Abstract
Dietary exposure of insects to a feeding deterrent substance for hours to days can induce habituation and concomitant desensitization of the response of peripheral gustatory neurons to such a substance. In the present study, larvae of the herbivore Helicoverpa armigera were fed on diets containing either a high, medium or low concentration of sucrose, a major feeding stimulant. The responsiveness of the sucrose-best neuron in the lateral sensilla styloconica on the galea was quantified. Results showed the response of the sucrose-best neuron exposed to high-sucrose diets decreased gradually over successive generations, resulting in complete desensitization in the 5th and subsequent generations. However, the sensitivity was completely restored in the ninth generation after neonate larvae were exposed to low-sucrose diet. These findings demonstrate phenotypic plasticity and exclude inadvertent artificial selection for low sensitivity to sucrose. No significant changes were found in the sensitivity of caterpillars which experienced low- or medium-sucrose diets over the same generations. Such desensitization versus re-sensitization did not generalise to the phagosimulant myo-inositol-sensitive neuron or the feeding deterrent-sensitive neuron. Our results demonstrate that under conditions of high sucrose availability trans-generational desensitization of a neuron sensitive to this feeding stimulant becomes more pronounced whereas re-sensitization occurs within one generation.
Similar content being viewed by others
Introduction
All animals have chemosensory receptor neurons that respond to phagostimulant compounds and potentially bitter tastants in foods1,2,3,4,5,6. The importance of food chemistry and chemosensory detection in the evolution and maintenance of animal-food associations has been well documented for rats7,8, mice9,10,11, Drosophila12, lepidopteran insects13,14,15,16, and other insect species17,18,19,20. An important issue is to understand the evolutionary role of phenotypic plasticity of sensory systems elicited by changing environmental factors, for example food availability and nutritional quality9,21,22,23,24. In particular, a period of exposure to specific compounds can profoundly alter subsequent responsiveness to chemical stimuli15,25,26. In most investigations, changes in sensitivity develop over a period of hours to days27,28,29. Little is known, however, about the effects of trans-generational exposure on chemosensory sensitivity. Here, we investigate the trans-generational exposure to diets differing in sugar content on the responsiveness of gustatory receptor neurons in a polyphagous insect herbivore.
It has been well documented that food discrimination of lepidopteran caterpillars is governed by the activity of gustatory neurons in the medial and lateral sensilla styloconica on the maxillary galea to stimulants and deterrents in host plants30. It is well established that the sensitivity of these gustatory neurons can be modified by dietary experience that also affected food selection behaviour13,31. In most cases, after a period of exposure to diets containing a chemical stimulus, in later larval instars the gustatory neurons exhibited reduced sensitivity to the stimulus27,28,29,32,33,34.
The process of gustatory desensitization of sensilla styloconica to feeding deterrent substances depends on the insect species and the deterrent itself. Some studies documented that the sensitivity of the deterrent neuron in the sensilla styloconica decreased significantly but not completely after a chronic dietary exposure to the deterrent substance from neonates to the final instar27,28. In other cases, several days of dietary exposure to a deterrent resulted in the complete desensitization of the deterrent neuron29,34,35. Such desensitization of peripheral gustatory neurons to feeding deterrent substances could be mediated by gustatory transduction pathways, centrifugal control by the central gustatory system or post-ingestive mechanisms21,29,36.
It is known that the maxillary sensilla styloconica of caterpillars of all lepidopteran species studied contain a “sucrose-best”neuron, the activation of which leads to stimulation of feeding behavior37,38,39,40,41,42. Compared to the number of reports on the plasticity of gustatory neurons in response to exposure to feeding deterrents, the effects of exposure to varying levels of phagostimulants on the gustatory sensitivity of plant-feeding insects have received little attention. After exposure for a few hours to food containing a high level of carbohydrates but a low level of protein, the responsiveness to sucrose in the blowfly Phormia regina, and the caterpillars of the lepidopterans Spodoptera littoralis and Grammia geneura exhibited desensitization to sucrose38,43,44. Little is known, however, about desensitization and resensitization of sucrose-best neurons in sensilla styloconica of caterpillars elicited by varying levels of dietary sucrose.
The cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), is a typical polyphagous species feeding on at least 160 plant species45,46,47. Taste neurons sensitive to sucrose and the deterrent azadirachtin are located in the lateral sensillum, whereas neurons responding to the sugar alcohol myo-inositol and the deterrents strychnine and strophanthin-K reside in the medial sensillum27,37,48,49,50. Deterrent-sensitive neurons in the maxillary sensilla of H. armigera caterpillars reared on artificial diets containing either strychnine or strophanthin-K from neonate to the 5th instar exhibited reduced sensitivity to the two chemicals compared with the caterpillars reared on normal diets27. Sinigrin was found to be deterrent to H. armigera caterpillars in dual-choice leaf disk assays and it excites the same deterrent neuron in the medial sensillum as strychnine and strophantin-K. (Tang et al., unpubl. results). In the present study, the electrophysiological activity of the sucrose neuron in the lateral sensillum of H. armigera larvae that experienced diets differing in the content of the major feeding stimulant sucrose, high-sucrose (HS), medium-sucrose (MS) and low-sucrose (LS) diets were investigated.
We addressed the following inter-related questions: (1) Can chronic exposure to HS diets during larval development induce desensitization of the sucrose-best neuron in the lateral sensillum styloconicum of H. armigera? (2) Can the chronic exposure to HS diets result in a complete desensitization of the sucrose-best neuron? If so, how long is needed to achieve this? (3) Can the sensitivity of the sucrose-best neuron be fully restored upon exposure to a normal dietary sucrose content and how long is needed for full restoration? (4) Does exposure of caterpillars to LS diets induce plasticity of the sucrose-best neuron?
Results
With the aid of Autospike software plus visual inspection, spikes in the responses from lateral sensilla of the 5th instar caterpillars of H. armigera with different feeding experiences were sorted out and assigned to different types of gustatory receptor neurons known to be present in each chemosensillum. By comparing to the responses to sucrose with those obtained to KCl, at least three types receptor neurons were identified as the “salt”, “sucrose” and “water” best responding unites (see exemplary traces in Fig. 1). In most traces obtained from the responses to sucrose, the dominating “medium-sized” spikes were from the sucrose-best neuron, the “largest-sized” spikes were from the “salt” best responding neuron, and few “small-sized” spikes were from the “water” best responding neurons (see exemplary traces in Fig. 1).
Representative recordings of electrophysiological activity of the gustatory receptor neurons in the lateral sensillum of larvae exposed to high-sucrose (HS) diet in successive generations.
Traces represent electrophysiological responses to 1 mM sucrose. Sucrose was dissolved in 2 mM KCl. The duration of each trace is 1 s (except those in rectangles). Spikes in each big rectangle show the expansions of part of the corresponding trace (the small rectangle), suggesting that different gustatory receptor neurons were activated. K: the “largest-sized”spikes from the salt responding neuron. S: the medium-sized spikes from the “sucrose-best” neuron; W: the small-sized spikes from the “water” responding neuron; Note that the medium-sized spikes from the sucrose-best neuron had the dominating activities in most traces.
Desensitization to sucrose after HS-exposure over generations
The sensitivity of the sucrose-best neuron in the lateral sensillum of H. armigera caterpillars exposed to HS diet was significantly affected by both generation and sucrose concentration (univariate ANOVA: generation, df = 7, F = 42.707, P < 0.001; sucrose concentration, df = 4, F = 20.598, P < 0.001). There was also a significant interaction between generation and sucrose concentration affecting response frequency (univariate ANOVA: generation × concentration, df = 28, F = 3.066, P < 0.001). The 5th instar caterpillars exposed to HS diet exhibited decreased sensitivity of the sucrose-best neuron in the lateral sensillum of Wild caterpillars (see exemplary trace “F1” in Fig. 1) (Fig. 2A). In subsequent generations sensitivity to sucrose decreased graduallyto a slight response in F5 generation. The response frequency of the sucrose-best neuron to sucrose from F5 to F8 generation were not significantly different from those to the control solvent KCl (Post-hoc SNK-test after univariate ANOVA: all P = 0.143) (see exemplary traces in Fig. 1) (Fig. 2A).
Dose–response curves of electrophysiological activity recorded from the sucrose-best neuron in the lateral sensillum of caterpillars reared on diets containing different sugar levels over successive generations.
