Abstract
Pesticide resistance represents a major challenge to global food production. The spread of resistance alleles is the primary explanation for observations of reduced pesticide efficacy over time, but the potential for gene-by-environment interactions (plasticity) to mediate susceptibility has largely been overlooked. Here we show that nutrition is an environmental factor that affects susceptibility to Bt toxins. Protein and carbohydrates are two key macronutrients for insect herbivores, and the polyphagous pest Helicoverpa zea self-selects and performs best on diets that are protein-biased relative to carbohydrates. Despite this, most Bt bioassays employ carbohydrate-biased rearing diets. This study explored the effect of diet protein-carbohydrate content on H. zea susceptibility to Cry1Ac, a common Bt endotoxin. We detected a 100-fold increase in LC50 for larvae on optimal versus carbohydrate-biased diets, and significant diet-mediated variation in survival and performance when challenged with Cry1Ac. Our results suggest that Bt resistance bioassays that use ecologically- and physiologically-mismatched diets over-estimate susceptibility and under-estimate resistance.
Similar content being viewed by others
Introduction
Acreage of transgenic Bt crops has increased over 60-fold since their introduction in 1996, with over 1 billion acres planted throughout the world1. Due to concerns about the evolution of insect resistance to this technology, the U.S. EPA mandates resistance monitoring for all Bt plant-incorporated protectants. Nevertheless, incidents of field-evolved resistance to Bt crops have been reported in 5 of the 13 insect pest species examined as of 20131. The overriding assumption in Bt resistance monitoring is that genetic factors are primarily responsible for the presence of resistant phenotypes2,3 (Fig. 1a), as evidenced by the fact that all definitions for the term resistance describe a change in susceptibility that is genetically-determined and heritable4. This is true even though the underlying genetics responsible for resistance are difficult to identify and rarely known in instances of field-evolved resistance, except for a few species/populations5,6,7,8,9,10,11,12,13. We should note, that our use of the term susceptibility throughout the paper refers only to the extent or likelihood of an organism being negatively affected by a Bt trait, and this usage makes no assumptions about the mode of susceptibility or its underlying heritability.
An alternative to this gene-centric view is to acknowledge that resistant phenotypes are not solely dependent on an individual’s genotype, but ultimately on gene expression. The regulation of all genes is, at least to some extent, dependent on environmental factors. These gene-by-environment interactions allow a single genotype (individual) to produce a range of phenotypes across different environmental conditions; a phenomenon referred to as phenotypic plasticity. As such, an insect could exhibit low susceptibility to Bt in one environment and high susceptibility in another (Fig. 1b). Phenotypic plasticity is widespread across both traits and organisms14,15,16,17,18 and is likely to occur when populations experience a variable environment with contrasting fitness advantages of specific phenotypes across different environments19,20,21,22,23,24,25. Nutrition is a pervasive environmental factor that meets both these criteria, as plant nutrient content is often highly variable26,27,28,29 and the nutritional quality of plant resources, particularly the concentration and balance of protein and digestible carbohydrates (henceforth carbohydrates), has strong effects on insect performance, including growth rate, reproduction30,31, and detoxification ability32,33. Furthermore, because insects actively regulate their intake of both protein (P) and carbohydrates (C), they can achieve an optimal balance (termed an intake target) by feeding selectively on plant tissues that most closely match their current nutritional needs, or mixing among those that are nutritionally complementary31,34,35. It is also the case that the concentration of plant defensive compounds within and between individual plants can be variable36,37,38,39,40,41 and that plant nutrient content can interact with plant defensive compounds to affect insect herbivore performance33,42. However, our focus for the current manuscript is on plant nutrients. Once this baseline has been established, it is then possible to study the effects of plant secondary compounds, and interactions between plant nutrients and plant secondary compounds, as environmental factors impacting susceptibility to Bt toxins.
Currently the effects of nutrition on insecticide susceptibility and resistance is poorly understood, particularly in agricultural systems where environmentally-mediated effects may have significant economic implications43,44,45. Helicoverpa zea, known as the cotton bollworm or corn earworm, is a widespread New World lepidopteran pest targeted by Bt transgenic plants. A recent re-examination of H. zea’s nutritional requirements highlights a critical disparity between their self-selected protein-to-carbohydrate ratio (P:C) and the P:C ratio of diets routinely used in diet-incorporation Bt resistance bioassays. The empirically-determined intake target P:C ratio for H. zea is slightly protein-biased, at 1.6:146, but the majority of rearing diets, including commercial diets, for H. zea are severely carbohydrate-biased, at around 0.4:129.
We conducted two experiments using H. zea as a model to test whether variation in diet protein and carbohydrates impacts susceptibility to Cry1Ac endotoxins, one of the major plant-incorporated insecticides widely expressed in Bt cotton and corn. First, we performed 7-day dose-response assays to calculate the LC50 for neonates fed either a commercially-available rearing diet or the same diet modified to match H. zea’s P:C intake target ratio of 1.6:1. We then expanded our study beyond 7 days and reared larvae on artificial diets created to mimic the range of P:C ratios and total macronutrient concentrations (P + C) present in different cotton tissues47. We hypothesized that diet protein-carbohydrate content would have strong effects on survival and performance across sub-lethal and lethal concentrations of Cry1Ac, and that the diet most closely matching the self-selected P:C ratio of 1.6:1 would confer the greatest survival and performance for larva when challenged with Cry proteins.
