Abstract
Therapeutic hypothermia is recommended for moderate and severe neonatal encephalopathy, but is being applied to a wider range of neonates than originally envisaged. To examine the clinical use of therapeutic hypothermia, data collected during the first 3 years (2012–2014) of the Baby Cooling Registry of Japan were analysed. Of 485 cooled neonates, 96.5% were ≥36 weeks gestation and 99.4% weighed ≥1,800 g. Severe acidosis (pH < 7 or base deficit ≥16 mmol/L) was present in 68.9%, and 96.7% required resuscitation for >10 min. Stage II/III encephalopathy was evident in 88.3%; hypotonia, seizures and abnormal amplitude-integrated electroencephalogram were observed in the majority of the remainder. In-hospital mortality was 2.7%; 90.7% were discharged home. Apgar scores and severity of acidosis/encephalopathy did not change over time. The time to reach the target temperature was shorter in 2014 than in 2012. The proportion undergoing whole-body cooling rose from 45.4% to 81.6%, while selective head cooling fell over time. Mortality, duration of mechanical ventilation and requirement for tube feeding at discharge remained unchanged. Adherence to standard cooling protocols was high throughout, with a consistent trend towards cooling being achieved more promptly. The mortality rate of cooled neonates was considerably lower than that reported in previous studies.
Similar content being viewed by others
Introduction
Hypoxic-ischaemic encephalopathy of the newborn remains an important cause of mortality and morbidity in newborns1. The results of large randomised controlled trials (RCTs) indicate that therapeutic hypothermia (TH), using either selective head cooling (SHC) or whole body cooling (WBC), reduces the incidence of death and severe disability following neonatal encephalopathy2,3,4,5,6,7,8. Based on these studies, TH initiated within 6 h of birth has been recommended since 2010 as the standard of care for newborn infants ≥36 weeks gestation and ≥1,800 g birth weight with moderate to severe neonatal encephalopathy9,10. Now that the technique has become a routine part of clinical practice, it is expected that TH will be used in a broader range of neonates than the recommendations suggest11. Indeed, a survey conducted in 330 North American neonatal intensive care units demonstrated that approximately 30% of the units administered TH to neonates ≤35 weeks gestation12. An extensive registry established in the United Kingdom for the TOBY Study suggested the presence of “therapeutic drift”, whereby TH was being used outside the originally intended indications. In approximately 10% of neonates registered between December 2006 and July 2011, cooling commenced >6 h after birth, and the extent of birth asphyxia suggested by initial blood base deficits and amplitude-integrated encephalography (aEEG) patterns gradually became less severe over the study period4.
Previously in Japan, the use of TH as a clinical strategy outside clinical trials had been determined by empirical rather than evidence-based indications and protocols13. To ensure evidence-based best practice, a series of substantive alterations were undertaken in 201014 to coincide with the release of the revised Consensus Statement and Treatment Recommendation on Cardiopulmonary Resuscitation (CoSTR)9. Owing to the nationwide campaign, adherence to evidence-based cooling protocols improved from 20.7% to 94.7% in less than 3 years14.
To further examine and monitor the use of TH in neonates, an online case registry was established in January 2012. The aims of this study were to examine adherence to the standard cooling protocol and illuminate changes in cooling practice during the first 3 years of the case registry.
Results
Clinical characteristics of registered patients
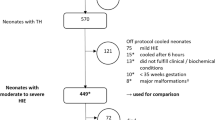
Over the 3-year period, the clinical information of 533 infants was submitted from 100 units; 17 were excluded from the analysis because of missing data (Fig. 1). Thirty-one neonates of gestational age 38.7 ± 2.0 weeks and birth weight 2,889 ± 509 g were considered for TH, but were not cooled because: (i) physicians considered that the risk of TH outweighed the potential benefits because of the patient’s clinical condition (n = 17); (ii) encephalopathy was mild at the time of admission (n = 8); (iii) gestational age was <36 weeks (n = 5), or (iv) the neonate was aged >6 h (n = 1). The remaining 485 neonates of gestational age 38.7 ± 3.5 weeks and birth weight 2,862 ± 465 g underwent TH (Table 1). Data from non-cooled neonates were not subject to further analysis, and the proportions presented in the following section are calculated based on the 485 cooled neonates unless otherwise stated.