Each point represents the mean response frequency +/− SE of the sucrose-best neuron of 5th instar H. armigera caterpillars in different generations (F1 to F8) in response to a concentration series of sucrose. (A) Reared on HS diet (high-sucrose artificial diet); (B) reared on LS diet (low-sucrose artificial diet); (C) reared on MS diet (medium-sucrose artificial diet). The tested number of caterpillars exposure to HS diet in each generation from Wild, F1, F2, F4, F6, F7 and F8 were 22, 20, 24, 20, 20, 20 and 20. And the tested number of caterpillars exposure to LS diet in each generation from Wild, F1 to F8 were 22, 12, 12, 15, 11, 13, 10, 18 16, respectively. The tested number of caterpillars exposure to MS diet in different generations from Wild, F1 to F8 were 14, 12, 14, 12, 13, 12, 13, 12 and 14 respectively.
The sucrose-best neuron in Wild caterpillars of H. armigera displayed obvious dose-dependent responses to a concentration series of sucrose from 0.001 mM to 10 mM (Wildin Fig. 2A). Similarly, caterpillars exposure to HS diet in F1, F2 and F4 generations also exhibited dose-dependent response patterns to sucrose but the sensitivity was significantly lower than that in Wild(Post-hoc SNK-test after univariate ANOVA: all P < 0.01) (F1,F2andF4in Fig. 2A). However, caterpillars exposed to HS diet from the 5th generation to the 8th generation did no longer display a dose-dependent response pattern to sucrose and the response intensity was significantly lower than that in the previous generations (Post-hoc SNK-test after univariate ANOVA: all P < 0.01) (Fig. 2A). So, it is apparent that the sucrose-best neuron of 5th instar larvae had become completely desensitized in the 5th generation since exposure to high-sucrose diets.
Responsiveness to LS/MS dietary exposure over generations
To establish that it was exposure to a HS diet across successive generations that produced the desensitization of the sucrose-best neuron, the responsiveness of the sucrose-best neuron in the lateral sensillum of H. armigera caterpillars exposed to either LS diet or MS diet over generations was also investigated (Fig. 2B,C). Firstly, the sucrose-best neuron of caterpillars exposed to LS diet displayed dose-dependent responses in each generation from Wild to F8 (Fig. 2B and see exemplary traces in Fig. 3). The sensitivity of the sucrose-best neuron in caterpillars reared on LS diet was significantly affected by both generation and sucrose concentration (univariate ANOVA: generation, df = 8, F = 2.855, P = 0.004; concentration, df = 4, F = 325.953, P < 0.001; generation × concentration, df = 32, F = 0. 468, P = 0.995). Compared to Wild, the response intensity of the sucrose-best neuron of caterpillars in F1-LS decreased significantly (Post-hoc SNK-test after univariate ANOVA: P < 0.01), whereas the sensitivity of the sucrose-best neuron inF2-LS was significantly higher than that of F1-LS (Post-hoc SNK-test after univariate ANOVA: P < 0.05) and was similar to that of the Wildand the F3~F8 generations (Post-hoc SNK-test after univariate ANOVA: P = 0.136) (Fig. 2B).
Representative recordings of electrophysiological activity obtained from the sucrose-best neuron in the lateral sensillum of caterpillars exposed to low-sucrose (LS) artificial diets in the generation F1-LS.
Recordings in this figure originated from caterpillars of the F1 generation exposed to LS diet (F1-LS). Each concentration of sucrose was dissolved in 2 mM KCl. The duration of each trace was 1 s.
Similarly, the responsiveness of the sucrose-best neuron in caterpillars exposed to MS diet also displayed dose-dependent responses from Wild to F8 generation (Fig. 2C). Generation of exposure to MS-diet had no significant effect on the sensitivity of the sucrose-best neuron (univariate ANOVA: generation, df = 8, F = 1.644, P = 0.110; concentration, df = 4, F = 373.503, P < 0.001; generation × concentration, df = 32, F = 0.490, P = 0.992).
