 in plant height and flowering time were restored to WT (wild type control) level and leaf area was also partially restored (Fig. 1b–d), which was similar to the effects of CBF1, -2 here as well as previously reported instances of CBF1 and DDF122,40. Next, we tested the endogenous bioactive gibberellin level in 4-week-old CBF1-ox, CBF2-ox and CBF3-ox plants. Consistently, GA1+3 levels of these plants were all significantly decreased (Fig. 1e). These suggested that CBF1, -2 or -3 genes similarly downregulate GA level in cold response. In particular, cbf3 mutant showed weaker growth reduction in leaf size and flowering time under low temperature, and these two indices of cbf3 were close to that of GA3 rescued Col plants at 12 °C (Fig. 1f,h). For plant height, no obvious difference between Col and cbf3 was observed, indicating that height can be affected by CBF3-independent pathways (Fig. 1g). Further, cbf3 showed less reduction of GA1+3 levels compared with Col in response to chilling temperature (Fig. 1i). Together, CBF3 participates in the control of GA repression and restrained growth in the face of cold stress.
(a) Representative phenotypes of 4-week-old CBF1-ox, CBF2-ox and CBF3-ox plants with or without GA3 application. Dwarfism caused by CBF1, -2, -3 overexpression can be partially rescued by 10−5 M GA3 application. Phenotypes including (b) the areas of fifth rosette leaves, (c) the final heights, (d) the rosette leaf numbers and (e) GA1+3 contents are shown. In cbf3 mutant cold induced growth repression and GA reduction are weakened according to (f–h) growth phenotypes and (i) GA1+3 contents. (SE, n = 20, *P < 0.05, **P < 0.01).
CBF3 represses plant growth through DELLA accumulation under low temperature
DELLA proteins are key growth inhibitors that can be accumulated in GA-deficient plants36. Since CBF3 reduced gibberellin levels, we assumed that it was also involved in DELLA regulation. According to the report that late flowering of A. thaliana at a low temperature of 12 °C could be obviously restored in della-global mutants41, we tested GFP:RGA fusion protein levels in plants with or without GA3 application at 22 °C or 12 °C. The GFP:RGA level was obviously enhanced at 12 °C as well as CBF1-ox, CBF2-ox and CBF3-ox background in 8-day-old roots (Fig. 2a). In 4-week-old leaves, similar elevation of GFP:RGA level was observed (Fig. 2b). The CBF3-ox plants showed a lower level of GFP:RGA compared with CBF1-ox and CBF2-ox in normal temperature, suggesting that in late growth stage CBF1 and CBF2 may have stronger effects in RGA level than CBF3. Moreover, GFP:RGA level was lower in cbf3 mutant than Col under low temperature, indicating the positive role in modulating RGA level of CBF3 (Fig. 2c). On the other hand, GA3 leaded to degradation of GFP:RGA both under cold condition and in CBF1-ox, CBF2-ox, CBF3-ox plants, suggesting that CBF1, -2 and -3 may not affect GID1 and SLY1 function. Next, to confirm the contribution of DELLAs to growth repression caused by CBF1, -2 or -3, we created transgenic plants that constitutively express CBF1, -2 or -3 in della-global (gai-t6; rga-t2; rgl1-1; rgl2-1; rgl3-1) background. Two lines with high transgenic expression level for each were used for further analysis (Fig. S2). Consistent with the study of Kumar et al.41, della-global mutation significantly weakened the growth retardation at 12 °C (Fig. 3a–c). Similar restoration was observed in CBF1-ox della-global, CBF2-ox della-global or CBF3-ox della-global plants (Fig. 3d–f and Fig. S3). The differences in leaf area, plant height and leaf number at flowering were strongly reduced by della-global mutation. These demonstrated that CBF3 inhibits plant growth through accumulating DELLAs under low temperature.
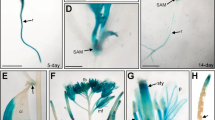
(a) GFP fluorescence in the root tip (first row) and elongating zone (second row) of 8-day-old of pRGA::GFP:RGA, CBF1-ox pRGA::GFP:RGA, CBF2-ox pRGA::GFP:RGA and CBF3-ox pRGA::GFP:RGA seedlings. Images are taken with identical parameters for comparison of fluorescence levels. (b) Immunoblot analysis of GFP:RGA levels in leaves from 4-week-old plants indicated. (c) GFP:RGA levels in 8-d-old seedlings treated at 12 °C for 4 h. The β-tubulin is used as loading control.
(a–c) Comparison of Ler and della-global plants. Up or down arrows represent increase or decrease relative to wild type, respectively. (d–f) Comparison between Ws and CBF1-ox, CBF2-ox and CBF3-ox plants as well as comparison between della-global and CBF1-ox della-global plants, CBF2-ox della-global plants, CBF3-ox della-global plants. (SE, n = 20, *P < 0.05, **P < 0.01).