Results
Experiment 1: Cry1Ac dose response assays on diets with different protein-carbohydrate content
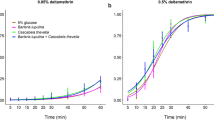
The LC50 concentration of Cry1Ac varied by approximately two orders of magnitude across the three diets (Table 1; Fig. 2). It was lowest on the commercial diet (CD; Southland Products, Lake Village, AR), which had a P:C ratio that was very carbohydrate-biased relative to the self-selected P:C observed for H. zea46; the total macronutrient content (P + C) of the CD was also high (62%) relative to what is typical for most plant vegetative tissues47. In contrast, the LC50 value was 75 times higher for neonates fed a modified commercial diet (MCD1) that had a P:C matching the self-selected ratio for H. zea, but a macronutrient content similar to the CD. Finally, LC50 values were 100 times higher (compared to the CD) when caterpillars were fed a modified diet (MCD2) that had a P:C matching the self-selected ratio of H. zea, but a total macronutrient content more in line with most plant vegetative tissues (P + C = 46%). Variability in LC50 values was evident between the three trials, but the relative effects of diet were consistent. The LC50 values for each diet, across trials one, two and three, were as follows: (1) CD – 0.08, 0.92, 0.08, (2) MCD1 – 4.2, 14.4, 8.5, and (3) MCD2 – 32.3, 18.6, 2.2. Despite this variability, the LC50 values for the CD were consistently and considerably lower than either of the MCDs across trials. In fact, the LC50 values for the MCD1 were between 15.7–106.5 times greater than the LC50 values of the CD values, while the LC50 values for the MCD2 were between 20.2–408.8 times greater compared to the CD.
Dose-response curves for H. zea neonates in diet-incorporated bioassays (Experiment 1). One of the diets was a commercially available (CD) carbohydrate-biased rearing diet commonly used for Bt resistance monitoring. The other two diets were modified (MCD1 and MCD2) to reflect protein-carbohydrate profiles that are more physiologically- and ecologically-relevant and to align with H. zea’s self-selected P:C intake target. Mortality represents the average for three trials; the LC50 concentrations are listed for each diet (N = 22–28 larvae per concentration).
Experiment 2: Interactions between Cry1Ac and food protein-carbohydrate content
We first examined the effects of Cry1Ac concentration on survival independent of diet protein-carbohydrate content (we pooled individuals from all diets together). Specifically, we employed a Kaplan-Meier survival analysis to analyze time until death; all individuals that pupated were right censored. As expected, there were significant Cry1Ac concentrations effects (X2 = 218.70, df = 4, P < 0.001). Post hoc tests indicated that time until death was similar on the control and 0.1 ppm treatments (X2 = 0.01, P = 0.924), but that it differed significantly between the 0.1 ppm and 0.6 ppm treatments (X2 = 13.60, P < 0.001), the 0.6 and 1 ppm treatments (X2 = 18.33, P < 0.001), and the 1 ppm and 3 ppm treatments (X2 = 17.34, P < 0.001).
To understand how diet protein-carbohydrate content might mediate these strong Cry1Ac concentration effects, we next compared survival between our four P:C diets within each Cry1Ac concentration. On the diets lacking Cry1Ac (Fig. 3a; X2 = 4.56, P = 0.207), and the diets with Cry1Ac at 0.1 ppm (Fig. 3b; X2 = 2.05, P = 0.563) and 0.6 ppm (Fig. 3c; X2 = 4.12, P = 0.249), there were no significant differences in time until death as a function of diet protein-carbohydrate content. In contrast, significant effects were observed when diets contained Cry1Ac at 1 ppm (X2 = 30.23, P < 0.001) and 3 ppm (X2 = 46.69, P < 0.001). On the treatments containing 1 ppm Cry1Ac (Fig. 3d), caterpillars lived longest on the p24:c18 and p39:c29 diets; the two diets with a P:C that most closely matched H. zea’s self-selected ratio46 (1.6:1). On the treatments with 3 ppm Cry1Ac (Fig. 3e), caterpillars lived longest on the p39:c29, the diet with a P:C closest to the H. zea intake target but a higher total macronutrient concentration, and shortest on the carbohydrate-biased diet (p12:c30). Larval survival was intermediate on diets that were protein-biased and had total macronutrient content of 42% (p24:c18 and p30:c12).