Responsiveness of caterpillars reared on HS diet and switched to LS or MS diets
The sensitivity of the sucrose-best neuron of caterpillars that experienced one diet over eight generations and then were switched to other diets in the ninth generation were tested to investigate whether the changing of diets could result in a taste change. Three groups of caterpillars experienced different artificial diets differing in sugar content were investigated as described in materials and methods. In Group I, the responsiveness of the sucrose-best neuron was significantly higher after caterpillars of H. armigera exposed to HS diet for eight generations were exposed to LS or MS diet (univariate ANOVA: experience, df = 3, F = 49.112, P < 0.001; concentration, df = 4, F = 23.262, P < 0.001; experience × concentration, df = 12, F = 12.742, P < 0.001) (see exemplary traces in Figs 4 and 5A). When neonate F9-caterpillars were exposed to LS diet (F8-HS + F9-LS), the response of the sucrose-best neuron of 5th instar caterpillars was significantly higher than that of F8-HS, F8-HS + F9-MS andF9-HS(Post-hoc SNK-test after univariate ANOVA: all P < 0.01) (Fig. 5A) and similar to that of Wild and F1-HS(Post-Hoc SNK Test after ANOVA: P = 0.075) (Fig. 5B).
Representative recordings of electrophysiological activity of the sucrose-best neuron in the lateral sensillum to 1 mM sucrose in caterpillars exposed to different artificial diets.
(A) Caterpillars exposed to HS diet over eight successive generations (F8-HS); (B) F9-caterpillars exposed to the LS diet (F8-HS + F9-LS); (C) F9-caterpillars exposed to the medium-sucrose artificial diet (F8-HS + F9-MS); (D) caterpillars exposed to HS diet over nine successive generations (F9-HS); Sucrose was dissolved in 2 mM KCl. The duration of each trace was 1 s.
Dose–response curves recorded from the sucrose-best neuron in the lateral sensillum of caterpillars exposure to HS diet over eight generations and then switched to other diets.
Each point represents the mean response frequency +/− SE of the sucrose-best neuron of 5th instar H. armigera caterpillars in the response to a series of sucrose concentrations. (A) F8-HS (n = 20), F8-HS + F9-LS (n = 22), F8-HS + F9-MS (n = 24) and F9-HS (n = 20) caterpillars; (B) responses to 1 mM sucrose in Wild (n = 22), F1-HS (n = 20) to F8-HS (n = 20), and F8-HS + F9-LS (n = 22) caterpillars. Means with different low case letters are significantly different (Post-hoc SNK-test: P < 0.05).
Similar to F8-HS + F9-LS, F9-neonate caterpillars derived from F8-HS exposed to MS diet (F8-HS + F9-MS) displayed higher responsiveness of the sucrose-best neuron in the 5th instra than F8-HS 5th instar caterpillars (Post-hoc SNK-test after univariate ANOVA: P < 0.01), but responsiveness was significantly lower than that of F8-HS + F9-LS caterpillars (Post-hoc SNK-test after univariate ANOVA: P < 0.01) (Fig. 5A).
Responsiveness of caterpillars reared on LS/MS diet and then switched to other diets
In Group II, after the F9-neonate caterpillars derived from generation F8-LS were exposed to HS diet or MS diet, the responsiveness of the sucrose-best neuron of the 5th instar caterpillars in F8-LS + F9-MS decreased but not significantly (Post-hoc SNK-test after univariate ANOVA: P = 0.292), while that of F8-LS + F9-HScaterpillars decreased significantly compared to that of F8-LSandF9-LS caterpillars (Post-hoc SNK-test after univariate ANOVA: all P < 0.05) (Fig. 6A). For example, 1 mM sucrose elicited 65.1 ± 9.34 spk s−1 in F8-LS + F9-HScaterpillars, which was significantly lower than 101.9 ± 8.66 spk s−1 recorded from F8-LS and F2-LS to F7-LS caterpillars (Post-Hoc SNK Tests after ANOVA: all P < 0.05) (Fig. 6A′).
Dose–response curves recorded from the sucrose-best neuron in the lateral sensilla of caterpillars exposed to LS or MS diet over eight generations and then switched to other diets.
Each point represents the mean response frequency +/− SE of the sucrose-best neuron of 5th instar caterpillars. (A) Responsiveness of caterpillars exposed to LS diet over eight generations (F8-LS, n = 16) and switched to MS (F8-LS + F9-MS, n = 12) or HS diets (F8-LS + F9-HS, n = 14; F9-LS, n = 10); (A′) Response curve to 1 mM sucrose in caterpillars exposed to LS diet over eight generations (replicates of caterpillars were the same as in Fig. 2B) and then exposed to HS diet (F8-LS + F9-HS, n = 14). (B) Caterpillars exposed to MS diet over eight generations (F8-MS, n = 14) and switched to HS/LS diets (F8-MS + F9-LS, n = 14; F8-MS + F9-HS, n = 13; F9-MS, n = 12); (B′) Response curve to 1 mM sucrose in caterpillars exposed to MS diet over eight generations (replicates of caterpillars were the same as in Fig. 2C) and then exposed to LS diet (F8-MS + F9-LS, n = 14). Different letters represent significant difference of the mean response frequency in different generations (Post-hoc SNK-test: P < 0.05).