CBF3 upregulates the DELLA and GA2ox genes expression
The GAs level in plants is homeostatically modulated through GA biosynthesis and deactivation pathways, two processes catalyzed by three categories of dioxygenases, which are respectively encoded by a small gene family42. GA 20-oxidases (GA20ox) and GA 3-oxidases (GA3ox) catalyze successive steps in the synthesis of bioactive GAs27, while GA 2-oxidases (GA2ox) deactivate bioactive GAs29. To further figure out the mechanism involved in GA decrease and DELLA increase, we tested the expression pattern of GA signaling and metabolic genes in CBF1-ox, CBF2-ox and CBF3-ox plants. Similar to CBF1-ox and CBF2-ox plants, CBF3-ox lines showed significantly higher transcript levels of RGL3, GA2ox3 and GA2ox7 than Ws plants (Fig. 4a). Meanwhile, RGL3 and GA2ox7 could also be induced by cold treatment, while GA2ox3 was slightly affected in Ws plants (Fig. 4b), suggesting that GA2ox3 might be affected by other regulators under low temperature. In addition, no GA20ox or GA3ox genes were repressed and RGA, GA20ox1, GA3ox1 and GA2ox6 expression were slightly enhanced between two and four folds in CBF1-ox, CBF2-ox, CBF3-ox lines (Fig. 4a). Previous work reported that GA20ox and GA3ox transcripts could be increased by DELLA accumulation due to a feedback mechanism26,29. In Col background, cold induction pattern of RGL3, GA2ox3 and GA2ox7 were similar to Ws and cbf3 mutation blocked elevation of RGL3 and GA2ox7 transcript levels in cold treatment (Fig. 4c). Interestingly, GA2ox3 had even a higher transcript level at 22 °C in cbf3 plants and showed a similar expression level in cold condition compared with WT, implying that GA2ox3 may be downregulated by CBF3 in the normal condition and CBF3 is not required for expression of GA2ox3 at low temperature. The enhancement of GA2ox3 expression in CBF3-ox plants can be due to indirect feedback mechanisms. In a word, CBF3 confers a transcriptional increase of RGL3 and GA2ox7 gene, which is consistent with the altered GA and DELLA levels.
(a) Relative expression levels of GA metabolism and signaling genes in Ws and CBF1-ox, CBF2-ox and CBF3-ox plants. (b) Relative expression levels of RGL3, GA2ox3 and GA2ox7 in Ws plants under 12 °C treatment. (c) Relative expression levels of RGL3, GA2ox3 and GA2ox7 in Col and cbf3 plants under 12 °C treatment. Data are means ± SE.
GA2ox7 is a CBF3 regulon
Since GA2ox7 and RGL3 were significantly induced by CBF3 overexpression, we decided to test whether they were the targets of CBF3. The binding sequence of CBF transcription factors in promoter regions is defined as CRT/DRE element with a core sequence of A/GCCGAC43,44,45. In the presumed promoter region of −0.2 kb to −0.35 kb from the initiation codon of RD29a, a well-known CBF regulated gene, there are three ACCGAC and one GCCGAC motifs. Thus this area can be a good positive control in ChIP-qPCR for detection of in vivo binding of transcription factor to the promoter. Meanwhile, one region without CCGAC in the GAI gene not induced by CBF was used as a negative control. There are three putative CRT-like elements (designated L1, L2 and L3) in −0.9 kb to −2.9 kb regions of GA2ox7 and among them only L2 has the exact CRT/DRE core sequence (GCCGAC) (Fig. 5a). Consistently, we observed the enrichment of CBF3 near L2 but not L1 or L3 according to ChIP-qPCR (Fig. 5b). Besides, there is no exact CRT/DRE core sequence in RGL3 promoter regions. We identified two similar elements with sequence of GTCGAC in −0.74 kb to −0.76 kb region of RGL3 instead. However, no recruitment of CBF3 was detected in these areas (data not shown).
(a) Schematic diagram of three CRT/DRE-like elements in the promoter region of GA2ox7. Core sequences of L1, L2 and L3 are shown. (b) ChIP qRT-PCR analysis of CBF3 binding to the three CRT/DRE-like elements of GA2ox7. The −0.2 kb to −0.35 kb promoter region of RD29a containing three CRT/DRE-like elements serves as positive control and one area without CRT/DRE-like elements in the CBF-noninduced gene GAI is used as negative control. Data are means ± SE. (c) Oligonucleotides of L2 and L2-m (mutated version) elements within the GA2ox7 promoter used in the EMSA. Underlined letters are core sequences of CRT/DRE. Three nucleotides are substituted in L2-m. (d) CBF3 binds to L2 element of GA2ox7 promoter in vitro. Unlabeled L2 and L2-m elements fragment are used as competitors. (e) Dual-LUC Assays using transient expression system in tobacco leaves. CBF3 driven by 35S promoter was served as the effector and LUC under control of GA2ox7 promoter truncations as indicated were reporters. The relative activity (LUC/REN) were shown. Reporters co-transformed with the blank pC1304 vector were used as controls. Data are means ± SE.
Subsequently we also used EMSA to confirm the binding of CBF3 to L2. The DNA fragments containing L2 or mutated version L2-m were used as probes (Fig. 5c). The 5′ biotin-labeled L2 was incubated with CBF3-His protein and several complexes were observed (Fig. 5d). Without competitors, all probes were bound to CBF3-His protein. Addition of increasing amounts of cold competitors with the same sequence weakened the complexes and released free probes, while competitor with mutated sequence did not abolish probe-bound complex bands, suggesting the specificity of the binding in the CBF3-L2 complex (Fig. 5d). However, the concentration of cold competitors needed for eliminating probe-bound complex bands was high (x300) and the binding affinity of CBF3 to L2 appeared to be somewhat low, which could be due to the in vitro reaction condition. For further validating the activation of GA2ox7 regulated by CBF3, we performed the in vivo dual-LUC assay using transient expression of CBF3 driven by 35S promoter (used as the effector) and LUC driven by truncated GA2ox7 promoter fragments (used as reporters) (Fig. 5e). The GA2ox7 promoter reporter containing L2 + L3 + L1 that was co-transformed with CBF3 showed highest relative LUC/REN activity. The fragments of L3 + L1 or L1 lacking L2 moderately upregulated LUC, which was nearly in a half level of L2 + L3 + L1 induction, and the truncation excluding all three elements showed lowest LUC intensity. Interestingly, the promoter harboring mutated L2 (L2m + L3 + L1) exhibited the LUC/REN ratio that was similar to L3 + L1 or L1, demonstrating the contribution of L2 in the activation of GA2ox7 by CBF3. The control groups without CBF3 all showed extremely low activity of LUC, indicating the weak basal transcription of GA2ox7. These verified the in vivo activity of CBF3 in the induction of GA2ox7, which is consistent with the qPCR results. Together, ChIP and EMSA analyses suggested the interaction of CBF3 and GA2ox7 promoter, and LUC assay indicated the function of CBF3 in transcriptional regulation of GA2ox7. We propose that GA2ox7 is a newly identified CBF3 regulon that can be upregulated by CBF3 through the CRT/DRE element in plants.