Survival plots for larvae reared on diets with different protein-carbohydrate content, at different Cry1Ac concentrations (Experiment 2). Four diets (p12:c30, p24:c18, p30:c12 and p39:c28) and five Cry1Ac concentrations were tested: (a) no Cry1Ac. (b) 0.1 ppm, (c) 0.6 ppm, (d) 1 ppm, and (e) 3 ppm (N = 16–34 larvae per treatment). Hatch marks indicate pupation events, and line endpoints indicate the point in time where all individuals had either pupated or died. Different letters indicate post hoc differences between the four diets within each Cry1Ac concentration (α = 0.05).
We also examined pupation success across all Cry1Ac concentrations and diet P:C combinations by scoring larvae as either pupating or not pupating, and then analyzed these data using a logistic regression analysis. We observed a significant Cry1Ac concentration effect (X2 = 292.51, P < 0.001), but there was no significant diet P:C effect (X2 = 0.000, P = 1.000), or Cry1Ac-by-diet P:C interaction (X2 = 15.10, P = 0.236). Odds ratios indicated pupal success was best in the absence of Cry1Ac (Table S1), and that all Cry1Ac treatments differed from one another except for the 1 and 3 ppm treatments (which had equally low pupation).
Because high mortality was observed on the 1 and 3 ppm treatments, analysis of developmental time from egg to pupation, pupal mass, and consumption was restricted to the controls (no Cry1Ac), 0.1 and 0.6 ppm treatments. Developmental time (egg to pupa) increased as Cry1Ac concentration increased (X2 = 260.56, P < 0.001), and each treatment was statistically different from one another (no Cry versus 0.1 ppm: X2 = 158.23, P < 0.001; 0.1 ppm versus 0.6 ppm: X2 = 77.17, P < 0.001). We thus examined development time on each diet P:C within each Cry1Ac concentration. In the control treatments (no Cry1Ac), a significant difference was observed (X2 = 9.32, P = 0.025). Development was fastest on the high macronutrient diet (p39:c29), and statistically similar for the three other diets, which each had a total macronutrient content of 42% (Fig. 4d). There was no difference in developmental time when diets contained 0.1 ppm of Cry1Ac (X2 = 1.93, P = 0.588; Fig. 4e), but at 0.6 ppm, significant diet P:C effects were observed (X2 = 13.46, P = 0.004). At 0.6 ppm Cry1Ac, development was fastest on the p39:c29 diet and intermediate on the p24:c18 diet (the two diets closest to the intake target P:C ratio), but was equally slow on the carbohydrate-biased p12:c30 and protein-biased p30:c12 diets (Fig. 4f).
Pupation success (N = 16–34 larvae per treatment), larval development time (N = 6–30 larvae per treatment), and pupal mass (N = 6–32 larvae per treatment) for larvae reared on diets with different protein-carbohydrate content, at different Cry1Ac concentrations (Experiment 2). Development (mean ± SEM) and pupal mass (mean ± SEM) were only recorded for larvae that pupated. Each column represents one of the three sub-lethal Cry1Ac concentrations: no Cry1Ac (panels a, d and g), 0.1 ppm (panels b, e and h) and 0.6 ppm (panels c, f and i). Within each panel the four diets are shown (p12:c30, p24:c18, p30:c12 and p39:c28). Different letters indicate significant differences between treatments (α = 0.05).
There was a significant effect of Cry1Ac concentration on pupal mass (F2 = 62.89, P < 0.001), but no diet effect (F3 = 0.13, P = 0.945), or interaction between diet P:C and Cry1Ac concentration (F6 = 0.81, P = 0.564). A post-hoc comparison between the controls, 0.1 ppm, and 0.6 ppm treatments showed that pupal mass decreased significantly as Cry1Ac concentration increased (Fig. 4d–f).
Effects of diet P:C and Cry1Ac concentration on consumption were also investigated; for these analyses only consumption from insects that pupated was analyzed. Total diet consumption was significantly affected by Cry1Ac concentration (ANOVA: F2 = 59.49, P < 0.001); it increased in the presence of Cry1Ac (Fig. 5a). Additionally, a significant interaction between diet P:C and Cry1Ac concentration was observed (ANOVA: F6 = 59.49, P = 0.014). In the absence of Cry1Ac, total consumption was not affected by diet P:C content. However, when Cry1Ac was present (0.1 and 0.6 ppm), consumption patterns differed depending on the P:C content of the food (Fig. 5a). Cry1Ac intake mirrored food consumption patterns (Fig. 5b), and as with consumption there was a significant interaction between diet P:C content and Cry1Ac concentration (ANOVA: F3 = 5.22, P = 0.002). Although total diet consumption increased with Cry1Ac concentration, mass-specific diet consumption, which accounts for larval size and duration of feeding period, revealed a significant interaction between P:C and Cry1Ac concentration (ANOVA: F6 = 2.18, P = 0.048). However, differences in mass specific consumption, as a function of diet P:C, were only observed on treatments with Cry1Ac at 0.1 ppm (Figure S1). On this Cry1Ac treatment, caterpillars eating the p24:c18 diet had the lowest consumption rate, while those eating the p39:c29 diet had the highest consumption; consumption rates on the other two diets were intermediate.