Similarly, in Group III after F9-neonate caterpillars derived from F8-MS were exposed to LS diet (F8-MS + F9-LS) or HS diet (F8-MS + F9-HS), the responsiveness of the sucrose-best neuron also differed (univariate ANOVA: experience, df = 3, F = 8.773, P < 0.001; concentration, df = 4, F = 162.671, P < 0.001; experience × concentration, df = 12, F = 1.136, P = 0.355). Firstly, compared to that in F8-MS and F9-MScaterpillars, the sensitivity of the sucrose-best neuron of F8-MS + F9-LS 5th instar caterpillars was significantly increased (Post-hoc SNK-test after univariate ANOVA: all P < 0.05) (Fig. 6B). On the contrary, the sucrose-best neuron of F8-MS + F9-HS caterpillars had significantly lower sensitivity compared to that of F8-MS and F9-MS caterpillars (Post-hoc SNK-test after univariate ANOVA: all P < 0.05) (Fig. 6B). However, 1 mM sucrose elicited similar response intensity in the sucrose-best neuron of Wild, F1-MS to F8-MS, and F8-MS + F9-LScaterpillars (Post-Hoc SNK Test after ANOVA: P = 0.445) (Fig. 6B′).
Effects of diets with different sucrose levels on the deterrent and myo-inositol neurons
The effects of HS diet or LS diet on the responsiveness of the myo-inositol-sensitive neuron and the sinigrin-sensitive neuron in the medial sensillum of H. armigera caterpillars were investigated. Results showed that the sensitivity of the myo-inositol-sensitive neuron in the medial sensillum of caterpillars in Wild was significantly higher than that of caterpillars in F1-HS, F9-HS and F9-LSin the response to 1 mM myo-inositol (Post-Hoc SNK Test after ANOVA: P < 0.05), while the response frequencies among the latter three groups were similar (Post-Hoc SNK Test, P = 0.840) (Fig. 7A,A′). On the other hand, 1 mM sinigrin elicited a similar response from the deterrent neuron in the medial sensillum of caterpillars from Wild, F1-HS, F9-HS and F9-LS (Post-Hoc SNK Test after ANOVA: P = 0.915) (Fig. 7B,B′).
Representative recordings of electrophysiological activity and comparison of response intensity of gustatory neurons in the medial sensillum to myo-inositol and sinigrin from caterpillars exposed to diets differing in sugar level.
(A) representative recording from neuron to myo-inositol (300 ms); (A′) comparison of response intensity from neuron to myo-inositol; (B) Representative recording from neuron to sinigrin (300 ms); (B′) comparison of response intensity from neuron to sinigrin. Columns represent the mean response frequency +/− SE in the response to 1 mM myo-inositol (A′) and to 1 mM sinigrin (B′). Numbers below the columns show the numbers of insects tested. SNK post hoc test for one-way ANOVA was used to compare mean spike frequencies of the sensilla across caterpillars with different feeding experiences (P < 0.05). Means with different lower case letters are differ significantly (P < 0.05).
Discussion
Sugars serve as universal sources of metabolic energy to organisms and most animals have the ability to taste sugars that in many species constitute primary stimulatory signals for feeding51. The sugar concentrations investigated here are within the range of reported levels in different plants and plant organs on which H. armigera is known to feed. The sugar content varies greatly in the principal host plants of the polyphagous herbivore H. armigera, e.g. 1.73~5.37% of dry weight in the green leaves of tobacco, ca. 8% in tomato fruits in green ripening stage, 16% in corn stalks, and up to 20.5% in floral buds of cotton52,53,54,55,56,57. We found that dietary exposure to the HS diet desensitized the sucrose-best gustatory neuron. The desensitization developed to stronger degrees over successive generations, reaching its maximum after five generations of exposure and remaining at this level in the next three generations. Once the caterpillars were exposed to the LS diet, the sucrose-best neuron was completely restored to its wildtype condition, showing that no selection for low sensitivity had occurred after eight generations. Taste plasticity induced by early exposure to stimuli within one generation was also reported in other insect species and animals. For example, honeybees Apis mellifera decreased the gustatory responsiveness to sucrose after switching to an sucrose-enriched diet for 6 h to 24 h18, and adult rats exposure to a sodium-deficient diet for 10 days selectively decreased NaCl responses in the chorda tympani nerve26. Mice that experienced unlimited exposure to sucrose diets in early life reduces motivation to acquire sucrose in adulthood58, but no similar findings have yet been reported on trans-generational taste plasticity in insects and other animals except Dias and Ressler reported that the fear odor could be inherited transgenerationally at behavioral, neuroanatomical and epigenetic levels from parental olfactory experiences via parental gametes59.
