Abstract
Cadmium (Cd2+) is a common toxic heavy metal ion. We investigated the roles of hydrogen sulfide (H2S) and cysteine (Cys) in plant responses to Cd2+ stress. The expression of H2S synthetic genes LCD and DES1 were induced by Cd2+ within 3 h, and endogenous H2S was then rapidly released. H2S promoted the expression of Cys synthesis-related genes SAT1 and OASA1, which led to endogenous Cys accumulation. The H2S and Cys cycle system was stimulated by Cd2+ stress, and it maintained high levels in plant cells. H2S inhibited the ROS burst by inducing alternative respiration capacity (AP) and antioxidase activity. H2S weakened Cd2+ toxicity by inducing the metallothionein (MTs) genes expression. Cys promoted GSH accumulation and inhibited the ROS burst, and GSH induced the expression of phytochelatin (PCs) genes, counteracting Cd2+ toxicity. In summary, the H2S and Cys cycle system played a key role in plant responses to Cd2+ stress. The Cd2+ tolerance was weakened when the cycle system was blocked in lcddes1-1 and oasa1 mutants. This paper is the first to describe the role of the H2S and Cys cycle system in Cd2+ stress and to explore the relevant and specificity mechanisms of H2S and Cys in mediating Cd2+ stress.
Similar content being viewed by others
Introduction
Cadmium (Cd2+) is a common toxic heavy metal ion in the environment. It greatly affects the growth and development of plants and is harmful to human health through the food chain1,2. Because of its carcinogenic properties and its detrimental effects on the growth of organisms, Cd2+ contamination of agricultural soil has become a critical concern. Preventing reduced growth and accumulation of Cd2+ in harvested organs of plants growing on Cd2+-contaminated soils has become an urgent task as it can contribute to food safety. Thus, it is important to explore plant stress defense mechanisms and to find ways to reduce the Cd2+ accumulation in grains.
As a heavy metal not participating in redox reactions, Cd2+ can easily dissolve in water and quickly be taken up by plant roots3,4. The physiological consequences of Cd2+ toxicity in plants are chlorosis, stunted growth, and cell death, among others5,6,7. At the cellular level, Cd2+ can alter protein structure and inhibit enzyme activity by binding to sulfhydryl and carbonyl groups and replacing essential co-factors of enzymes7,8,9. The overproduction of reactive oxygen species (ROS) is the primary response of plants to Cd2+ with negative impact on cell function10. Further damage can be caused by ROS-independent, secondary mechanisms. Lipid peroxidation is the most deleterious effect caused by Cd2+-induced ROS4. Malondialdehyde (MDA), one of the decomposition products of lipid peroxidation, can modify active substrates in plant cells, including nucleic acids, proteins and saccharides11. To become resistant to Cd2+ toxicity, plants have developed several strategies, such as inducing the alternative respiratory pathway11, activating antioxidants and glutathione (GSH)12, and regulating the influx and efflux of heavy metals13,14, as well as regulating the levels of heavy metal chelators, phytochelatins (PCs)15 and metallothioneins (MTs)16.
Hydrogen sulfide (H2S) has been considered toxic gas for many years, which inhibits cytochrome oxidase activity in animal cell17. Recently, it has emerged as the third endogenous gasotransmitter, following the discovery of nitric oxide and carbon monoxide17. In plant systems, endogenous H2S is generated through enzymatic pathways. Cysteine (Cys) desulfhydrases (CDes) are key enzymes involved in H2S generation18. Cys synthesis occurs via two sequential phases catalyzed by serine acetyltransferase (SAT) and O-acetylserine(thiol)lyase (OAS-TL), both of which are encoded by multigene families19. L-Cys desulfhydrase (LCD) is the most understood CDes in Arabidopsis; it regulates L-Cys degradation into pyruvate, ammonia and H2S20. OAS-TL regulates that H2S and O-acetylserine (OAS) synthetise L-Cys20. These physiological processes form H2S - Cys cycle system in cell. Recently, based on the sequence characteristics of DES1, it has been classified as an OAS-TL21. However, functional analysis of this enzyme revealed that DES1 has a higher affinity for L-Cys and degrades it to generate H2S21.
The alternative respiratory pathway is a unique pathway in the mitochondrial electron transport chain in higher plants that is regulated by alternative oxidase (AOX)22. A large body of evidence suggests that the enhanced alternative pathway could improve stress tolerance through limiting the ROS burst22,23. Recent research indicated that respiratory activity is regulated by endogenous H2S in Escherichia coli24. However, the relationship between H2S and the alternative respiratory pathway in plant responses to Cd2+ stress is unclear.
Sulfur is an essential element that is taken up by plants in its oxidized state, reduced to H2S, and first incorporated into Cys before it is used in metabolic processes. The products of sulfur metabolism, such as Cys, GSH, PCs, MTs and H2S, have biological functions in plant responses to heavy metal stress and oxidative stress25. Recently, positive effects of H2S in response to several types of abiotic stress in plants have been found, such as osmotic stress, salt stress, heat shock stress and heavy metal stress26,27,28,29. It has been reported that a cross-talk between H2S and nitric oxide is responsible for the increased Cd2+ tolerance in alfalfa and Bermuda grass plants30,31. In addition, H2S alleviates Cd2+ toxicity by regulating cadmium transport in Populus euphratica cells32. H2S is also involved in the growth and development of plants through its effects on stomatal closure and seed germination and by increasing the growth rate33,34,35,36. Cys acts as a functional precursor for many important biologic activators, such as PCs and GSH, which can enhance the tolerance of plants to heavy metal stress25,37. In addition, Cd2+ tolerance significantly decreases when Cys biosynthesis is blocked in oasa1-1 and oasa1-2 mutants37.
Compelling evidence has suggested that H2S and Cys are involved in plant tolerance to heavy metal stress. The biosynthesis of H2S and Cys are interrelated. H2S is involved in the uptake of SO42− and in the biosynthesis of Cys. Cys is also involved in the generation of H2S. Nevertheless, the relation and interaction between H2S and Cys are yet unknown under Cd2+ stress. In this study, we aimed to clarify the common and independent functions of H2S and Cys in Cd2+ stress. Additionally, we sought to demonstrate the working mechanisms of the H2S and Cys cycle system response to Cd2+ stress in Arabidopsis.