DELLAs contribute to cold induction of CBF3
Interplay between GA and cold responsive signaling raises the question of how DELLAs regulate CBFs. The fact that CBF genes are transiently induced to a peak after around 3 h of cold application and DELLAs accumulation stays in a high level decreases the possibility that DELLAs directly target CBF genes. Indeed, no DELLA binding activity was detected in CBF gene regions. It has been reported that MeJA modulates CBF signaling through degradation of JASMONATE ZIM-domain (JAZ)s, a repressor of INDUCER OF CBF EXPRESSION 1 (ICE1)46. ICE1 plays a central role in CBF3 cold induction. At the same time, DELLAs can regulate JA signaling via interaction with JAZs to release MYC2, a key transcription activator in JA signaling47,48. Since the direct bindings between DELLAs and JAZs as well as JAZs and ICE1 have been revealed, ICE1 can also be released from JAZs binding by DELLAs and strongly induce CBF3 expression when cold temperature comes down. As the next step, to investigate the potential regulation of CBFs by DELLAs we measured cold induction of CBF1, -2, -3 genes when GA3 and MeJA were applied. Compared with WT, the cold induction of three CBF genes were all significantly weaker in della-global mutants. Same changes happened when MS medium contained 10−5 M GA3 (Fig. 6a–c). Nevertheless, when MeJA was present, influence by GA3 treatment or DELLA mutation were eliminated and the cold induced transcript levels of CBF1, -2, -3 were even higher than control. Likewise, after a transient induction at 12 °C CBF3 showed a higher peak under 4 °C treatment and MeJA application enhanced this induction while della-global mutation partially blocked it (Fig. 6d). Coordinately, JA positively regulates DELLAs accumulation according to increased GFP:RGA (Fig. S4) and RGL3-GFP levels after MeJA treatment48. Interestingly, without cold treatment at 22 °C, CBF1, -2, -3 did not have obvious difference of expression level in comparison between Ler and della-global seedlings with these kinds of GA3 or MeJA application (Fig. 6e), suggesting that GA3 or MeJA might not affect CBF1, -2, -3 in normal temperature. These demonstrated that DELLAs played a positive role in cold induction of CBF1, -2, -3 involving JA signaling, which was consistent with our hypothesis.
(a–c) Altered cold induction levels of CBF1, -2, -3 in Ler and della-global plants under GA3, MeJA, GA3 together with MeJA or 0.1% ethanol treatments. (d) Expression level of CBF1, -2, -3 in Ler and della-global plants under GA3 or MeJA treatments at 22 °C. (e ) CBF3 expression level under 12 °C and 4 °C in seedlings indicated. Data are means ± SE.
Discussion
The CBF signaling pathway is conserved in higher plant species14. For modulation of freezing tolerance and cold acclimation, overexpression of three CBF genes in A. thaliana results in enhanced freezing tolerance49, whereas cbf1 or cbf3 loss-of-function single mutant increases plant sensitivity to freezing stress after cold acclimation50, the cbf2 mutant shows a freezing tolerance phenotype with or without cold acclimation51. These results indicate CBF1 and CBF3 play a different role than CBF250,51. For growth restriction and late flowering, phenotype caused by CBF1 overexpression is mainly mediated by GA/DELLA signaling22. The dwarfism conferred by CBF3 and CBF2 would appear to involve either different mechanism(s) or same from that reported for CBF1. Here we determine the matching function of CBF1, -2, -3 involved in the crosstalk with GA/DELLA signaling and cause of growth reduction, in agreement with the analysis in CBF1-ox, CBF2-ox, CBF3-ox lines4,13. Increased CBF3 expression level has the same effects compared with CBF1 and CBF2 according to decreased GA levels and abundant DELLA proteins. Meanwhile, DELLAs play a positive role in cold induced expression of CBF1, CBF2 and CBF3 through interacting with JA signaling (Fig. 7). Recent reports also confirmed that CBF1, CBF2 and CBF3 transcription factors regulate very similar gene sets52. Contrary to our results, Kasuga et al.17 showed that 104 M GA3 caused no reversal of growth reduction in CBF3-ox tobacco plants and only enlarged leaf area under GA3 application was observed in CBF3-ox Arabidopsis. Moreover, Cong et al.53 also reported that CBF3-ox tobacco leaves were enlarged and petioles were lengthened by 10−4 M GA353. In our case, lower concentration of GA3 (10−5 M and 10−6 M) promotes growth in both WT and transgenic plants; nevertheless, the percentages of changes in transgenic plants are significantly higher (Fig. 1b–d). We also show that three kinds of overexpression Arabidopsis plants have a similar response to continuous application of GA3 on plates. These indicate that different treatment conditions including concentration of GA3, treatment timing or time duration can lead to diverse phenotypes in different plant materials.