The exposure of caterpillars to HS diet for successive generations resulted in a gradual desensitization of the sucrose-best neuron, while the exposure to either LS diet or MS diet for successive generations failed to elicit significant change in the sensitivity of the sucrose-best neuron of H. armigera. On the other hand, after the neonate caterpillars exposed to the same diet for eight generations were exposed to another diet, the sensitivity of the sucrose-best neuron in the 5th instar caterpillars demonstrated significant plasticity in most situations. For example, the exposure to LS diet elicited stronger responses of F8-HS + F9-LS or F8-MS + F9-LS caterpillars compared to that of F8-HS or in F8-MScaterpillars (Figs 5A and 6B), while the exposure to HS diet desensitized the sucrose-best neuron of caterpillars in F8-LS + F9-HS or in F8-MS + F9-HScompared to that in F8-LS or in F8-MS(Fig. 6A,B). This plasticity of the sucrose-best neuron of H. armigera caterpillars was at least in part consistent with plasticity in the chemosensitivity to sucrose in the locust Locusta migratoria, the blowfly P. regina, and the caterpillars S. littoralis and G. geneura exposed to high-sucrose/low-protein diets or to low-sucrose/high-protein diets38,43,44,60.
The current findings also show that different sucrose contents in artificial diets elicited different degrees of desensitization or resensitization of the sucrose-best neuron in H. armigera. For example, LS diet elicited a stronger response in caterpillars of F8-HS + F9-LS than the response elicited by MS diet in F8-HS + F9-MS (Fig. 5A). On the contrary, the HS diet significantly desensitized the sucrose-best neuron of caterpillars in F8-HS + F9-HS(F9-HS) compared to the response of caterpillars in F8-HS + F9-MS elicited by MS diet (Fig. 5A). Similarly, HS diet elicited a significantly lower response in the caterpillars of F8-LS + F9-HS than MS did in F8-LS + F9-MS (Fig. 6A). Similar findings on the plasticity of the sensitivity to sucrose were also found in S. littoralis exposed to diets differing in sucrose and protein content for hours61.
The shifts in carbohydrate/protein ratio in the HS/LS/MS artificial diet in the current experiment might have had effects on the development of H. armigera caterpillars as reported for Heliothis virescens62 and Helicoverpa zea63. However, we found that caterpillars of H. armigera exposed to HS and LS diets in our rearing colonies had similar pupal development time, pupal mass, and pupal survival (% eclosing) as well as larval development time, while larval survival (% pupating) and the number of eggs produced by F9-HS individuals (larval survival: 69.53%; number of eggs/female: 344.84) was lower than those of the F9-LS individuals (larval survival: 85.41%; number of eggs/female: 460.47) and the F9-MS individuals (larval survival: 93.26%; number of eggs/female: 622.83). On the contrary, F9-MS individuals had higher pupal survival (% eclosing) (92.08%) than that of F9-HS individuals (74.55%) as well as F9-LS individuals (84.10%). The current data suggest that the plasticity of the sucrose-best neuron in H. armigera depends on dietary sucrose content rather than on protein/sucrose ratio or altered development. We consider this explanation plausible for two reasons. First, the absolute content of protein in LS, HS and MS diets was similar and the protein/carbohydrate ratio in the current study was not so unbalanced as that in the studies on H. virescens, H. zea and G. geneura38,62,63. Bernays et al.38 regarded the sensitivity changes of taste neurons to sucrose and amino acids in G. geneura to reflect carbohydrate but not protein imbalance. Second, the HS diet or the LS artificial diet in the current experiment did decrease the sensitivity of the neuron sensitive to myo-inositol in F1 generation, but did not change further on the basis of sugar concentration or trans-generationally, indicating artificial diets differing in sugar concentrations and generational exposure had no bearing on overall plasticity. Third, the sensitivity of the deterrent neuron to sinigrin in H. armigera did not exhibit any plasticity to any sugary diets in any tested generations, suggesting that there was no general physiological effect on H. armigera caterpillars experiencing LS diet or HS diets for eight generations.