Results
Effect of Cd2+ on root elongation, MDA and EL in Arabidopsis roots
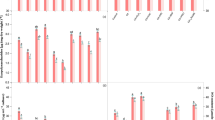
Arabidopsis seedlings (7-d-old) were transferred aseptically to 1/2 MS medium-containing CdCl2, and the lengths of the primary roots were measured 5 d later. Cd2+ stress led to toxicity symptoms and inhibited the elongation of Arabidopsis roots in a dose-dependent manner. As shown in Fig. 1a and b, root growth was slightly inhibited under 25 μM Cd2+, but root elongation was significantly inhibited under 50 to 150 μM Cd2+, exhibiting 53.5% to 34.9% inhibition, respectively. Malondialdehyde (MDA) and electrolyte leakage (EL) are considered as good indicators of stress-induced cell damage. Cd2+ stress caused lipid peroxidation and MDA accumulation. When plants were treated with 50 to 150 μM Cd2+, the MDA content of the roots increased by 117% to 200%, respectively (Fig. 1c). In the presence of 50 to 150 μM Cd2+, EL increased by 131% to 217%, respectively (Fig. 1d). The 50 to 150 μM Cd2+ treatment had significant effects on Arabidopsis roots. 100 and 150 μM Cd2+ concentrations were too violent for plant growth and 150 μM Cd2+ concentrations lead to the seedling etiolation in Fig. 1a. Thus, 50 μM Cd2+ was chosen for further study of Cd2+ stress.
The effect of Cd2+ on root length, MDA and EL in Arabidopsis roots.
(a) Phenotype of root growth in WT seedlings. Bar = 1 cm. (b) The root lengths of WT seedlings (n > 25). (c) MDA contents in WT roots stressed by various concentrations of Cd2+. (d) EL in WT roots stressed by various concentrations of Cd2+. 7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates supplied with 0–150 μM Cd2+ for 5 d, and the lengths of the primary roots, MDA contents and EL were recorded. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
Effect of Cd2+, NaHS and Cys on the H2S and Cys cycle system
To explore the working mechanisms of the H2S and Cys cycle system’s response to Cd2+ stress in Arabidopsis roots, a time-course analysis of endogenous H2S and Cys contents was performed. Endogenous H2S and Cys contents undulated along with the time of Cd2+ stress. H2S content was rapidly induced after treatment with Cd2+ for 3 h, reached the highest level at 9 h, and then decreased at 12 h, but had another increase at 36 h (Fig. 2a). Treatment with Cys could enhance the H2S level and maintain H2S content at a high level in Cd2+ stress (Fig. 2a). The Cys level slightly decreased in the initial stage under Cd2+ treatment, but it increased after treatment with Cd2+ for 12 h and maintained a high level from 24 to 36 h (Fig. 2b). Treatment with NaHS promoted Cys accumulation and a high level of Cys was maintained during Cd2+ stress (Fig. 2b). When plants were treated with 50 or 100 μM Cd2+ for a long time, both H2S and Cys levels were enhanced in Arabidopsis roots (Fig. 2c and d). As mentioned above, H2S and Cys contents were elevated by Cd2+ stress, and H2S appeared to be an important mediator in the Cd2+-induced increase of Cys, and the H2S and Cys cycle system was enhanced.
Analysis of endogenous H2S and Cys contents in WT roots of Arabidopsis.
(a) Time-course of H2S content. (b) Time-course of Cys content. 7-d-old WT seedlings were treated with 50 μM Cd2+, 50 μM Cd2+ + 1 mM Cys and 50 μM Cd2+ + 50 μM NaHS for 0 to 48 h. (c) Changes of H2S content in various Cd2+ concentrations. (d) Changes of Cys content in various Cd2+ concentrations. 7-d-old WT seedlings were supplied with 0–150 μM Cd2+ for 5 d. Mean values and SE are calculated from three replicates. Within each set of experiments, bars with different letters are significant different (P < 0.05, Duncan’s multiple range tests).
The effects of Cd2+, NaHS and Cys on synthetic genes of H2S and Cys
To study the direct effects of Cd2+, NaHS and Cys on genes regulating the synthesis of H2S and Cys, the Arabidopsis seedlings were exposed to various treatments for 3 h. The expressions of H2S synthetic genes LCD and DES1 were markedly induced by Cd2+ and Cys (Fig. 3a and b), but the expression of D-CDES was not significantly affected by Cd2+ and Cys treatments (Fig. 3c). SATs and OASs are the important synthetic genes of Cys, but they had different responses to Cd2+, NaHS and Cys. The expression levels of SAT1 and OASA1 were slightly increased in Cd2+ stress, but they were markedly induced by treatment with NaHS (Fig. 3d and g). Additionally, the expression of SAT5 was inhibited by Cys (Fig. 3f). SAT3, OASB and OASC did not respond to Cd2+, NaHS or Cys (Fig. 3e,h and i).
qRT-PCR analysis the synthesis genes of H2S and Cys in Arabidopsis WT roots.
Relative expression levels were normalized with the internal standard EF1a. 7-d-old WT seedlings were grown on agar plates supplemented with 50 μM Cd2+, 50 μM NaHS, 1 mM Cys, 50 μM Cd2+ plus 50 μM NaHS, and 50 μM Cd2+ plus 1 mM Cys for 3 h, respectively. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significant different (P < 0.05, Duncan’s multiple range tests).