Total food consumption and total Cry1Ac consumption for larvae reared on diets with different protein-carbohydrate content, at different Cry1Ac concentrations (Experiment 2). Consumption (mean ± SEM) was only recorded for larvae that pupated (N = 7–28 larvae per treatment). Different letters indicate significant differences between treatments (α = 0.05).
Discussion
Our study shows that nutrition can mediate the susceptibility of an economically important insect pest to a Bt toxin. We found that both diet P:C and total macronutrient concentration had effects on H. zea susceptibility to Cry1Ac, but also that these effects were dependent on Cry1Ac concentration. The importance of diet protein-carbohydrate content was most pronounced in our dose-response assay (Experiment 1). Here we showed that simply changing the P:C from carbohydrate-biased (similar to diets typically used in resistance bioassays) to the ratio that H. zea actively selects for caused a 100-fold increase in the LC50 of Cry1Ac. Our subsequent study, which examined diet P:C and Cry1Ac interactions throughout the entire larval stage (Experiment 2), showed that diet protein-carbohydrate content mediates larval performance in the presence of sub-lethal and lethal Cry1Ac concentrations. When Cry1Ac was absent, diet macronutrients had minimal impacts on survival and larval performance, which is consistent with other nutritional studies investigating generalist caterpillars48,49,50, including H. zea46. In contrast, when Cry1Ac was present, larvae reared on the diets that most closely matched their self-selected P:C46 showed faster development and better survival.
Originally, Waldbauer et al.51 inferred that H. zea self-selected a P:C of 4:1. However, Deans et al.46 recently revisited P:C regulation in H. zea, using a more robust experimental approach, and recorded a self-selected P:C of 1.6:1. This ratio is more in-line with P:C regulation reported for other lepidopterans31,46. The p24:c18 and p39:c29 diets in the current study were the closest to this self-selected P:C, and correspondingly H. zea larvae on these two diets, when challenged with Cry1Ac, showed the best survival and performance. Simpson and Raubeheimer29 reported similar results in locusts, where nymphs forced to consume diets with the allelochemical tannic acid had better survival and performance on the diet that most closely matched their self-selected P:C ratio. In the current study, dietary total macronutrient concentration also emerged as an important variable mediating the effects of Cry1Ac, as larvae on both the p24:c18 and p39:c29 diets at 1 ppm performed best and had similar survival curves. However, when Cry1Ac was present at 3 ppm, larvae on the higher macronutrient p39:c29 diet took significantly longer to die than those on the other three diets. This has potential implications in the field, because delayed mortality and an overall delay in development for larvae exposed to Cry1Ac, suggests that larvae feeding on higher macronutrient tissues47 (e.g., cotton seed) may produce greater crop damage. This is supported by our consumption data, which shows that larvae consume more total diet when Cry1Ac is present. However, our mass-specific consumption data suggests this is a function of the extended developmental time associated with Cry1Ac ingestion, as opposed to an increase in feeding rate (Figure S1).
While the current study shows that diet protein-carbohydrate content had significant effects on the survival and performance of a susceptible lab strain of H. zea, additional research is necessary to determine if this is likely for other H. zea strains. Orpet et al.45 examined the effect of diet P:C on Cry1Ac susceptibility in two strains of H. zea – one selected for susceptibility, the other to be genetically-resistant. They found that the resistant strain had better survival in the presence of Cry1Ac, but that diet P:C did not significantly affect survival for either strain. Shikano and Cory43 did find differences in the effect of diet P:C on Bt-related mortality for a susceptible and resistant strain of Trichoplusia ni. In their study, survival of the susceptible strain increased as diet P:C increased in the presence of B. thuringiensis, while the resistant strain had higher mortality on the most protein-biased diet. It is difficult to make direct comparisons between our study and Orpet et al.45, as they based their diet treatments around an optimal P:C of 4:1 (as documented in Waldbauer et al.51) rather than the updated 1.6:1 ratio31,46. Additionally, both Orpet et al.45 and Shikano and Cory43 used spore/protoxin formulations of Bt rather than activated toxin, which could have produced different physiological responses in the larvae (i.e., triggering an immune response in addition to detoxification pathways). In any case, the results of these studies indicate that genetic background may alter the impact that diet protein-carbohydrate content has on susceptibility to Cry1Ac, highlighting the need for further investigation into the relative roles of genetic and environmental factors on Bt resistance using standardized methodologies that are more physiologically- and ecologically-relevant.
In terms of phenotypic plasticity, environmental effects can have a genetic basis and vary among genotypes16,52,53. This means that while it’s difficult to tie plastic phenotypes to specific genes because they have low heritability, the ability of a phenotype to be plastic (vary along environmental gradients) is in itself a trait, that can be heritable. In fact, this kind if phenotypic flexibility may play an important role as an adaptive intermediate stage in evolution16,54. As such, observations of differential susceptibility to Cry1Ac exhibited by different genotypes in response to variation in nutritional environment are consistent with the notion of environmentally-mediated plasticity in response to Cry1Ac.