Indications for cooling
Seventeen neonates (3.5%) were <36 weeks gestation, and three (0.6%) weighed <1,800 g. Apgar scores at 10 minutes were recorded for 369 neonates; scores ≤5 were present in 220 cases (59.6%). Severe acidosis (pH < 7 or base deficit ≥16 mmol/L) for the cord or first blood gas analysis within 1 h of birth was confirmed in 334 neonates (68.9%). The majority (469 neonates, 96.7%) had required persistent resuscitation for >10 minutes after birth. Four neonates (0.8%) neither required continuous resuscitation for >10 minutes, nor had severe acidosis or 10-minute Apgar scores ≤5; however, two had clinical seizure activity. Of 471 neonates with completed Sarnat encephalopathy stage data at admission, 288 (61.1%) and 128 (27.2%) neonates had stage II and III neonatal encephalopathy, respectively. Fifty-five (11.7%) had stage I encephalopathy; however, abnormal primitive reflexes (for example weak/absent sucking, rooting and the Moro reflex) were observed in all 55 cases, and signs suggestive of moderate encephalopathy, such as moderate/severe hypotonia (n = 21), clinical seizure activity (n = 4) and moderately/severely abnormal aEEG (n = 4) were observed in the majority. An aEEG was obtained in 295 (60.8%) and 201 (41.4%) neonates at admission and on the day of rewarming, respectively. The use of aEEG was more common for neonates who were cooled by WBC than those cooled by SHC at admission (66.1% for WBC versus 53.9% for SHC, P = 0.016) and on the day of rewarming (49.8% for WBC versus 30.0% for SHC, P < 0.001).
Therapeutic hypothermia
In 408 neonates with temperature data available at admission, mean body temperature was 35.9 ± 1.5 °C (Table 2). Body temperature at admission was ≥38 °C in 14 (3.4%) neonates, whereas hypothermia (<35 °C) was recorded in 73 cases (17.9%, Fig. 2). In 468 cases with data available, cooling was commenced on average 222 ± 93 minutes after birth and 105 ± 87 minutes after admission. Cooling was commenced within 6 h of birth in 452 cases (96.6%). After the commencement of cooling, the target core body temperature was achieved in an average of 94 ± 154 minutes, leading to the completion of cooling initiation on average 312 ± 183 minutes after birth.
Among 476 neonates with complete data recorded over the first 4 days, SHC and WBC were used in 181 (38.0%) and 295 (62.0%) neonates, respectively. The mean core body temperature during cooling (from 6–72 h after the commencement of cooling) was 34.0 ± 0.6 °C and 33.7 ± 0.5 °C for SHC and WBC, respectively. The mean heart rates during SHC and WBC were 114 ± 15 beats/min and 112 ± 14 beats/min, respectively, and the mean blood pressure was 49 ± 6 mmHg and 48 ± 6 mmHg, respectively (Supplemental Table 1).
Adjuvant neuroprotective therapies were used in 148 cases (30.5%) using magnesium sulphate (n = 114), erythropoietin (n = 16), edaravone (n = 12), osmotic diuretics (n = 8), phenobarbital (n = 5) and/or ulinastatin (n = 3).
Outcome at discharge
Of 474 neonates whose short-term outcome was confirmed, 430 (90.7%) were discharged home after 59.3 ± 122.3 days, 13 (2.7%) died before discharge and 31 (6.5%) were referred to other hospitals or transitional centres for further medical care (Table 3). The majority (86.3%) were successfully weaned from mechanical ventilation after 11.2 ± 27.0 days, whereas 46 (9.7%) required persistent mechanical ventilation, even at the time of discharge or transfer to another institution. Full oral feeding was established in 386 (81.4%) cases during initial hospital admission.
Adverse events
Serious adverse events were observed in 371 cases, and consisted of: hypotension (34.8%); clinically diagnosed seizures (24.7%); coagulation disorders (13.2%); arrhythmia (1.4%); hypoglycaemia (1.0%); septicaemia (0.8%) and subcutaneous fat necrosis (0.4%) (Table 3).