The effects of Cd2+, NaHS and Cys on root elongation, MDA and EL in lcddes1-1 and oasa1 mutants
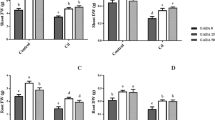
Five-day-old Arabidopsis seedlings were transferred aseptically to Cd2+-containing 1/2 MS medium, and the lengths of the primary roots were measured one week later. The root elongation of lcddes1-1 mutant was shorter compared to WT root elongation under control conditions, but the root elongation of oasa1 was the same as WT (Fig. 4a and b). The lcddes1-1 and oasa1 mutants were more sensitive to Cd2+ stress. Application of NaHS or Cys recovered the Cd2+-induced growth inhibition in WT. NaHS markedly recovered the effect of Cd2+ in lcddes1-1, but it only partly recovered the effect of Cd2+ in oasa1 (Fig. 4a and b). On the contrary, treatment with Cys slightly recovered the effect of Cd2+ in lcddes1-1, but it significantly recovered the effect of Cd2+ in oasa1 (Fig. 4a and b). Cys-mediated partial recovery the root length may be due to an independent physiological action of Cys in lcddes1-1 because H2S production was blocked in the double mutant. NaHS or Cys treatment markedly decreased the EL level and the content of MDA under Cd2+ stress in WT (Fig. 4c and d). NaHS strongly reduced the MDA content and the EL in lcddes1-1 and oasa1 (Fig. 4c and d). Cys prevented the effects of Cd2+ on the MDA content and EL in oasa1 but partly weakened the effects of Cd2+ on the MDA content and EL in lcddes1-1 (Fig. 4c and d).
The effect of H2S and Cys on root length, MDA and EL in lcddes1-1 and oasa1 mutant plants under Cd2+ stress.
(a) Phenotype of Arabidopsis root growth. Bar = 1 cm. (b) The root lengths of Arabidopsis seedlings (n > 25). (c) MDA contents in Arabidopsis roots. (d) EL in Arabidopsis roots. 7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates supplied with 50 μM Cd2+, 50 μM Cd2+ plus 50 μM NaHS and 50 μM Cd2+ plus 1 mM Cys for 5 d respectively, and the lengths of the primary roots, MDA contents and EL were recorded. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
The effects of Cd2+, NaHS and Cys on the alternative respiratory pathway
The alternative respiratory pathway plays an important role in plant stress resistance by limiting the ROS burst38. In this study, we sought to elucidate the roles of NaHS and Cys in the alternative respiratory pathway under Cd2+ stress. In general, the alternative pathway operates at a low level under normal conditions, but it can be significantly induced when plants are stimulated by various environmental stresses23. We first checked the expression of AOX genes after Cd2+, NaHS and Cys treatments for 3 h. The expression of AOX1A, AOX1C and AOX2 were increased in Cd2+ stress (Fig. 5a,b and c). Interestingly, NaHS and Cys treatments also markedly enhanced the expression levels of AOX1A, AOX1C and AOX2 in both control and Cd2+ stress conditions (Fig. 5a,b and c). Furthermore, the total respiration capacity (TP), cytochrome respiration capacity (CP) and alternative respiration capacity (AP) were analyzed in WT and mutant plants. TP was slightly enhanced by 25 or 50 μM Cd2+, but it was inhibited by 100 or 150 μM Cd2+ in WT (Fig. 5d). Under Cd2+ stress, CP was inhibited in a dose-dependent manner; however, AP was increased under Cd2+ stress, and AP achieved its maximum induction with the 50 μM Cd2+ treatment (Fig. 5e). Similar to the pattern of expression of the AOX genes in response to NaHS or Cys treatments under Cd2+ stress, AP was increased by NaHS or Cys under Cd2+ stress (Fig. 5f). However, it was different in the mutant plants. Under Cd2+ stress, the effects of Cys on AP were not observed in lcd and des1-1, and they were especially decreased in lcddes1-1 (Fig. 5g,h and i). In oasa1, the effects of NaHS and Cys were the same as in WT under Cd2+ stress (Fig. 5g).
Effect of H2S and Cys on the expression of AOX genes and the activity of TP, CP and AP in Cd2+ stress.
(a–c) The expression of AOX1A, AOX1C and AOX2. 7-d-old WT seedlings were grown on 1/2 MS agar plates supplied with 50 μM Cd2+, 50 μM NaHS, 1 mM Cys, 50 μM Cd2+ plus 50 μM NaHS, and 50 μM Cd2+ plus 1 mM Cys for 3 h, respectively. (d,e) Changes in TP, CP and AP activity in various Cd2+ concentrations for 5 d in WT. (f–l) AP activity in WT and mutants. 7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates treated with 50 μM Cd2+, 50 μM Cd2+ plus 50 μM NaHS and 50 μM Cd2+ plus 1 mM Cys for 5 d. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
The effects of Cd2+, NaHS and Cys on antioxidant enzyme activity and GSH level, and the relationship among AP, antioxidant enzyme activity, and GSH level in Cd2+ stress
Antioxidant enzymes depress the level of ROS. A previous study showed that H2S could enhance antioxidase activity in rice39. In addition, many studies suggested that AOX was important in maintaining the homeostasis of the redox state22,38. Therefore, the effects of Cd2+, NaHS, Cys and AP on antioxidant enzyme activity were analyzed. As shown in Fig. 6a and c, after 12 h of 50 μM Cd2+ treatment, the activities of SOD and CAT in plants were significantly higher than in the control plants in WT. NaHS or Cys treatments could enhance the antioxidase activity under unstressed conditions (Fig. 6b and d), and this enhancement was further strengthened under Cd2+ stress in WT (Fig. 6a and c). However, treatment with n-propyl gallate (nPG) had no significant effect on the antioxidase activity of the plants either under Cd2+ stress or under unstressed conditions. Furthermore, nPG did not affect the elevated antioxidase activity of the NaHS- and Cys-treated plants under Cd2+ stress (Fig. 6a and c). The effects of Cd2+ and Cys were altered in lcddes1-1; treatment with Cd2+ or Cys did not enhance the antioxidase activity in lcddes1-1 (Fig. 6b and d). Additionally, the effects of Cd2+ on the SOD activity were also weakened, and CAT activity was negligible in oasa1 (Fig. 6d). Contrarily, treatment with NaHS still enhanced the antioxidase activity in mutant plants (Fig. 6b and d).
Effect of H2S and Cys on antioxidant enzymes activity and GSH level in Cd2+ stress.