It is known that the myo-inositol serves as a phagostimulant and sinigrin as a deterrent to non-adapted lepidopteran caterpillars64,65,66. Our data demonstrated that neither exposure of F1 caterpillars nor trans-generational exposure to HS/LS diets desensitized the sinigrin-sensitive neuron in the medial sensillum of H. armigera. On the other hand, the sensitivity of the myo-inositol-sensitive neuron in the medial sensillum was lower in F1-HS caterpillars compared to that in Wildcaterpillars, but did not exhibit significant plasticity in successive generations regardless of the diets experienced. We interprete this as evidence for specificity of desensitization of the sucrose-best neuron caused by exposure to the HS diet across successive generations. Specificity in the desensitization of gustatory neurons was also reported for other caterpillars, e.g. a gustatory neuron in the medial sensillum of caterpillars of G. geneura exposed to a high-sucrose diet was less responsive to sucrose while the response to fructose in the lateral sensillum was constant38. Similarly, dietary exposure to caffeine in Manduca sexta caterpillars desensitized a caffein-sensitive neuron but neither the sucrose neuron nor the myo-inositol-sensitive neuron were affected29.
It was unexpected that not only the sensitivity of the sucrose-best neuron in F1-HS caterpillars decreased compared to that in Wild caterpillars but also that of F1-LS and F1-MS caterpillars. Decreased sensitivity was also observed in the myo-inositol-sensitive neuron of caterpillars in generation F1-HS. We postulate that F1caterpillars had not yet completely adapted to the artificial rearing diet but F2caterpillars had, as judged by the recovered sensitivity of the sucrose-best neuron in F2-LS(Fig. 2B) or F2-MScaterpillars (Fig. 2C). Another sign of ongoing adaptation was the observation that 4th or the 5th instar caterpillars of H. armigera captured directly from the natural fields had a higher mortality than that of second or third instar caterpillars if reared on normal artificial diets (unpublished data).
Desensitization of the deterrent-sensitive neurons in H. armigera and other caterpillar species in general took 2 h to 24 h, however, it was not complete27,28,34,36. Our results show that it took five generations of exposure to a high dietary sucrose concentration to completely desensitize the sucrose-best neuron. Transgenerational experiments on desensitization to deterrents have not been performed to our knowledge. Several mechanisms may explain exposure-elicited desensitization, e.g. a reduced number of membrane-bound gustatory receptor proteins10,67,68,69 and/or down-regulated secondary messenger signal-transduction pathways70,71,72. The sensitivity of the sucrose-best neuron in the last instar caterpillars of H. armigera declined gradually over successive generations, suggesting that the reduced sensitivity could be transferred from one generation to the next and may involve an epigenetic phenomenon. Based on the full recovery of electrophysiological sensitivity within one larval generation, genetic selection on low sensitivity to sucrose can be excluded.
The regulation of gustatory plasticity in insects is clearly very complex. Notable in these responses is the observation that, after exposure to HS diet over generations, caterpillars of H. armigera showed lower responsiveness to sucrose, while after switching after eight generations to a LS diet higher responsiveness to sucrose was found. It seems likely that this restoration of the sensitivity of the sucrose-best neuron is driven, at least in part, by low a carbohydrate level in the haemolymph. Therefore, we consider the operation of a post-ingestive centrifugal nutritional feedback mechanism involving the central nervous system as the most parsimonious explanation for the sucrose exposure-elicited gustatory de- and resensitization. The exposure to a high dietary level of sucrose may result in a reduced sensitivity of the sucrose-best neuron in H. armigera since abundant dietary sucrose may not require gustatory sensitivity to sucrose and may save investments in maintaining sucrose chemoreception. Moreover, it was reported that the biogenic amines, c.a. the octopamine and serotonin in extracellular sensillum lymph, could adjust the sensitivity of olfactory receptor neurons of M. sexta by modulating the transepithelial potential of the accessory cells in the sensillum73. Therefore, it is possible to explore whether such neurotransmitters or hormone in extracellular sensillum a affected by artificial diets, resulting in direct or indirect plasticity of the sucrose-best neuron.