In warm temperature, DELLAs interact with JAZs to prevent JAZs binding to ICE1. Meanwhile, DELLAs are degraded through GA mediated signaling. In cold temperature, ICE1 is modified to gain the function for activation of CBF3 transcription. CBF3 activates GA2ox7 to decrease the bioactive GA level and subsequently promotes the accumulation of DELLAs. Increased DELLAs release more ICE1 to enhance next round of CBF3 cold induction.
Consistent with some previous reports, we also show that RGL3 can be significantly induced by CBF1, -2 and -3 overexpression22,54. Surprisingly, no binding activity of CBF3 protein is detected in the putative CRT/DRE-like elements of RGL3 promoter in our assay. It has been acknowledged that CBF proteins do not bind equally to all CRT/DRE-like elements51,52. In any case, the induction of RGL3 by CBFs could be indirect. In other DELLA genes or GA metabolic genes, no CRT/DRE-like elements are observed8 and the only identified regulatory node in the crosstalk between CBFs and DELLAs is GA2ox7. It has been reported that DDF1, a homolog of CBFs in A. thaliana that regulates high-salinity response, also binds to promoter region of GA2ox7 and therefore reduces GA level45. Thus GA2ox7 can be a key component of CBF/DREB1 signaling pathway modulating GA and DELLAs in response to multiple abiotic stresses, which is a good candidate of modification target in the genetic engineering and molecular breeding of stress tolerant crops without yield penalty.
There is an evidence supporting that the activation of CBF regulons by CBF1 is in a DELLA-independent fashion - when CBF1 was overexpressed in normal temperature, transcript levels of CBF regulons are similar in WT and DELLA mutants22. The present work reveals that although DELLAs do not affect CBFs regulation in CBF regulons transcription, they contribute to the cold induced expression of CBF1, -2, -3 instead. The CBF genes expression in warm temperature and cold induction are in different regulatory routes. ICE1, the key activator of CBF genes in cold induction, is constitutively expressed and can only be modified to gain function for activation of CBF genes under low temperature2,46. Previous analysis of DELLA direct targets did not detect binding of DELLAs to CBF1, -2, -3 promoter regions55, thus DELLAs can regulate cold induction of CBF1, -2, -3 through ICE1. Recent studies on JA signaling provide a clue connecting ICE1, JAZs and DELLAs. JAZs, a major repressor in JA signaling, directly targets ICE1 to inhibit the activation of CBFs, while DELLAs competitively bind to JAZs to release MYC2 to activate JA response46,47,48. Meanwhile, MYC2 also interacts with ICE1 to enhance CBF genes transcription in cold condition56. Indeed, our work shows that MeJA treatment, which degrades JAZs and activates MYC2, eliminates the effects from GA and della-global mutation and increases CBF1, -2, -3 cold induced expression levels (Fig. 6a–d). Notably, due to the transient induction of CBF1, -2, -3 under low temperature, the abundance of DELLAs caused by CBFs unlikely have a direct feedback to induce CBFs. Consistently, neither GA nor JA change CBF1, -2, -3 expression in warm temperature, suggesting that DELLAs strengthen “priming” of induction of CBF genes before cold application through JA-dependent pathway. When DELLAs are abundant, the subsequent induction of CBF genes can be enhanced (Fig. 7).
In addition, the present work shows that GA application or della-global mutation does not completely recover the growth repression of CBF1-ox, CBF2-ox or CBF3-ox plants, especially for CBF3-ox seedlings. Hence there are still some DELLA-independent pathways involved in CBF-caused growth retardation. Analysis in a CBF gene from Capsella bursa-pastoris revealed that CbCBF also affected cell cycle signaling besides antagonizing with GA23. In A. thaliana, although microarray has been used in some work about CBF signaling, more powerful tools such as deep RNA-seq will be needed to uncover more details. In summary, the present knowledge of positive regulation between DELLAs and CBF transcription factors as well as the investigation in additional unknown mechanism of how CBFs restrain growth can contribute to the accurate genetic control in molecular breeding of tolerant crops.
Methods and Materials
Plant materials and treatments for phenotyping
The A. thaliana seeds were grown in pots at 22 °C under 16-h-light/8-h-dark cycle. The cbf3 knock-out line (SAIL_244_D02)57, della-global mutant (gai-t6; rga-t2; rgl1-1; rgl2-1; rgl3-1)58 and pRGA::GFP:RGA line (Col and Ler)59 were obtained from Arabidopsis Biological Resource Center. To generate CBF1-ox della-global, CBF2-ox della-global, CBF3-ox della-global, CBF1-ox pRGA::GFP:RGA, CBF2-ox pRGA::GFP:RGA and CBF3-ox pRGA::GFP:RGA plants, the full length of the CBF1, CBF2 or CBF3 coding sequence was cloned into pCAMBIA1304 vector using primers listed in Table S1 and transformed into della-global and pRGA::GFP:RGA plants. The cbf3 knock-out line (SAIL_244_D02) was crossed with pRGA::GFP:RGA line (Col) and cbf3 pRGA::GFP:RGA line was isolated from F3 progeny. CBF1-ox, CBF2-ox and CBF3-ox plants in Ws background were previously described4. For phenotyping in low temperature, 14-d-old plants growing at 22 °C were transferred to 12 °C and applied to the measurements when they were 4-week-old. Gibberellin spray treatments were performed as previously described23. For phenotyping on plates, GA3 with concentration of 10−5 and 10−6 M was added to Murashige and Skoog (MS) agar medium and seedlings were grown at 22 °C for 3 weeks. For leaf area analysis, the fifth rosette leaves of 4-week-old plants were collected and determined for size with IMAGEJ (http://rsbweb.nih.gov/).