The implications of our data are perhaps most important for informing resistance monitoring methodologies29. We now know that H. zea larvae in the field are capable of regulating their ingestion of plant macronutrients, and likely do so to match a P:C of 1.6:146. It is also apparent that caterpillars can detect Cry endotoxins in plants and often feed selectively to avoid ingestion of these toxins, increasing the likelihood of sub-lethal exposure55,56,57,58.For resistance bioassays to accurately estimate susceptibility in the field, they must adequately simulate the nutritional conditions in the field. However, most diet-based resistance bioassays use diets with extremely carbohydrate-biased ratios. This includes commonly used rearing diets as well as commercially-available wheat-germ-based diets26. In fact, Deans et al.26 showed that all of the most recent studies assessing Bt resistance in H. zea used carbohydrate-biased diets with P:C ratios below 0.5259,60. Based on our data, carbohydrate-biased diets such as these have likely overestimated susceptibility in H. zea by confounding nutritional stress with Cry toxicity, thereby compromising our ability to detect resistance in the field.
Our study highlights two important and often overlooked characteristics of Bt susceptibility. First, susceptibility to Bt endotoxins can be a plastic phenotype that is not exclusively genetically-determined. Secondly, heterogeneity in plant protein-carbohydrate content, combined with the ability of insects to regulate protein-carbohydrate intake, provides an opportunity for crop pests to mediate the toxic effects of Bt endotoxins. These gene-by-environment interactions have significant implications for how we define, monitor, and manage resistance in the field. Genetic mutations are not the only mechanisms that can reduce susceptibility to Bt crops, and while these plastic responses do not currently fall under the definition of resistance, they can produce resistant phenotypes capable of undermining the efficacy of Bt technology, and ultimately costing growers money. This is particularly true for H. zea, which is a species that exhibits highly variable regional responses to Bt crops, but the same principles may be broadly applicable to other insects targeted by Bt crops.
Methods
Insects
Caterpillar eggs (H. zea) were purchased from Benzon Research (Carlisle, PA). Upon hatching, neonates were individually transferred, using a fine-tipped paint brush, into their experimental containers. For Experiment 1, a colony was started with one batch of eggs from Benzon Research and larvae were collected from different generations for use in each trial. All larvae were housed in a growth chamber (Model I-66VL; Percival Scientific, Perry, IA, USA) set at 28 °C with a 12:12 L:D cycle for the duration of the study. For Experiment 2, larvae were housed in a growth chamber (Model I-66VL; Percival Scientific, Perry, IA, USA) set at 25 °C with a 14:10 L:D cycle for the duration of the study.
Cry1Ac Solutions
Trypsin-activated HPLC purified Cry1Ac was produced in the Pusztai-Carey Lab at Case Western Reserve University (Cleveland, OH) and stored at −80 °C. Because Cry endotoxins degrade over time at room temperature, each artificial diet was refrigerated and then thoroughly mixed with the appropriate amount of Cry1Ac stock solution just before feeding.
The concentrations of Cry1Ac stock solutions were standardized so that the same amount of solution was needed to achieve the desired Cry1Ac concentration in the diets. This controlled for the amount of water being added to the diets across Cry1Ac treatments. During diet preparation, the total amount of diet needed to feed all replicates within a single treatment was weighed, and the amount of the corresponding Cry1Ac stock solution needed to achieve the overall Cry1Ac concentration in the diet (ug of Cry1Ac per g of diet) was calculated. Stock solutions were then thawed, the appropriate amounts were added to each diet, and the diet thoroughly mixed before being portioned into each rearing container. Water was added to the control diets in the same amounts as the Cry1Ac treatments.
Experiment 1: Cry1Ac dose response curves on diets with different protein-carbohydrate content
Experimental diets
In this experiment we measured the LC50 for Cry1Ac across three diets that differed in their protein-carbohydrate content. We tested a common commercial diet (CD; Southland (Lake Village, AR)), similar in nutritional quality to those used in resistance monitoring assays for H. zea. This diet had a carbohydrate-biased P:C ratio of 0.43 and a total macronutrient concentration (P + C) of 62%. This protein-carbohydrate profile is similar to other wheat-germ-based rearing diets36. We modified the CD to produce two additional diets. The first modified commercial diet (MCD1) had a P:C ratio of 1.6:1, matching the self-selected P:C ratio for H. zea46, and the total macronutrient content was kept at 62%. The second modified commercial diet (MCD2) also had a P:C ratio of 1.6:1 but a total macronutrient content of 46%, which approximates the total macronutrient content of cotton vegetative tissue47. Modifications were achieved through the addition of cellulose, vitamin-free casein, anti-microbials, and agar. Casein provided a protein source, thus its addition increased the percent protein in the diet. Agar and cellulose were used to dilute the total macronutrient concentration. To maintain similar water content the ratio of dry ingredients to water was standardized according to the control diet. Sorbic acid, methylparaben, and chlortetracycline hydrochloride were added to match the concentrations in the control diet.