7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates supplemented with 50 μM Cd2+, 50 μM NaHS, 1 mM Cys, and 200 μM nPG for 6 h, and SOD activity (a,b), CAT activity (c,d) and GSH content (e,f) were recorded. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
GSH is the product of sulfur metabolism, and it has positive biological functions in plant responses to heavy metal stress and oxidative stress25. As shown in Fig. 6e, the GSH content was increased in Cd2+ stress. NaHS and Cys also enhanced the GSH level in WT (Fig. 6e and f). Specially, Cys had a significant promoting effect on GSH content. The oasa1 mutant did not respond to Cd2+ and NaHS, and even had a reduced GSH level, but Cys still increased the GSH content in oasa1 (Fig. 6f). Additionally, the effect of Cd2+, NaHS and Cys on the GSH content in lcddes1-1 was the same as WT plants (Fig. 6f).
Effect of NaHS and Cys on ROS, and the relationship between AP and ROS in Cd2+ stress
To estimate the potential role of the H2S and Cys cycle in ROS homeostasis, we visualized the production of H2O2 in the roots under Cd2+ stress. Over-accumulation of H2O2 was visualized by fluorescence labeling in the roots subjected to Cd2+ stress (Fig. 7a). Conversely, NaHS or Cys treatment considerably diminished the accumulation of H2O2 in Cd2+ stress (Fig. 7a and b). Additionally, inhibiting the alternative respiratory pathway with nPG caused an over-accumulation of H2O2 under Cd2+ stress. The effects of NaHS and Cys were partly averted and slightly weakened by nPG in Cd2+ stress, respectively (Fig. 7a and b). As shown in the time-course of H2O2, the ROS burst occurred during the early phase of Cd2+ stress. Then, high levels of ROS were maintained from 4 to 8 h and declined after 12 h. H2S supplementation could maintain H2O2 at a low level during Cd2+ stress. Treatment with Cys did not alter the burst of H2O2 in the early phase, but it prevented the over-accumulation of H2O2 after 6 h (Fig. 7c).
The changes of the endogenous H2O2 level in Arabidopsis root.
(a) H2O2 H2DCF-DA fluorescence in WT roots (bar = 100 μm). (b) Quantification of H2O2 H2DCF-DA fluorescence intensity. (c) Time-course of H2O2 level. 7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates supplemented with 50 μM Cd2+, 50 μM NaHS, 1 mM Cys, and 200 μM nPG for 6 h (a,b), 0–24 h (c). H2DCF-DA fluorescence intensity data represents mean grey values. SE was calculated from measurements of at least five roots for each treatment, and the experiments were repeated three times. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
Effect of NaHS and Cys on Cd2+ accumulation
The role of H2S and Cys in Cd2+ homeostasis was investigated by measuring the percentage of Cd2+ in the root. The results in Fig. 8a show that Cd2+ accumulation increased in roots under Cd2+ stress in WT and in the mutants, but the mutants accumulated more Cd2+ than the WT. NaHS or Cys supplementation had inhibitory effects on Cd2+ uptake and accumulation in WT and oasa1. Nevertheless, lcddes1-1 did not respond to the effect of NaHS under Cd2+ stress, but the effect of Cys on Cd2+ uptake and accumulation was only partially reduced in lcddes1-1.
Effect of NaHS and Cys on Cd2+ percentage and qRT-PCR analysis the expression of heavy metal chelators genes in Arabidopsis roots.
(a) Cd2+ accumulation in Arabidopsis roots. (b) The expression of PCS1, PCS2, MT1A, MT1B and MT2B in WT. (c) Time-course of PCS1 gene expression. (d) Time-course of MT1A gene expression. 7-d-old Arabidopsis seedlings were grown on 1/2 MS agar plates supplied with 50 μM Cd2+, 50 μM NaHS and 1 mM Cys for 5 d (a), 3 h (b), 0–12 (c,d). Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters are significantly different (P < 0.05, Duncan’s multiple range tests).
Effect of NaHS and Cys on the expression of heavy metal chelator genes
When plants were treated with Cd2+ for 3 h, the expression of the heavy metal chelator genes PCS1, PCS2, MT1A, MT1B and MT2B was significantly up-regulated in WT. Cys supplementation promoted the expression of PCS1 and PCS2, and NaHS promoted the expression of MT1A, MT1B and MT2B (Fig. 8b). To further study the effect of the H2S and Cys cycle system on the heavy metal chelator genes, the time-course of PCS1 and MT1A gene expression was investigated. Cd2+ was found to up-regulate the expression of PCS1 and MT1A genes at 0.5 h, which then remained at a high expression level. Cys enhanced the expression of the PCS1 gene at 0.5 h, which reached a maximum by 1 h, but NaHS enhanced the expression of the PCS1 gene at 6 to 12 h. The expression of MT1A was different from PCS1. After 3 h of Cys supplementation, the expression of MT1A started to increase, reaching a maximum at 6 h, and NaHS enhanced the expression of MT1A gene at 0.5 h (Fig. 8c and d).
Discussion
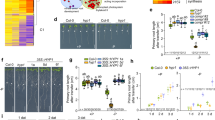
The root is the primary organ that plants deploy to accumulate most of the heavy metals to which they are exposed40,41. Sulfur metabolism is required for the growth and development of plants, and the production of sulfur metabolites also plays a critical role in plant responses to heavy metal-induced stress25. H2S and Cys are important sulfur metabolism products that participate in suppressing heavy metal stress in plants40. In previous reports, H2S and Cys were always studied separately in plant responses to abiotic stress39,42. Recently, H2S and H2S-induced Cys accumulation were reported to be critical in imparting Cr6+ tolerance in Arabidopsis43. Therefore, the H2S and Cys cycle is an important system for regulating H2S and Cys functions in heavy metal stress. In this study, we used the lcddes1-1 and oasa1 Arabidopsis mutants to block the H2S and Cys cycle system. Then, we intensively researched the relevant and specificity roles of H2S and Cys in Cd2+ stress. Our results indicated that Cd2+ can rapidly accumulate in Arabidopsis roots and inhibit the primary root growth in a Cd2+ concentration-dependent manner (Figs 1a and 7a), suggesting that Cd2+ is easily absorbed and highly toxic. We observed that endogenous H2S and Cys levels undulate from 3 to 48 h under Cd2+ stress (Fig. 2). However, the endogenous patterns of change were different for H2S and Cys levels. Endogenous H2S was first induced by Cd2+ stress, and then Cys levels increased. On this account, we suppose that H2S is produced rapidly under Cd2+ stress and that it acts as second messenger to activate the synthesis of Cys, implying that Cd2+ stress could be the direct cause of endogenous H2S release but that Cys accumulation is a secondary effect of Cd2+ stress. Data for the expression of H2S and Cys synthetic genes supports this hypothesis. The expression of H2S synthetic genes was directly induced by Cd2+, and then, exogenous H2S supplementation induced the upregulation of Cys synthetic-related genes (Fig. 3). Additionally, exogenous H2S or Cys supplementation during Cd2+ stress could rapidly induce mutual endogenous levels of the other contents in Cd2+ stress. These results suggested that the H2S - Cys cycle system was triggered by Cd2+ and that H2S and Cys could promote the production of each other, forming a cycle of activation. Finally, treatment with Cd2+ for 5 d, H2S and Cys contents increased significantly in Arabidopsis roots.