Measurements of endogenous gibberellin contents in Arabidopsis
The endogenous gibberellin level in A. thaliana was measured using enzyme linked immunosorbent assay (ELISA) as described elsewhere23. Briefly, samples were extracted in cold 80% (v/v) methanol with 1 mM butylated hydroxytoluene overnight at 4 °C. After centrifugation at 10, 000 g for 20 min, the extracts were passed through a C18Sep-Pak cartridge (Waters, Milford, MA, USA) and residues were dissolved in 10 mM PBS buffer (pH 7.4). Meanwhile, the 96-well microtitration plates (Nunc, Denmark) was coated with synthetic GA1-ovalbumin conjugates in 50 mM NaHCO3 buffer (pH 9.6) overnight at 37 °C. Samples were incubated with HRP-labeled goat anti-rabbit immunoglobulins for 1 h at 37 °C and ovalbumin solution (10 mg/mL) was used to block nonspecific binding. The enzyme-substrate reaction was carried out in the dark and data were calculated according to absorbance of 490 nm. The cross-reactivity of antibodies raised against GA1-ovalbumin to GA3-ovalbumin was 32% based on previous report60.
Cold and phytohormone treatments for transcript and protein level tests
For gene expression tests in Ws and Col plants, 14-d-old seedlings growing in soil at 22 °C were transferred to 12 °C and rosette leaves were collected. For Ler and della-global plants, 4-d-old seedlings grown in MS medium containing 10 μM GA3, 5 μM MeJA46, GA3 together with MeJA or 0.1% ethanol at 22 °C were transferred to 12 °C. For pRGA::GFP:RGA lines, 8-d-old seedlings were incubated in 50 mM MeJA48 for time designed. The plant materials were collected immediately in liquid nitrogen at each time point of treatments as indicated and stored at −80 °C until use.
Quantitative real-time PCR
Total RNA was extracted using Plant RNA Mini Kit (Watson Biotechnologies, Inc, China). RNA concentration was estimated by spectrophotometer (WFZUV-2100, UnicoTM Instruments Inc.) and genomic DNA was removed using DNAase I (Promega, Madison, WI, USA). Approximately 1 μg RNA was reverse transcribed using PrimeScript® RT Master Mix (Takara, China) at 37 °C for 20 min. The PCR amplification reactions were carried out using SYBR® Premix Ex Taq™ II (Perfect Real Time) (Takara, China) with three replicates for each sample and the Actin2 gene (AY096381) was used as internal control. The 2−△△Ct method was used to determine the relative mRNA abundance and primers used in this work are all listed in Table S1.
Confocal Microscopy analysis
The 8-d-old seedlings of pRGA::GFP:RGA, CBF1-ox pRGA::GFP:RGA, CBF2-ox pRGA::GFP:RGA and CBF3-ox pRGA::GFP:RGA grown on MS plates were sprayed with 10−5 M GA3 or 0.1% ethanol at 22 °C or 12 °C, respectively. After 4 h the root tip and elongating zone were excised with razor blade. GFP:RGA fusion protein level was detected by confocal laser scanning microscopy (Leica TCS NT, Wetzlar, Germany) as mentioned previously61.
Protein extraction and western blot
Proteins were extracted using Plant Protein Extraction Reagent kit (CWBIO, China). Approximate 1 g plant tissues were used for each sample. Protein samples were separated by SDS-PAGE on 10% polyacrylamide gels and transferred onto PVDF membranes according to standard protocols. Membranes were probed with anti-GFP antibody (Beyotime Biotechnology, China) at a 1:2000 dilution and signals were visualized on a Typhoon system (GE Healthcare, http://www.gelifesciences.com/).