Experimental protocol
Newly hatched neonates were individually placed into wells in a 96 well plate. Each well contained one of the three diets outlined above with one of the following seven concentrations of Cry1Ac incorporated into the diet: 0, 0.01, 0.1, 1.0, 10, 100 and 1000 ppm (ug/g). For the 0 ppm treatment, an equivalent amount of distilled water was added to the diet. We performed three trials and had 10 neonate replicates per Cry1Ac concentration for each diet in each trail.
Experiment 2: Interactions between Cry1Ac and food protein-carbohydrate content
Experimental diets
To maintain relevance to natural conditions, the protein (P) and carbohydrate (C) content, as well as the total macronutrient concentrations (P + C), of our experimental diets in this experiment were selected to match the nutritional value of different cotton plant tissues available to H. zea larvae in a typical cotton field47. We tested three diet P:C ratios, which contained a total macronutrient concentration (P + C) of 42% (by dry mass). The first diet (p24:c18) contained protein and carbohydrates in a ratio that approximated the published self-regulated P:C intake target for H. zea46. The second (p12:c30) and third (p30:c12) diets were carbohydrate-biased and protein-biased, respectively, to the first diet. We also tested a fourth diet (p39:c29). The P:C ratio of this diet matched that of the 24:18 diet but contained a higher total macronutrients concentration (P + C = 68%) relative to the other three diets (P + C = 42%).
Experimental protocol
Upon hatching, neonates were individually placed, using a fine-tipped paint brush, into 1 oz. clear condiment cups with mesh lids. Each cup contained one of four experimental diets that varied in protein-to-carbohydrate ratio (P:C), and one of four different Cry1Ac concentrations (see below), resulting in a total of twelve different treatments (16–34 larvae per treatment).
Within each of our four diets we tested four different Cry1Ac concentrations: 0.1, 0.6, 1.0 and 3.0 ppm (ug/g), which simulated a range of lethality based on preliminary studies using the same strain of H. zea. Cry1Ac stock solutions were mixed with fresh diet at each feeding to achieve the desired concentration of Cry1Ac within the diet, and larvae were given fresh diets a minimum of every four days.
Larvae were fed on their respective diets from hatching, and monitored daily until pupation. Survival was recorded for all treatments, and for larva that pupated we recorded pupation success, larval developmental time, and pupal mass. Consumption was also recorded for all larvae that pupated. Although caterpillars consumed wet agar blocks, consumption was calculated on a dry weight basis (=start dry mass − end dry mass). We recorded the wet mass of each diet block per replicate and then the dry mass of any uneaten diet. Separately, we created a wet-to-dry mass regression by recording the wet mass of diet blocks (not used in the experiment) and their subsequent dry mass after drying in a freeze-dryer. We then used this regression equation to back-calculate the starting dry mass of the diet initially supplied to each replicate. We calculated consumption by subtracting the dry mass of any uneaten diet from the calculated initial dry mass.
Data Analysis
For Experiment 1, the LC50 estimates and dose response curves were calculated using a simple probit analysis in JMP® (SAS Institute). For Experiment 2, a Kaplan-Meier survival analysis (Mantel-Cox test) was used to determine differences in larval survival distributions and developmental time (time to pupation) across treatments. This allowed us to look for the main effects of diet, Cry1Ac concentrations, and interactions between the two. A two-way ANOVA was used to test for differences in pupal mass and consumption. A logistic regression was used to determine the effects of diet and Cry1Ac concentration on pupation success. All these analyses were done using SPSS version 21 for Windows (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Deans, C. A. et al. Nutrition affects insect susceptibility to Bt toxins. Sci. Rep. 7, 39705; doi: 10.1038/srep39705 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Tabashnik, B. E., Brévault, T. & Carrière, Y. Insect resistance to Bt crops: lessons from the first billion acres. Nature Biotechnol. 31, 510–521 (2013).
Tabashnik, B. E. Evolution of resistance to Bacillus thuringiensis . Ann. Rev. Entomol. 39, 47–79 (1994).
Moar, W. et al. Field-evolved resistance to Bt toxins. Nature 26, 1072–1074 (2008).
Tabashnik, B. E., Mota-Sanchez, D., Whalon, M. E., Hollingworth, R. M. & Carrière, Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 107, 496–507 (2014).
McGaughey, W. H. Insect resistance to the biological insecticide Bacillus thuringiensis . Science 229, 193–194 (1985).
Tabashnik, B. E., Cushing, N. L., Finson, N. & Johnson, M. W. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 83, 1671–1676 (1990).
Shelton, A. M. et al. Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J. Econ. Entomol. 86, 697–705 (1993).
Janmaat, A. F. & Myers, J. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni . Proc. R. Soc. Lond. [Biol.] 270, 2263–2270 (2003).
Baxter, S. W., Zhao, J. Z., Gahan, L. J., Shelton, A. M., Tabashnik, B. E. & Heckel, D. G. Novel genetic basis of field-evolved resistance to Bt toxins in Plutella xylostella . Insect Mol. Biol. 14, 327–334 (2005).