Changes in practice over 3-year study period
Apgar score at 10 minutes, cord or first blood pH and base deficit, Sarnat encephalopathy stage and Thompson encephalopathy score at admission did not change significantly in the first 3 years of the registry (Table 1). Similarly, body temperature at admission and the time to start cooling (after birth) remained unchanged over the 3 years (Table 2). In contrast, the mean time to reach the target temperature after initiation of cooling fell significantly from 104 ± 141 minutes in 2012 to 66 ± 71 minutes in 2014 (P = 0.004). The proportion undergoing SHC fell and the proportion undergoing WBC rose over time, so that WBC accounted for 81.6% of cases in 2014 (P < 0.001 compared with 2012). In 2012, the proportion of neonates undergoing aEEG at admission was 44.1%, with 27.4% undergoing aEEG on the day of rewarming, which increased to 79.3% and 46.3%, respectively, in 2014 (both P < 0.001).
Short-term outcomes, including duration of mechanical ventilation, duration of hospital stay, requirement for tube feeding at discharge, requirement for mechanical ventilation at discharge or referral to other centres, and mortality rate during the initial hospital stay remained unchanged over the study period (Table 3).
Discussion
Adherence to standard cooling protocols was maintained at a high level in Japan, even after the conclusion of the nationwide implementation campaign that ran between 2010 and 201214. During the first 3 years of the Baby Cooling Register, there was a substantial change in cooling technique from SHC to WBC. A consistent trend towards more prompt initiation of cooling was also observed. The mortality rate of cooled neonates before discharge was 2.7%, substantially lower than previous clinical trials and registers2,3,4.
Despite the established efficacy of TH for neonatal encephalopathy15, approximately 40% of neonates do not respond, and consequently many will go on to develop permanent neurological impairments or succumb16. To further improve the cooling protocol, a recent large-scale RCT17 examined the potential benefits of deep (32.0 °C) and prolonged (120 h) hypothermia; however, there was a trend towards increased short-term mortality rates with deep/prolonged cooling compared with the current standard cooling protocol. Several other RCTs of TH with revised cooling indications/protocols are underway, but further improving the therapeutic efficacy of TH might be challenging considering the body of clinical evidence that has informed the choice of the current standard cooling criteria and protocol. Nevertheless, large-scale clinical databases of cooled neonates can now be established relatively easily, which may help identify novel techniques or refine cooling strategies that further improve outcomes in cooled neonates. The UK TOBY Register and the Vermont Oxford Network Neonatal Encephalopathy Registry were the first major attempts to collect a large-scale clinical dataset18,19. Although these registries recorded that the standard TH protocol was generally followed outside RCTs, the application of TH to neonates with less severe asphyxia, to those <36 weeks gestational age and/or >6 h after birth, was already evident. A more recent survey in California found that the proportion of neonates with mild encephalopathy who were cooled increased from 38% to 55% between 2010 and 201220.
In contrast with the therapeutic drift observed in Western countries18,19,20, we found that, even up to 4 years after the initial recommendation of TH for neonatal encephalopathy9, the standard cooling criteria and protocol were followed in most neonatal care centres in Japan. When TH was newly recommended as a standard of care in 20109, a substantial proportion of Japanese neonatal intensive care centres had already started cooling encephalopathic neonates according to empirically-acquired cooling indications and protocols13. To disseminate evidence-based best practice for TH for newborn infants, a dynamic nationwide campaign was conducted between 2010 and 2012, leading to a dramatic improvement in adherence to the standard cooling criteria and protocol14. The Baby Cooling Registry of Japan was opened in 2012 to disseminate evidence-based cooling practice by monitoring the clinical use of TH, and by giving feedback to participating units. As well as ensuring adherence to the standard cooling protocol, we found that cooling was initiated more promptly over the first 3 years of the registry, which may explain the increase that we observed in the number of neonates in whom TH was achieved within the therapeutic time window.