The expression levels of the Cys synthesis-related genes OASA1 and SAT1 were up-regulated significantly by H2S treatment, and the H2S synthesis genes LCD and DES1 were up-regulated significantly by Cys treatment (Fig. 3). OASA1 directly regulated Cys synthesis, and LCD and DES1 directly regulated H2S synthesis; thus, lcd, des1-1, lcddes1-1 and oasa1 were used to study the H2S and Cys cycle system in Cd2+ stress. The Cd2+-induced root shortening and increases in MAD and EL were markedly enhanced in mutant plants, suggesting that the Cd2+ resistance was weakened when the H2S and Cys cycle was blocked. Exogenous H2S or Cys supplementation only partly restored the root growth, MAD and EL levels, suggesting that H2S or Cys alone could not replace the function of the H2S and Cys cycle in plant cells. Additionally, the H2S and Cys system is also important for stress caused by other heavy metals, such as Cr6+; it was reported that NaHS treatment increases the expression levels of the Cys synthesis-related genes43. However, different heavy metal stress condition lead to the difference genes expression include MTs genes43, thus Cd2+ and Cr6+ condition also could lead to the difference MTs genes expression. The details regarding the mechanism of H2S in heavy metal resistance requires further study.
Excessive Cd2+ can induce the production of ROS, which is highly toxic to biomembranes, nucleic acids and proteins11. The alternative respiratory pathway plays an important role in stress conditions by repressing the production of ROS22,23,42. Our study also found that the CP and AP activities were altered by Cd2+ stress (Fig. 5e). Plant signaling molecules, such as nitric oxide, can regulate the alternative respiratory pathway in stress conditions44. Whether an H2S signal or Cys could affect AP activity was not previously known; our analysis found that exogenous H2S or Cys supplementation could further induce the activity of AP in Cd2+ stress. However, in H2S synthesis mutants, the effect of Cys was negligible, and in Cys synthesis mutants, the effect of H2S was not altered. These data imply that H2S is a direct trigger of AP activity and that Cys might play an indirect role in Cd2+ stress. The expression of AOX genes was also induced by H2S within 3 h, but not by Cys.
Antioxidases are also one of the central elements in maintaining ROS levels in plant cells45. We investigated the connection between the alternative respiratory pathway and antioxidases, but we found that the activities of SOD and CAT were not altered when the alternative respiratory pathway was inhibited by nPG (Fig. 6a and c), suggesting that the alternative respiratory pathway and antioxidases have independent functions in response to Cd2+ stress. The activities of SOD and CAT were induced by Cd2+ and increased Cd2+ resistance (Fig. 6). H2S or Cys biosynthesis was necessary for the increase in SOD and CAT activities in response to Cd2+ stress because Cd2+-induced activities of SOD and CAT were weakened in H2S and Cys synthesis mutants. We further studied the relationship of H2S and Cys in this physiological process. H2S supplementation could remedy the deficiency of Cys biosynthesis and increase the activities of SOD and CAT in oasa1 mutants, but Cys supplementation could not. These data suggest that the activities of SOD and CAT are directly regulated by H2S and that Cys indirectly affects the activities of SOD and CAT by promoting the generation of H2S.
GSH performs numerous physiological functions in the plant response to heavy metal stress46. Cys is a precursor of GSH, which stores and transports GSH via the γ-glutamyl cycle47. In this study, supplementation with exogenous H2S or Cys strengthened Cd2+-mediated GSH elevation in WT plants (Fig. 6f). It is interesting that the effects of Cd2+ and H2S were reversed in oasa1, but the effects of Cd2+ and H2S were not altered in lcddes1-1 (Fig. 6f). These results suggest that Cys is a direct regulatory factor of GSH, and H2S affects GSH levels indirectly. Additionally, the GSH content was not altered by nPG (Fig. 6e), suggesting that the alternative respiratory pathway and GSH are not related in their responses to Cd2+ stress.
Cd2+ enrichment was also observed in this study (Fig. 8a). Inhibiting Cd2+ uptake and enhancing Cd2+ efflux are the main defense strategies that plant cells use to prevent Cd2+ toxicity. Exogenous H2S or Cys supplementation effectively inhibited the accumulation of Cd2+ (Fig. 8a). When endogenous H2S or Cys synthesis was blocked, Cd2+ over-accumulation occurred (Fig. 8a), suggesting that the H2S and Cys cycle system is important for inhibiting Cd2+ uptake or enhancing Cd2+ efflux. Additionally, the effect of Cys was partly inhibited in the lcddes1-1 mutant, implying that the role of H2S in the H2S and Cys cycle might be to directly regulate Cd2+ uptake or efflux.