Yang, Y., Chen, H., Wu, Y., Yang, Y. & Wu, S. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl. Environ. Microbiol. 73, 6939–6944 (2007).
Farias, J. R. et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Protection 64, 150–158 (2014).
Jakka, S. R. et al. Field-evolved mode 1 fall armyworm resistance to Bt corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Appl. Environ. Microbiol. AEM-02871 (2015).
Monnerat, R. Evidence of field-evolved resistance of Spodoptera frugiperda to Bt corn expressing Cry1F in Brazil that is still sensitive to modified Bt toxins. PLoS One 10, e0119544 (2015).
Travis, J. Evaluating the adaptive role of morphological plasticity. In Ecological Morphology: Integrative Organismal Biology (eds Wainwright, P. C. & Reilly, S. M. ) 99–122 (University of Chicago Press, Chicago, IL, 1994).
Schlichting, C. D. & Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective. (Sinauer Associates Incorporated, Sunderland, MA, 1998).
Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture (JHU Press, Baltimore, MD, 2001).
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford University Press, Oxford, UK, 2003).
Gibson, G. The environmental contribution to gene expression profiles. Nat. Rev. Genet. 9, 575–581 (2008).
Bradshaw, A. D. Evolutionary significance of phenotypic plasticity plants. In Advances in Genetics (eds Caspari, E. W. & Thoday, J. M. ) 115–155 (Academic Press, New York, NY, 1965).
Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (Princeton University Press, Princeton, NJ, 1968).
Via, S. & Lande, R. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522 (1985).
Lively, C. M. Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572 (1986).
Gomulkiewicz, R. & Kirkpatrick, M. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390–411(1992).
Moran, N. A. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 (1992).
Ghalambor, C. K., McKay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (2007).
Elser, J. J. et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 (2000).
Güswell, S. N:P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266 (2004).
Lenhart, P. A., Eubanks, M. D. & Behmer, S. T. Water stress in grasslands: dynamic responses of plant and insect herbivores. Oikos 124, 381–390 (2015).
Deans, C. A., Sword, G. A. & Behmer, S. T. Nutrition as a neglected factor in insect herbivore susceptibility to Bt toxins. Curr. Opin. Insect Sci. 15, 97–103 (2016a).
Simpson, S. J. & Raubeheimer, D. The geometric analysis of feeding and nutrition: a user’s guide. J. Insect Physiol. 41, 545–553 (1995).
Behmer, S. T. Insect herbivore nutrient regulation. Ann. Rev. Entomol. 54, 165–187 (2009).
Simpson, S. J. & Raubenheimer, D. The geometric analysis of nutrient-allelochemical interactions: a case study using locusts. Ecology 82, 422–439 (2001).
Behmer, S. T., Simpson, S. J. & Raubenheimer, D. Herbivore foraging in chemically heterogeneous environments: nutrients and secondary metabolites. Ecology 83, 2489–2501 (2002).
Raubenheimer, D. & Simpson, S. J. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10, 151–179 (1997).
Simpson, S. J., Sibly, R. M., Lee, K. P., Behmer, S. T. & Raubenheimer, D. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1211 (2004).
Gutbrodt, B., Dorn, S. & Mody, K. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod Plant Interact. 6, 171–179 (2012).
Inbar, M., Doostdor, H. & Mayer, R. T. Suitability of stressed and vigorous plants to various insect herbivores. Oikos 94, 228–235 (2001).
Han, P., Lavoir, A., Le Bot, J., Amiens-Desneux, E. & Desneux, N. Nitrogen and water availability to tomato plants triggers bottom-up effects on the leafminer Tuta absoluta . Sci. Rep. 4, 4455 (2014).
Han, P., Dong, Y., Lavoir, A., Adamowicz, S., Bearez, P., Wajnberg, E. & Desneux, N. Effect of plant nitrogen and water status on the foraging behavior and fitness of an omnivorous arthropod. Ecol. Evol. 5, 5468–5477 (2015a).
Han, P., Bearez, P., Adamowicz, S., Lavoir, A., Amiens-Desneux, E. & Desneux, N. Nitrogen and water limitations in tomato plants trigger negative bottom-up effects on the omnivorous predator Macrolophus pygmaeus . J. Pest. Sci. 88, 685–691 (2015b).
Larbat, R., Adamowicz, S., Robin, C., Han, P., Desneux, N. & Le Bot, J. Interrelated responses of tomato plants and the leafminer Tuta absoluta to nitrogen supply. Plant Biol. 18, 495–504 (2015).
Simpson, S. J. & Raubenheimer, R. Herbivore foraging in chemically heterogeneous environments: nutrients and secondary metabolites. Ecology 83, 2489–2501 (2002).
Shikano, I. & Cory, J. S. Dietary mechanisms behind the cost associated with resistance to Bacillus thuringiensis in the cabbage looper, Trichoplusia ni . PLoS ONE 9, e105864 (2014a).