Electrophoretic mobility shift assay
CBF3 cDNA was cloned into pET-28a vector using primers CBF3-HisF and CBF3-HisR (Table S1). Soluble CBF3-His protein was expressed in Escherich coli strain BL21 and purified using HisPur™ Cobalt Resin (Thermo, USA). The 5′ biotin-labeled double-stranded oligonucleotides GA2ox7-L2 was used as a probe while non-labeled GA2ox7-L2 and GA2ox7-L2-m (mutated) were used as competitors, respectively. The labeled probes (3 pM) with or without unlabeled competitors were incubated for 20 min at room temperature with 4 μg of purified CBF3-His fusion protein in binding buffer (25 mM HEPES-KOH buffer at pH 7.9, containing 50 mM KCl, 0.5 mM EDTA, 0.5 mM DTT and 10% glycerol) supplemented with 20 pM poly (dI-dC). The resulting DNA-protein complexes were resolved by electrophoresis on a 6% non-denaturing polyacrylamide gel in 0.5 × TBE buffer and transferred by electroblotting to PVDF membranes. After crosslinked under UV (120 mJ/cm2, 254 nm), the signal was visualized according to manufacturer instruction of LightShift Chemiluminescent EMSA kit (Thermo, USA).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed as previously described55. Briefly, 30 μL protein A-agarose beads (Epigentek) were incubated with 5 μg anti-CBF3 antibody at 4 °C for overnight. Soluble CBF3-His protein described above was used to immunize two rabbits to obtain antiserum. The immunoglobulin G (polyclonal antibodies) fraction was purified by affinity chromatography using protein G-agarose (Sigma-Aldrich, USA). Preserum was used as a background control. Around 3 g of washed 4-week-old plants were submerged in 50 mL of crosslinking buffer (10 mM Tris-HCl, pH8, 0.4M Suc, 1 mM PMSF, 1 mM EDTA and 1% formaldehyde) and vacuum infiltrated for 5 min at room temperature. The cross-linking was stopped by transferring plant materials to 250 mM Gly and vacuum infiltration for 5 min. Plant tissues were ground in liquid nitrogen and resuspended in 25 mL cold nuclei isolation buffer (15 mM PIPES, pH 6.8, 0.25 M Suc, 5 mM MgCl2, 60 mM KCl, 15 mM NaCl, 1 mM CaCl2, 1% Triton X-100, 20 mM sodium butyrate, 1 mM PMSF, 2 μg/mL pepstatin A and 2 μg/mL aprotinin). The homogenized slurry was filtered and centrifuged at 3800 g for 20 min to get the pellet (nuclei). The nuclei were resuspended in 1.5 mL of lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.1% sodium deoxycholate, 1% Triton X-100, 20 mM sodium butyrate, 1 μg/mL pepstatin A and 1 μg/mL aprotinin). DNA was sheared into 200 bp to 1000 bp fragments by 6–10 min of 5 sec pause sonication at 40% amplitude using a TekMar TM-100 sonic disruptor (TekMar). After centrifugation at 13,800 g for 10 min, the supernatant was diluted for five folds with nuclei lysis buffer. The prepared mixture of protein A-agarose beads and antibody was added and the sample was incubated at 4 °C for overnight with gentle rotation. After 3800 g centrifugation for 2 min, the agarose beads were sequentially washed with low salt wash buffer (20 mM Tris-HCl, pH 8, 150 mM NaCl, 0.2% SDS, 0.5% Triton X-100, 2 mM EDTA), high salt wash buffer (20 mM Tris-HCl, pH 8, 500 mM NaCl, 0.2% SDS, 0.5% Triton X-100, and 2 mM EDTA), LiCl wash buffer (10 mM Tris-HCl, pH 8, 0.25 M LiCl, 1% sodium deoxy-cholate, 1% Nonidet P-40, and 1 mM EDTA), and TE buffer (twice; 1 mM EDTA and 10 mM Tris-HCl, pH 8). The immunocomplexes were eluted with freshly made elution buffer (0.1 M NaHCO3 and 0.5% SDS) and incubated at 65 °C for 15 min. The crosslink was reversed by incubation at 65 °C for overnight in the presence of 250 mM NaCl. Proteins were digested by adding 20 mL of 1 M Tris-HCl, pH 6.5, 10 mL of 0.5 M EDTA, and 2 mL proteinase K (10 mg/mL) and incubated at 45 °C for 2 h. Immunoprecipitated DNA was purified using a mixture of phenol:chloroform:isoamylalcohol (25:24:1) and subsequently used for qRT-PCR with the primers listed in Table S1.
Dual-Luciferase Assays
Coding regions of CBF3 was cloned into the pCAMBIA1304 binary vector as the effector plasmid, in which CBF3 was driven by 35S promoter. Four fragments of GA2ox7 promoter truncations were inserted into the pGreenII-0800-LUC as the reporter plasmids. All constructs were introduced into Agrobacteria strain GV3101, respectively. The pSoup-P19 helper plasmid was co-transformed with pGreenII- 0800-LUC vectors. The reporter and effector Agrobacteria were mixed with a ratio of 2:8 and infiltrated into 3-week-old Nicotiana benthamiana leaves. Three days after infiltration, leaf discs were collected and the luciferase activity of extracts was analyzed using a Dual-Luciferase Assay Kit (Promega) as previously described62. Three replicates for each co-transformation were carried out.
Additional Information
Accession codes: Sequence data used in this work can be found in the GenBank libraries with the following accession numbers: CBF1 (AT4G25490), CBF2 (AT4G25470), CBF3 (AT4G25480), GID1a (AT3G05120), GID1b (AT3G63010), GID1c (AT5G27320), GAI (AT1G14920), RGA (AT2G01570), RGL1 (AT1G66350), RGL2 (AT3G03450), RGL3 (AT5G17490), SLY1 (AT4G24210), GA20ox1 (AT4G25420), GA20ox2 (AT 5G51810), GA20ox3 (AT5G07200), GA3ox1 (AT1G15550), GA3ox2 (AT1G80340), GA2ox1 (AT1G78440), GA2ox2 (AT1G30040), GA2ox3 (AT2G34555), GA2ox4 (AT 1G47990), GA2ox6 (AT1G02400), GA2ox7 (AT1G50960).
How to cite this article: Zhou, M. et al. Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci. Rep. 7, 39819; doi: 10.1038/srep39819 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Knight, M. R. & Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol 195, 737–751, doi: 10.1111/j. 1469-8137.2012. 04239.x (2012).
Zhou, M. Q., Shen, C., Wu, L. H., Tang, K. X. & Lin, J. CBF-dependent signaling pathway: A key low temperature responder in plants. Crit Rev Biotechnol 31, 186–192, doi: 10.3109/07388551.2010.505910 (2011).
Gilmour, S. J. et al. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16, 433–442, doi: 10.1046/j.1365-313x.1998. 00310.x (1998).
Gilmour, S. J., Fowler, S. G. & Thomashow, M. F. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54, 767–781 (2004).
Liu, K. R., Gilmour, S. J., Zarka, D. G., Schabenberger, O. & Thomashow, M. F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106, doi: 10.1126/science.280.5360.104 (1998).