The generation of chelators is also an effective pathway in plant cells for avoiding Cd2+ toxicity. PCS1, PCS2, MT1A, MT1B and MT2B are mainly expressed in roots and regulate PCs and MTs synthesis; the expression of these chelators is generally induced by numerous heavy metal ions42,43,48. Interestingly, the expression of PCS1 and PCS2 was found to be induced by Cys in a very short time, and the expression of MT1A, MT1B and MT2B was induced by H2S (Fig. 8b). However, only long-term supplementation of Cys or H2S induced the expression of PCS1 and MT1A (Fig. 8c,d). These data suggest that the generation of chelators can be regulated differently in plant cells. Cys and H2S played different roles in the physiological process, but when combined Cys and H2S mutually promoted the expression of chelator synthesis genes to a level higher than when they were used as separate supplements.
Based on the data described above, a signal pathway model was developed and is depicted in Fig. 9. It shows the specific roles of H2S and Cys in regulating plant responses to Cd2+ stress and their interaction. H2S is activated much earlier than Cys in plant responses to Cd2+ stress, acting as a secondary messenger to increase Cys accumulation by regulating the transcription levels of SAT1 and OASA1. In addition, the production of H2S might deplete the endogenous Cys pool, which might subsequently increase the expression of SAT1 or OASA1. Furthermore, once the H2S and Cys cycle is initiated, it works to maintain elevated H2S and Cys levels. H2S inhibits the ROS burst by promoting CP and antioxidase activities, and it weakens Cd2+ ion toxicity by inducing the gene expression of MTs. Cys acts as a precursor of GSH to promote GSH accumulation, which then contributes to inhibiting the ROS burst. GSH also induces genes expression of PCs, leading to raised PC activity, which counteracts Cd2+ ion toxicity. In sum, the H2S and Cys cycle system is a key regulator of the response to Cd2+ stress in plants that acts to induce and maintain levels of bioactive molecules (H2S, Cys, GSH, PCs, and MTs) that improve plant resistance to Cd2+ stress.
Materials and Methods
Plant material and chemical treatments
This study was carried out on Arabidopsis thaliana, including wild ecotypes Columbia (Col-0) and the lcd (SALK_082099), des1-1 (SALK_103855), lcddes1-1 and oasa1 (SALK_074242c) mutants. Seeds were surface sterilized with 70% ethanol for 30 s and 15% sodium hypochlorite for 15 min and were washed at least five times with sterilized water before sowing on solid 1/2 Murashige and Skoog (MS) medium (pH 5.7), which contained 1% (w/v) sucrose, and 0.8% (w/v) agar. After that, the seeds were vernalized for 48 h at 4 °C. Then, the seedlings were grown in a growth room, which had the temperature set at 22 °C and a 14/10 h light/dark photoperiod under a photon flux density of 120 mmol m−2s−1. The Arabidopsis plants used throughout this work were grown routinely in a growth chamber under 50–60% humidity.
Following 7 d growth, Arabidopsis seedlings were transferred to the following mediums: (1) 1/2 MS agar medium, (2) 1/2 MS agar medium containing 25–150 μM CdCl2, 50 μM sodium hydrosulfide (NaHS), 1 mM Cys, or 200 μM n-propyl gallate (nPG), respectively. The H2S donors NaHS, Cys and nPG were purchased from Sigma (USA).
Root elongation assays
Seven-day-old Arabidopsis seedlings grown on the vertical 1/2 MS agar plates were transferred to the 1/2 MS agar medium containing various chemicals for the different treatments. Root elongation was measured after 5 d of various treatments. All experiments were repeated at least three times, with photographs collected at 7 d from one representative experiment being shown. The root length was measured with ImageJ.
Electrolyte leakage assay
Measurement of ion leakage was determined according to Sairam and Srivastava (2002) with some modifications43. The 7-d-old Arabidopsis seedlings were treated for 5 d on the 1/2 MS agar medium containing different chemicals. Following the treatments, the roots were collected and washed in deionized water three times to remove surface-adhered electrolytes. Then, they were immersed in 10 ml deionized water for 3 h at 25 °C in test tubes. After the incubation, the conductivity in the bathing solution was determined (C1), and the conductivity of deionized water was also determined (C0). The samples were heated in boiling water for 1 h before the total conductivity was measured in the bathing solution (C2). Relative ion leakage was expressed as a percentage of the total conductivity after heating in boiling water [relative ion leakage = (C1 − C0)/(C2 − C0) × 100].
MDA and GSH content assays
The chemical treatments were the same as the measurements of ion leakage. Following the treatments49, Arabidopsis roots were collected. Lipid peroxidation of the roots was measured by estimating the MDA content according to the method of Heath and Packer. The GSH content was measured based on a previously described method49.
Measurement of H2S content
H2S quantification was performed as described by Nashef et al.50. The chemical treatments were the same as the methods of ion leakage. Following the treatments, the seedling roots were collected with liquid nitrogen and ground into fine powder with mortar and pestle; 0.3 g of frozen tissue was homogenized in 1 ml 100 mM potassium phosphate buffer (pH = 7), which contained 10 mM ethylenediaminetetra-acetic acid (EDTA). The homogenates were centrifuged at 15,000 × g for 20 min at 4 °C, and 100 μl of supernatant was used for the quantification of H2S in an assay mixture containing 1,880 μl extraction buffer and 20 μl of 20 mM 5,5′-dithiobis (2-nitrobenzoic acid), for a total volume of 2 ml. The assay mixture was incubated at room temperature for 2 min, and the absorbance was read at 412 nm. H2S was quantified based on a standard curve of known concentrations of NaHS.
Measurement of the Cys content
The chemical treatments were the same as the measurements of ion leakage. Following the treatments, Arabidopsis roots were collected. Cys can react specifically with acid ninhydrin, and the product was extracted by methylbenzene, which has a maximum absorbance at 560 nm. The reaction is highly sensitive for Cys determination. Thus, the Cys content could be determined as described previously51.