Shikano, I. & Cory, J. S. Genetic resistance to Bacillus thuringiensis alters feeding behavior in the cabbage looper, Trichoplusia ni . PLoS ONE 9, e85709 (2014b).
Orpet, R. J. et al. Effects of dietary protein to carbohydrate ratio on Bt toxicity and fitness costs of resistance in Helicoverpa zea . Entomol. Exp. Appl. 156, 28–36 (2015).
Deans, C. A., Sword, G. A. & Behmer, S. T. Revisiting macronutrient regulation in the polyphagous herbivore Helicoverpa zea (Lepidoptera: Noctuidae): new insights via nutritionally geometry. J. Insect Physiol. 81, 21–27 (2015).
Deans, C. A., Behmer, S. T., Fiene, J. & Sword, G. A. Spatio-temporal, genotypic, and environmental effects on plant soluble protein and digestible carbohydrate content: implication for insect herbivores with cotton as an exemplar. J. Chem. Ecol. 1–13 (2016b).
Lee, K. P., Behmer, S. T., Simpson, S. J. & Raubenheimer, D. A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J. Insect Physiol. 48, 655–665 (2002).
Lee, K. P., Behmer, S. T. & Simpson, S. J. Nutrient regulation in relation to diet breadth: a comparison of Heliothis sister species and a hybrid. J. Exp. Biol. 209, 2076–2084 (2006).
Roeder, K. A. & Behmer, S. T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 28, 1135–1143 (2014).
Waldbauer, G. P., Cohen, R. W. & Friedman, S. Self-selection of an optimal nutrient mix from defined diets by larvae of the corn earworm, Heliothis zea (Boddie). Physiol. Zool. 57, 590–597 (1984).
Scheiner, S. M. & Lyman, R. F. The genetics of phenotypic plasticity. I. Heritability. J. Evol. Biol. 2, 95–107 (1989).
Scheiner, S. M. & Samue, l M. Genetics and evolution of phenotypic plasticity. Ann. Rev. Ecol. Syst. 24, 35–68 (1993).
Pigliucci, M. & Murren, C. J. Perspective: Genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57, 1455–1464 (2003).
Gore, J., Leonard, B. R., Church, G. E. & Cook, D. R. Behavior of bollworm (Lepidoptera: Noctuidae) larvae on genetically engineered cotton. J Econ. Entomol. 95, 763–769 (2002).
Zhang, J. H., Wang, C. Z., Qin, J. D. & Guo, S. D. Feeding behavior of Helicoverpa armigera larvae on insect-resistant transgenic cotton and non-transgenic cotton. J. Appl. Entomol. 128, 218–225 (2004).
Singh, G., Rup, P. J. & Koul, O. Selective feeding of Helicoverpa armigera (Hübner) and Spodoptera litura (Fabricius) on meridic diet with Bacillus thuringiensis toxins. J. Insect Behav. 21, 407–421 (2008).
Han, P., Velasco-Hernández, M. C., Ramirez-Romero, R. & Desneux, N. Behavioral effects of insect-resistant genetically modified crops on phytophagous and beneficial arthropods: a review. J. Pest Sci. 89, 859–883 (2016).
Luttrell, R. G., Wan, L. & Knighten, K. Variation in susceptibility of noctuid (Lepidoptera) larvae attacking cotton and soybean to purified endotoxin proteins and commercial formulations of Bacillus thuringiensis . J. Econ. Entomol. 92, 21–32 (1999).
Ali, M. I., Luttrell, R. G. & Young, S. Y. III. Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J. Econ. Entomol. 99, 164–175 (2006).
Acknowledgements
We would like to thank all those who have provided technical assistance or general feedback, particularly William Moar and Heiko Vogel. This work was supported in part by the C. Everette Sayler Fellowship, the Charles R. Parencia Endowment through the Entomology Department at Texas A&M University, and Biotechnology Risk Assessment Grant Program competitive grant no. 2015-33522-24099 from the U.S. Department of Agriculture (awarded to GAS and STB, MP-C).
Author information
Authors and Affiliations
Contributions
C.D., S.B., and G.S. wrote the main manuscript and prepared all figures except Fig. 2, which was prepared by A.T. All authors, including P.T. and M.P., edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Deans, C., Behmer, S., Tessnow, A. et al. Nutrition affects insect susceptibility to Bt toxins. Sci Rep 7, 39705 (2017). https://doi.org/10.1038/srep39705
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39705
This article is cited by
-
Effectiveness of the natural resistance management refuge for Bt-cotton is dominated by local abundance of soybean and maize
Scientific Reports (2021)
-
Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris rapae
Scientific Reports (2019)
-
A new artificial diet for western corn rootworm larvae is compatible with and detects resistance to all current Bt toxins
Scientific Reports (2018)
-
Herbivore-Induced Defenses in Tomato Plants Enhance the Lethality of the Entomopathogenic Bacterium, Bacillus thuringiensis var. kurstaki
Journal of Chemical Ecology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.