Our study also identified an increase in the use of WBC over time. Although SHC is non-inferior to WBC in its therapeutic benefit, it is more difficult to perform cot-side examinations, such as cranial ultrasound and electroencephalogram, when SHC is used. This might explain our observation that a smaller proportion of SHC-cooled neonates underwent aEEG compared with those cooled by WBC. Moreover, the concept of simultaneous head cooling and body warming is sometimes misleading. Indeed, our previous nationwide survey in Japan found that a substantial proportion of units were undertaking SHC using relatively warm cap temperatures >25 °C, which were servo-controlled with reference to the nasopharyngeal temperature13. As this empirical protocol was in widespread use in Japan by 2010, the Neonatal Hypothermia Task Force Japan decided to promote WBC rather than disseminating the correct protocol for SHC. This is likely to have contributed to the reduction in the use of SHC over time.
With regard to the short-term outcome of cooled neonates, it is known that the mortality rates of cooled neonates vary between RCTs, as seen in the CoolCap (33%)2, NICHD (24%)3 and UK TOBY (26%) studies4, despite the use of similar inclusion criteria. A more recent clinical study showed a relatively lower short-term mortality rate of 7% in neonates who were randomised to WBC to 33.5 °C for 72 h17. In our cohort, the mortality rate of cooled neonates was 2.7%. This might not fully be explained by the difference in severity of neonatal encephalopathy considering that standard cooling indications were followed closely. Global comparative studies are needed to illuminate the factors associated with short- and long-term outcomes of cooled neonates.
Several limitations of the current study must be acknowledged. First, we were not able to analyse follow-up data of our cohort of cooled neonates. For most participating units, only one or two cases were cooled each year, which will likely influence the level of experience within each unit, and the subsequent outcome of cooled neonates. The Baby Cooling Registry of Japan is currently collecting data after hospital discharge to investigate the outcome of neonates cooled in different types of units using various therapeutic regimens. Second, although we provided reference values for the heart rate and blood pressure in neonates cooled with SHC and WBC, we did not collect information regarding the use of inotropes. Third, the number of registered cases is smaller than might be expected from the approximately 1,000,000 births in Japan per annum that were registered between 2012 and 2014. We would expect to see 1,000–2,000 cases of neonatal encephalopathy each year given that the incidence of neonatal encephalopathy is approximately 1–2 per 1,000 live births in developed countries21, but just over 500 cases were recorded in the registry over 3 years. Hayakawa and colleagues have, however, estimated that the incidence of moderate to severe neonatal encephalopathy in Japan is 0.37 per 1,000 live births22. Considering that 219 of 287 registered level II-III neonatal intensive care centres participated in the current registry (76.3%), our database is likely to have included the majority of eligible neonates. Nevertheless, caution is required to avoid drawing conclusions from studies based on case registries, which may be influenced by selection bias.
In conclusion, a national registry of TH in neonatal encephalopathy has been successfully established in Japan and maintained for 3 years. During this period, TH was applied and conducted in close concordance with the criteria and protocols used in previous large-scale RCTs. There was some improvement in practice, such as the time required for the initiation of TH. The mortality rate during the initial hospital stay was considerably lower than previous reports. International comparative studies may help identify factors associated with short-term outcome that would help investigators refine and improve the TH protocol.
Methods
This study was conducted in compliance with the Declaration of Helsinki. The protocols of the registry were approved by the Ethics Committees of Kurume University School of Medicine and Saitama Medical University, Japan. Since no patient identifiers were or are collected, the Ethics Committees advised that there is no statutory requirement for parental consent for data collection, and consent was not sought for the current registry.
The Neonatal Hypothermia Task Force Japan was formed by the Japan Society of Perinatal and Neonatal Medicine (JSPNM) and the Clinical Guidelines Committee for Neonatal Resuscitation in Japan (Neonatal Research Network Japan, Ministry of Health, Labour and Welfare [MHLW]) in June 2010 to implement evidence-based TH practice in Japanese neonatal intensive care centres. The Task Force launched an online case registry to monitor newborn TH practice in January 2012. The TH strategy was derived from the JSPNM and MHLW Japan Working Group Practice Guidelines Consensus Statement, which in turn had been developed by summarising and integrating the indications and cooling protocols used in large-scale RCTs2,3,4. All Japanese level II or III neonatal intensive care centres registered as designated hospitals for postgraduate clinical training with the Japanese Society for Neonatal Health and Development were invited to join the registry. Basic, mandatory clinical information for each case was input online via the official website of the Baby Cooling Registry of Japan. A unique identification number was allocated to each registered case. Data were anonymised so that patients could not be identified, obviating the need for individual consent for data collection. Although the registry guidance emphasised the importance of observing standard cooling protocols, participating units were requested to register all neonates (i) who underwent TH regardless of adherence to the guidance, and (ii) who were referred to the unit for consideration of TH but ultimately did not undergo cooling.