RNA isolation and qRT-PCR
Seven-day-old Arabidopsis seedlings were transferred to the 1/2 MS agar medium containing different chemicals and treated for 0–12 h. Following the treatments, roots of Arabidopsis were harvested to extract total RNA for real-time polymerase chain reaction (RT-PCR). Total RNA was extracted using an RNAprep pure plant kit (Tiangen, Beijing) and was treated with RNase-free DNase reagent (RNase-free DNase kit, Tiangen). The total RNA was reverse-transcribed into first-strand cDNA using PrimeScript™ Reverse Transcriptase (Takara, Japan) and Oligo (dT)15 primer (Takara) following the manufacturer’s instructions. The samples were amplified using SYBR Green I (SYBR® Premix Ex Taq™ Kit, Takara). The housekeeping gene EF1A was used as an internal control. The thermal cycle used was as follows: 95 °C for 10 s, and 40 cycles of 95 °C for 5 s and 59 °C for 25 s. This was followed by 80 cycles of 10 s during the time elapsed at 55–95 °C. The PCR amplifications for each gene were performed in triplicate. The results were analyzed by Rotor-Gene Real-Time Analysis Software 6.1 (Build 81).
Extraction and assay of antioxidant enzymes
Seven-day-old Arabidopsis seedlings were transferred to the 1/2 MS agar medium containing different chemicals and treated for 6 h. Following the treatments, Arabidopsis roots were collected and enzymes extracted according to the method of Mostofa et al.51. Activities of antioxidase and glyoxalase were determined by the standard methods reported in Mostofa and Fujita52 for SOD (EC 1.15.1.1) and CAT (EC 1.11.1.6). The protein standard was bovine serum albumin (BSA), which was employed to determine the protein content.
Determination of H2O2 contents
H2O2 was visualized using the specific H2O2 fluorescent probe dichlorofluorescein diacetate (H2DCF-DA) according to the method described by Maffei et al.53. Seven-day-old Arabidopsis seedlings were transferred to the 1/2 MS agar medium containing different chemicals and treated for 0–24 h. Following the treatments, Arabidopsis seedlings were incubated in the reaction buffer containing 10 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES)-NaOH (pH 7.5) and 10 μM H2DCF-DA for 15 min at 25 °C. Thereafter, the roots were washed three times with the HEPES-NaOH buffer (pH 7.4) prior to visualization using a laser confocal scanning microscope (Leica SM IRBE Multisync FE 1250). Excitation was at 480 nm and emission was at 520 nm. Images were processed and analyzed using the Leica Tcs SP2 software.
Statistical analysis
Each experiment was repeated at least three times and with three replications each time. Values were expressed as the mean ± SE. Experiments that required an analysis of variance were analyzed using SPSS 17.0 for one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc multiple comparisons. The confidence coefficient was set at 0.05.
Additional Information
How to cite this article: Jia, H. et al. Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 6, 39702; doi: 10.1038/srep39702 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bolan, N. S. et al. Chapter Four—Cadmium Contamination and Its Risk Management in Rice. Ecosystems Adv. Agron. 119, 183–273 (2013).
Sun, H. et al. Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere. 156, 392–397 (2016).
Kan, Q. et al. Nitrate reductase-mediated NO production enhances Cd accumulation in Panax notoginseng roots by affecting root cell wall properties. J. Plant Physiol. 193, 64–70 (2016).
Sandalio, L. M., Dalurzo, H. C., Gómez, M., Romero-Puertas, M. C. & del Río, L. A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52, 2115–2126 (2001).
Ortega-Villasante, C., Rellán-Álvarez, R., Del Campo, F. F., Carpena-Ruiz, R. O. & Hernández, L. E. Cellular damage induced by cadmium and mercury in Medicago sativa. J. Exp. Bot. 56, 2239–2251 (2005).
Wang, J. L., Li, T., Liu, G. Y., Smith, J. M. & Zhao, Z. W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: physiological, cytological and genic aspects. Sci Rep. 6, 22028 (2016).
Sharma, S. S., Dietz, K. J. & Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 39, 1112–1126 (2016).
Elobeid, M., Gobel, C., Feussner, I. & Polle, A. Cadmium interferes with auxin physiology and lignification in poplar. J. Exp. Bot. 63, 1413–1421 (2012).
Qin, W., Bazeille, N., Henry, E., Zhang, B., Deprez, E. & Xi, X. G. Mechanistic insight into cadmium-induced inactivation of the Bloom protein. Sci Rep. 6, 26225 (2016).
Schützendübel, A. et al. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol. 127, 887–898 (2001).
Stohs, S. J. & Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18, 321–336 (1995).
Semane, B. et al. Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol. Plant. 129, 519–528 (2007).
Migocka, M. et al. Cucumber metal tolerance protein CsMTP9 is a plasma membrane H+-coupled antiporter involved in the Mn2+ and Cd2+ efflux from root cells. Plant J. 84, 1045–1058 (2015).
Greger, M., Kabir, A. H., Landberg, T., Maity, P. J. & Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 211, 90–97 (2016).
Vatamaniuk, O. K., Mari, S., Lang, A., Chalasani, S., Demkiv, O. L. & Rea, P. A. Phytochelatin synthase, a dipeptidyktransferase that undergoes multisite acylation with -glutamylcysteine during catalysis. J. Biol. Chem. 279, 22449–22460 (2004).
Cobbett, C. & Goldsbrough, P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53, 159–182 (2002).
Tan, B. H., Wong, P. T. H. & Bian, J. S. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem. Int. 56, 3–10 (2010).
Papenbrock, J., Riemenschneider, A., Kamp, A., SchulzVogt, H. N. & Schmidt, A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants-from the field to the test tube and back. Plant Biol. 9, 582–588 (2007).
Sirko, A., Blaszczyk, A. & Liszewska, F. Overproduction of SAT and/or OASTL in transgenic plants: a survey of effects. J. Exp Bot. 55, 1881–1888 (2004).
Kopriva, S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 97, 479–495 (2006).
Alvarez, C., Calo, L., Romero, L. C., Garcia, I. & Gotor, C. An O-acetylserine(thiol) lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 152, 656–669 (2010).
Zhang, L. & Liu, J. Enhanced fatty acid accumulation in Isochrysis galbana by inhibition of the mitochondrial alternative oxidase pathway under nitrogen deprivation. Bioresour Technol. 211, 783–786 (2016).
Giraud, E. et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 147, 595–610 (2008).
Korshunov, S., Imlay, K. R. & Imlay, J. A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol Microbiol., doi: 10.1111/mmi. (2016).