Wingler, A. Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Front Plant Sci 5, 794, doi: 10.3389/fpls.2014.00794 (2015).
Wang, Y. & Hua, J. A moderate decrease in temperature induces COR15a expression through the CBF signaling cascade and enhances freezing tolerance. Plant J 60, 340–349, doi: 10.1111/j.1365-313X.2009.03959.x (2009).
Maruyama, K. et al. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J 38, 982–993, doi: 10.1111/j.1365-313X.2004. 02100.x (2004).
Rasmussen, S. et al. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol 161, 1783–1794, doi: 10.1104/pp.112.210773 (2013).
Stockinger, E. J., Gilmour, S. J. & Thomashow, M. F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94, 1035–1040 (1997).
Jaglo-Ottosen, K. R., Gilmour, S. J., Zarka, D. G., Schabenberger, O. & Thomashow, M. F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science, 280, 104–106, doi: 10.1126/science.280.5360.104 (1998).
Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K. & Shinozaki, K. Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17, 287–291, doi: 10.1038/ 7036 (1999).
Gilmour, S. J., Sebolt, A. M., Salazar, M. P., Everard, J. D. & Thomashow, M. F. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124, 1854–1865, doi: 10.1104/pp.124.4.1854 (2000).
Kurepin, L. V. et al. Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int J Mol Sci 14, 12729–12763, doi: 10.3390/ijms140612729 (2013).
Hsieh, T. H. et al. Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 129, 1086–1094, doi: 10.1104/pp.003442 (2002).
Lee, S. C., Huh, K. W., An, K., An, G. & Kim, S. R. Ectopic expression of a cold-inducible transcription factor, CBF1/DREB1b, in transgenic rice (Oryza sativa L). Mol Cells 18, 107–114 (2004).
Kasuga, M., Miura, S., Shinozaki, K. & Yamaguchi-Shinozaki, K. A combination of the Arabidopsis DREB1a gene and stress-inducible RD29a promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45, 346–350, doi: 10.1093/pcp/pch037 (2004).
Benedict, C. et al. The CBF1-dependent low temperature signaling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ 29, 1259–1272, doi: 10.1111/j.1365-3040.2006.01505.x (2006).
Pino, M. T. et al. Ectopic AtCBF1 over-expression enhances freezing tolerance and induces cold acclimation-associated physiological modifications in potato. Plant Cell Environ 31, 393–406, doi: 10.1111/j.1365-3040.2008. 01776.x (2008).
Bhatnagar-Mathur, P. et al. Transgenic peanut overexpressing the DREB1A transcription factor has higher yields under drought stress, Mol Breeding 33, 327–340, doi: 10.1007/s11032-013-9952-7 (2014).
Achard, P. et al. Integration of plant responses to environmentally activated phytohormonal signals. Science 331, 91–94, doi: 10.1126/science.1118642 (2006).
Achard, P. et al. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20, 2117–2129, doi: 10.1105/tpc.108.058941 (2008).
Zhou, M. Q. et al. CbCBF from Capsella bursa-pastoris enhances cold tolerance and restrains growth in Nicotiana tabacum by antagonizing with gibberellin and affecting cell cycle signaling. Plant Mol Biol 85, 259–275, doi: 10.1007/s11103-014-0181-1 (2014).
Feng, S. H. et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479, doi: 10.1038/nature06448 (2008).
Lucas, M. et al. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484, doi: 10.1038/nature06520 (2008).
Xu, Y. L., Li, L., Gage, D. A. & Zeevaart, J. A. Feedback regulation of GA5 expression and metabolic engineering of gibberellin levels in Arabidopsis . Plant Cell 11, 927–936, doi: 10.1105/tpc.11.5.927 (1999).
Chiang, H. H., Hwang, I. & Goodman, H. M. Isolation of the Arabidopsis GA4 locus. Plant Cell 7, 195–201, doi: 10.1105/tpc.7.2.195 (1995).
Cowling, R. J., Kamiya, Y., Seto, H. & Harberd, N. P. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis . Plant Physiol 117, 1195–1203, doi: 10.1104/pp.117.4.1195 (1998).
Thomas, S. G., Phillips, A. L. & Hedden, P. Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96, 4698–4703, doi: 10.1073/ pnas.96.8.4698 (1999).
Dill, A., Thomas, S. G., Hu, J. H., Steber, C. M. & Sun, T. P. The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 16, 1392–1405, doi: 10.1105/tpc.020958 (2004)
Fu, X. et al. The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16, 1406–1418, doi: 10.1105/tpc.021386 (2004).
Peng, J. et al. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11, 3194–3205, doi: 10.1101/gad.11.23.3194 (1997).
Silverstone, A. L., Ciampaglio, C. N. & Sun, T. P. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10, 155–169, doi: 10.1105/tpc.10.2.155 (1998).
Wen, C. K. & Chang, C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14, 87–100, doi: 10.1105/tpc.010325 (2002).
Lee, S. et al. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibitions. Genes Dev 16, 646–658, doi: 10.1101/gad.969002 (2002).
Zhang, Z. L. et al. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 108, 2160–2165, doi: 10.1073/pnas.1012232108. (2011).
Silverstone, A. L. et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13, 1555–1566, doi: 10.1105/TPC.010047 (2001).
Wilson, R. N., Heckman, J. W. & Somerville, C. R. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100, 403–408, doi: 10.1104/pp.100.1.403 (1992).