Case record forms were developed based on the format used in the UK TOBY Register4, including patient characteristics, clinical condition at birth, severity of encephalopathy assessed by established scales23,24, patterns of aEEG25, core body temperature, cardiovascular and respiratory parameters, supportive treatments, use of sedatives and analgesics, clinical complications, and short-term outcomes at hospital discharge. Adverse events were reported in accordance with the guidance and protocol provided on the official website of the Baby Cooling Registry of Japan (Supplemental Table 2). Participating units were encouraged to obtain magnetic resonance images before discharge and perform developmental assessments at around 12 months and 2 years of age.
Statistical analysis
In this observational study, descriptive data analysis was performed for the dataset compiled during the first 3 years of the registry (between 1st January 2012 and 31st December 2014). For the current analysis, only data collected during the primary hospital stay were analysed. Each record was inspected for case duplication, apparent input errors or excessive unexplained missing data (>5% of the individual dataset without plausible explanations). To assess changes in patient characteristics and practice with time, data were grouped into 12-month periods according to the date of birth of each neonate, and the data from 2013 and 2014 were compared with those from 2012 using either the chi-squared test or Student’s t-test with the Bonferroni correction. Values are shown as number (proportion, %) for categorical variables or mean ± standard deviation for normally distributed continuous variables.
Additional Information
How to cite this article: Tsuda, K. et al. Therapeutic hypothermia for neonatal encephalopathy: a report from the first 3 years of the Baby Cooling Registry of Japan. Sci. Rep. 7, 39508; doi: 10.1038/srep39508 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Liu, L. et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440, doi: 10.1016/s0140-6736(14)61698-6 (2015).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670, doi: 10.1016/s0140-6736(05)17946-x (2005).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine 353, 1574–1584, doi: 10.1056/NEJMcps050929 (2005).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England journal of medicine 361, 1349–1358, doi: 10.1056/NEJMoa0900854 (2009).
Simbruner, G., Mittal, R. A., Rohlmann, F. & Muche, R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126, e771–778, doi: 10.1542/peds.2009-2441 (2010).
Jacobs, S. E. et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Archives of pediatrics & adolescent medicine 165, 692–700, doi: 10.1001/archpediatrics.2011.43 (2011).
Tagin, M. A., Woolcott, C. G., Vincer, M. J., Whyte, R. K. & Stinson, D. A. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Archives of pediatrics & adolescent medicine 166, 558–566, doi: 10.1001/archpediatrics.2011.1772 (2012).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. The Cochrane database of systematic reviews 1, CD003311, doi: 10.1002/14651858.CD003311.pub3 (2013).
Perlman, J. M. et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122, S516–538, doi: 10.1161/circulationaha.110.971127 (2010).
Papile, L. A. et al. Hypothermia and neonatal encephalopathy. Pediatrics 133, 1146–1150, doi: 10.1542/peds.2014-0899 (2014).
Austin, T., Shanmugalingam, S. & Clarke, P. To cool or not to cool? Hypothermia treatment outside trial criteria. Archives of disease in childhood. Fetal and neonatal edition 98, F451–453, doi: 10.1136/archdischild-2012-302069 (2013).
Harris, M. N. et al. Perceptions and practices of therapeutic hypothermia in American neonatal intensive care units. American journal of perinatology 31, 15–20, doi: 10.1055/s-0033-1334454 (2014).