Takahashi, H., Kopriva, S., Giordano, M., Saito, K. & Hell, R. Sulfur Assimilation in Photosynthetic Organisms: Molecular Functions and Regulations of Transporters and Assimilatory Enzymes. Annu. Rev. Plant Biol. 62, 157–184 (2011).
Christou, A., Manganaris, G. A., Papadopoulos, I. & Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 647, 1953–1966 (2013).
Li, J. S., Jia, H. L., Wang, J., Cao, Q. & Wen, Z. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251, 899–912 (2014).
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X. & Shen, Z. Z. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell and Environ. 36, 1564–1572 (2013).
Chen, J. et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362, 301–318 (2013).
Li, L., Wang, Y. & Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. BioMetals. 25, 617–631 (2012).
Shi, H., Ye, T. & Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol. Biochem. 74, 99–107 (2014).
Sun, J., Wang, R., Zhang, X., Yu, Y., Zhao, R., Li, Z. & Chen, S. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 65, 67–74 (2013).
Zhang, H. et al. Hydrogen sulfide promotes wheat seed germination and alleviates the oxidative damage against copper stress. J. Integr. Plant Biol. 50, 1518–1529 (2008).
Jia, H., Hu, Y., Fan, T. & Li, J. Hydrogen sulfide modulates actin-dependent auxin transport viaregulating ABPs results in changing of root development in Arabidopsis. Sci. Rep. 5, 8251 (2015).
Dooley, F. D., Nair, S. P. & Ward, P. D. Increased growth and germination success in plants following hydrogen sulfide administration. Plos one 8, 62048–62052 (2013).
Zhang, H. et al. Hydrogen Sulfide Promotes Root Organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J. Integr. Plant Biol. 51, 1086–1094 (2009).
López-Martín, M. C., Becana, M., Romero, L. C. & Gotor, C. Knocking Out Cytosolic Cysteine Synthesis Compromises the Antioxidant Capacity of the Cytosol to Maintain Discrete Concentrations of Hydrogen Peroxide in Arabidopsis. Plant Physiol. 147, 562–572 (2008).
Wang, J. & Vanlerberghe, G. C. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol. Plant 149, 461–473 (2013).
Mostofa, M. G., Rahman, A., Ansary, M. M., Watanabe, A., Fujita, M. & Tran, L. S. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 5, 14078 (2015).
Mostofa, M. G., Hossain, M. A., Fujita, M. & Tran, L. S. Physiological and biochemical mechanisms associated with trehaloseinduced copper-stress tolerance in rice. Sci. Rep. 5, 11433 (2015).
Flores-Cáceres, M. L., Hattab, S., Boussetta, H., Banni, M. & Hernández, L. E. Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 233, 165–173 (2015).
Li, Y., Chen, Y. Y., Yang, S. G. & Tian, W. M. Cloning and characterization of HbMT2a, a metallothionein gene from Hevea brasiliensis Muell. Arg differently responds to abiotic stress and heavy metals. Biochem. Biophys. Res. Commun. 461, 95–101 (2015).
Fang, H., Liu, Z., Jin, Z., Zhang, L., Liu, D. & Pei, Y. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ. Pollut. 213, 870–877 (2016).
Jian W. et al. Alternative oxidase pathway is involved in the exogenous SNP-elevated tolerance of Medicago truncatula to salt stress. J. Plant Physiol. 193, 79–87 (2016).
Sairam, R. K. & Srivastava, G. C. Changes in antioxidant activity in subcellular fraction of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 162, 897–904 (2002).
Freeman, J. L. et al. Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16, 2176–2191(2004).
Seth, C. S. et al. Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ. 35, 334–346 (2012).
Zimeri, A. M., Dhankher, O. P., McCaig, B. & Meagher, B. M. The plant MT1 metallothioneins are stabilized by binding cadmium and are required for cadmium tolerance and accumulation. Plant Mol. Biol. 58, 839–855 (2005).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968).
Nashef, A. S., Osugam, D. T. & Feeney, R. E. Determination of hydrogen sulfide with 5,5β′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal. Biochem. 79, 394–405 (1977).
Gaitonde, M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104, 627–633 (1967).
Mostofa, M. G. & Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22, 959–973 (2013).
Mostofa, M. G., Yoshida, N. & Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 73, 31–44 (2014).
Acknowledgements
This work was supported by the Natural Science Foundation of China (NSFC No. 31400246), the China Postdoctoral Science Foundation (No. 2015T81052), Shaanxi Natural Science Basic Research Project (No. 2015JQ3087), Northwest A&F University basic research Foundation (No. 2014YB039), Natural Science Foundation of Shaanxi University of Science & Technology (No. 2016GBJ-01) and the Start-up Fund for the introduction of talent from Northwest A&F University (Z111021602). Thanks Johannes Liesche, Northwest A&F University, for language modification of the manuscript.
Author information
Authors and Affiliations
Contributions
J.S.L. and H.L.J. designed research; J.S.L. and H.L.J. performed research; X.F.W., Y.H.D. and D.L. screened mutant plants; H.L.J., W.T.S., H.F., C.Z., S.L.C. and J.J.X. analyzed data; and J.S.L. and H.L.J. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jia, H., Wang, X., Dou, Y. et al. Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci Rep 6, 39702 (2016). https://doi.org/10.1038/srep39702
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39702
This article is cited by
-
Role of methylglyoxal and glyoxalase in the regulation of plant response to heavy metal stress
Plant Cell Reports (2024)
-
The effect of sulphur supplementation on cadmium phytotoxicity in wheat and lettuce: changes in physiochemical properties of roots and accumulation of phytochelatins
Environmental Science and Pollution Research (2024)
-
Effects of Different Forms of Sulfur on Plant Growth and Soil Properties in Cadmium-Contaminated Soils
Journal of Soil Science and Plant Nutrition (2024)
-
Combined transcriptomic and metabolomic analyses elucidate key salt-responsive biomarkers to regulate salt tolerance in cotton
BMC Plant Biology (2023)
-
Comparative transcriptome analysis uncovers roles of hydrogen sulfide for alleviating cadmium toxicity in Tetrahymena thermophila
BMC Genomics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.