Wilson, R. N. & Sommerville, C. R. Phenotypic suppression of the gibberellins insensitive mutant (gai) of Arabidopsis. Plant Physiol 108, 495–502 doi: 10.1104/pp.108.2.495 (1995).
Magome, H., Yamaguchi, S., Hanada, A., Kamiya, Y. & Oda, K. Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37, 720–729, doi: 10.1111/j.1365-313X.2003. 01998.x (2004).
Kumar, S. V. et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245, doi: 10.1038/nature10928 (2012).
Hedden, P. & Phillips, A. L. Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5, 523–530 (2000).
Yamaguchi-Shinozaki, K. & Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low temperature, or high-salt stress. Plant Cell 6, 251–264, doi: 10.1105/tpc.6.2.251 (1994).
Sakuma, Y. et al. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold- inducible gene expression. Biochem Biophys Res Commun 290, 998–1009, doi: 10.1006/bbrc. 2001.6299 (2002).
Magome, H., Yamaguchi, S., Hanada, A., Kamiya, Y. & Oda, K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56, 613–626, doi: 10.1111/j.1365-313X.2008.03627. x (2008).
Hu, Y., Jiang, L., Wang, F. & Yu, D. Jasmonate regulates the Inducer of Cbf Expression–C-Repeat Binding Factor/Dre Binding Factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25, 2907–2924, doi: 10.1105/tpc.113.112631 (2013).
Hou, X. L., Lee, L. Y. C., Xia, K., Yan, Y. Y. & Yu, H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19, 884–894, doi: 10.1016/j. devcel.2010.10.024 (2010).
Wild, M. et al. The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell, 24, 3307–3319, doi: 10.1105/tpc.112.101428 (2012).
Thomashow, M. F. So what’s new in the field of plant cold acclimation? Lots! Plant Physiol 125, 89–93, doi: 10.1104/pp.125.1.89 (2001).
Novillo, F., Medina, J. & Salinas, J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA 104, 21002–21007, doi: 10.1073/pnas. 0705639105 (2007).
Novillo, F., Alonso, J. M., Ecker, J. R. & Salinas, J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101, 3985–3990, doi: 10.1073/pnas.0303029101 (2004).
Park, S. et al. Regulation of the Arabidopsis CBF regulon by a complex low- temperature regulatory network. Plant J 82, 193–207, doi: 10.1111/tpj.12796 (2015).
Cong, L., Zheng, H. C., Zhang, Y. X. & Chai, T. Y. Arabidopsis DREB1A confers high salinity tolerance and regulates the expression of GA dioxygenases in tobacco. Plant Sci 174, 156–164, doi: 10.1016/j.plantsci.2007.11.002 (2008).
Siddiqua, M. & Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ 34, 1345–1359, doi: 10.1111/j.1365-3040.2011. 02334.x (2011).
Zentella, R. et al. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19, 3037–3057, doi: 10.1105/tpc.107.054999 (2007).
Zhao, M. L. et al. Induction of jasmonate signaling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ 36, 30–51, doi: 10.1111/j.1365-3040.2012. 02551.x (2012).
Su, Z. et al. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25, 3785–3807, doi: 10.1105/tpc.113.115428 (2013).
Hong, G. J., Xue, X. Y., Mao, Y. B., Wang, L. J. & Chen, X. Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24, 2635–2648, doi: 10.1105/tpc.112.098749 (2012).
Dill, A., Jung, H. S. & Sun, T. P. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98, 14162–14167, doi: 10.1073/pnas.251534098 (2001).
Shan, D. P. et al. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176, 70–81, doi: 10.1111/j.1469-8137.2007.02160.x (2007).
Zhou, M. Q., Wu, L. H., Liang, J., Shen, C. & Lin, J. Cold-induced modulation of CbICE53 gene activates endogenous genes to enhance acclimation in transgenic tobacco. Mol Breeding 30, 1611–1620, doi: 10.1007/s11032-012-9744-5 (2012).
Cui, J. et al. Feedback regulation of DYT1 by interactions with downstream bHLH factors promotes DYT1 nuclear localization and anther development. Plant Cell 28, 1078–1093, doi: 10.1105/tpc.15.00986 (2016).
Acknowledgements
The seeds of CBF1-ox, CBF2-ox and CBF3-ox lines are kind gifts from Prof. Ilha Lee (Seoul National University). We are also grateful for the financial support from the Natural Science Foundation of China (31370346) and the National High Technology Research and Development Program of China (2008AA10Z105).
Author information
Authors and Affiliations
Contributions
J.L. conceived the study and designed the experiments. M.Z., H.C., and H.W. performed the experiments. H.M. gave very helpful suggestions to the manuscript. M.Z. and J.L. wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, M., Chen, H., Wei, D. et al. Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci Rep 7, 39819 (2017). https://doi.org/10.1038/srep39819
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39819
This article is cited by
-
Jasmonate resistant 1 and ethylene responsive factor 11 are involved in chilling sensitivity in pepper fruit (Capsicum annuum L.)
Scientific Reports (2022)
-
Interaction of gibberellin and other hormones in almond anthers: phenotypic and physiological changes and transcriptomic reprogramming
Horticulture Research (2021)
-
Co-regulation Role of Endogenous Hormones and Transcriptomics Profiling Under Cold Stress in Tetrastigma hemsleyanum
Journal of Plant Growth Regulation (2021)
-
AtLRRop2, an leucine-rich repeat-only protein, mediates cold stress response in Arabidopsis thaliana
Plant Biotechnology Reports (2021)
-
AP2/ERF, an important cold stress-related transcription factor family in plants: A review
Physiology and Molecular Biology of Plants (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.