Iwata, O. et al. Hypothermia for neonatal encephalopathy: Nationwide Survey of Clinical Practice in Japan as of August 2010. Acta paediatrica 101, e197–202, doi: 10.1111/j.1651-2227.2011.02562.x (2012).
Iwata, O. et al. The baby cooling project of Japan to implement evidence-based neonatal cooling. Therapeutic hypothermia and temperature management 4, 173–179, doi: 10.1089/ther.2014.0015 (2014).
Roka, A. & Azzopardi, D. Therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy. Early human development 86, 361–367, doi: 10.1016/j.earlhumdev.2010.05.013 (2010).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. Bmj 340, c363, doi: 10.1136/bmj.c363 (2010).
Shankaran, S. et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. Jama 312, 2629–2639, doi: 10.1001/jama.2014.16058 (2014).
Azzopardi, D. et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK–analysis of national data. PloS one 7, e38504, doi: 10.1371/journal.pone.0038504 (2012).
Pfister, R. H. et al. The Vermont Oxford Neonatal Encephalopathy Registry: rationale, methods, and initial results. BMC pediatrics 12, 84, doi: 10.1186/1471-2431-12-84 (2012).
Kracer, B., Hintz, S. R., Van Meurs, K. P. & Lee, H. C. Hypothermia therapy for neonatal hypoxic ischemic encephalopathy in the state of California. The Journal of pediatrics 165, 267–273, doi: 10.1016/j.jpeds.2014.04.052 (2014).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric research 74 Suppl 1, 50–72, doi: 10.1038/pr.2013.206 (2013).
Hayakawa, M. et al. Incidence and prediction of outcome in hypoxic-ischemic encephalopathy in Japan. Pediatrics international: official journal of the Japan Pediatric Society 56, 215–221, doi: 10.1111/ped.12233 (2014).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology 33, 696–705 (1976).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta paediatrica 86, 757–761 (1997).
Thoresen, M., Hellstrom-Westas, L., Liu, X. & de Vries, L. S. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 126, e131–139, doi: 10.1542/peds.2009-2938 (2010).
Acknowledgements
The authors are grateful to the staff of participating neonatal intensive care centres, and the infants and their parents who provided data to the Registry. This work was supported by the Japan Society of Perinatal and Neonatal Medicine, and the Ministry of Health, Labour and Welfare, Japan (H27-001, Special research in perinatal medicine). Dr Tsuda was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research B26860856). Dr Iwata S was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C24591533 and C15K09733). Dr Takenouchi was funded by Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics and Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research 24791121). Dr Iwata O was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C16K09005). The Baby Cooling Registry of Japan Collaboration Team participated in the study and contributed to patient recruitment and data collection for the registry.
Author information
Authors and Affiliations
Consortia
Contributions
K.T., S.I., and O.I. designed the study and the survey items. K.T., T.M., J.S., T.T., T.I., S.H., N.Y., A. Takahashi, A. Takeuchi, and T.T. participated in the data collection. K.T., T.M., S.I., Y.A., and O.I. performed the statistical analyses. K.T., S.I., T.T., Y.A., H.S., M.T., S.H., M.N., and O.I. contributed to the interpretation of the results. K.T., S.I., and O.I. drafted the manuscript, which was critically reviewed and revised by T.M., T.T., H.S., M.T., S.H., and M.N. All authors have seen and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tsuda, K., Mukai, T., Iwata, S. et al. Therapeutic hypothermia for neonatal encephalopathy: a report from the first 3 years of the Baby Cooling Registry of Japan. Sci Rep 7, 39508 (2017). https://doi.org/10.1038/srep39508
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39508
This article is cited by
-
Risk factors for unfavorable outcome at discharge of newborns with hypoxic-ischemic encephalopathy in the era of hypothermia
Pediatric Research (2023)
-
Predictive value of the Thompson score for short-term adverse outcomes in neonatal encephalopathy
Pediatric Research (2023)
-
Three-year outcome following neonatal encephalopathy in a high-survival cohort
Scientific Reports (2022)
-
Effect of Hypothermia on Myocardial Depolarization and Repolarization in Neonates with Hypoxic–Ischemic Encephalopathy Due to Asphyxia
Pediatric Cardiology (2022)
-
Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.