A nutritional feedback mechanism has been shown to operate within 5 min in response to injection of a mixture of amino acids into the haemocoel in the orthopteran L. migratoria and resulted in a reduced response of taste receptors to several amino acids74. Similarly, the gustatory response of labellar taste sensilla in the blowfly P. regina to glucose decreased significantly two hours after the injection of 1 M trehalose into the haemolymph75. Therefore, we hypothesize that the plasticity of the sucrose-best neuron in caterpillars of H. armigera induced by diets differing in sucrose content is related to variations in the levels of sugars in the haemolymph. In a next study, the levels of sugars in the haemolymph of H. armigera caterpillars after exposure to different levels of sucrose in the artificial diet will be quantified to test this hypothesis.
Materials and Methods
Insects
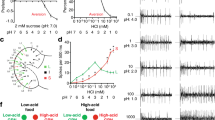
Helicoverpa armigera larvae were collected from a tomato field in Zhengzhou, Henan province, China, and were divided into different experimental groups. Based on the diet composition described by Wu & Gong76, three diets differing in sucrose concentration, high-sucrose (HS), medium-sucrose (MS) and low-sucrose (LS), were prepared to rear the experimental larvae. Including the sucrose in wheat bran, soybean powder and other dietary contents, the percentages of sucrose in the HS, MS and LS diets were 17.15%, 5.69% and 2.65% of dry weight, respectively which were within the range of sugar contents reported for natural host plants of H. armigera caterpillars (see Discussion). All colonies of H. armigera were maintained in the laboratory under controlled photoperiod (L16:D8) and temperature (27 ± 1 °C). Adults were supplied with a 10% v/v solution of sucrose in water.
Three groups of H. armigera caterpillars exposed to the three dietary sucrose levels were used in this experiment. Every group consisted of caterpillars captured directly from the field (Wild) as described above, caterpillars exposed to each diet for eight successive generations and caterpillars that were switched to the other two diets in the ninth generation as shown in Fig. 8.
Chemicals
Sucrose, myo-inositol and sinigrin were obtained from Sigma Chemical Co. (purity > 99.5%). For electrophysiological tests, sucrose was presented in a series of concentrations from 0.001 to 10 mM dissolved in 2 mM KCl, an appropriate electrolyte for the electrophysiology of Helicoverpa caterpillars27,77. As a control for the specificity of effects of diets on the sensitivity of the sucrose-best neuron, we also tested whether diets containing different contents of sucrose have effects on the sensitivity of another phagostimulant neuron and a deterrent neuron, i.e. the independent phagostimulant neuron to myo-inositol and the deterrent neuron to sinigrin in the medial sensillum of H. armigera caterpillars37. Concentration of both chemicals was 1 mM, a concentration often used in lepidopteran taste studies64,78.
Electrophysiological recording
The tip recording technique79,80 was used to investigate the electrophysiological activity of neurons in the lateral sensilla styloconica on the galea of H. armigera larvae. In brief, 5th instar larvae between 24 h and 36 h (during the photophase) since the penultimate moult were transected between the first and second pair of thoracic legs and the excised head capsule was mounted on a silver wire electrode that was connected to the input of a pre-amplifier (Syntech Taste Probe DTP-1, Hilversum, The Netherlands). The maxillary lateral sensilla styloconica were investigated for the sensitivity to sucrose of caterpillars that had fed on diets differing in sucrose content, while the medial sensilla were stimulated to assess the sensitivity of the myo-inositol-sensitive neuron or the deterrent neuron. Recordings of electrophysiological activity were obtained from the sensillum styloconicum of one side of the mouthparts of each caterpillar to different concentrations of sucrose or myo-inositol or sinigrin using a glass microelectrode (tip diameter ca. 30 μm) filled with stimulus solution. Each caterpillar was only tested by one of the three stimuli with series concentration and then discarded. The order in which the series of sucrose concentrations were applied to a single sensillum was from low to high concentration of sucrose. The duration of each stimulation was 10 s and an interval of at least 3 min between two stimulations was observed. At least 10 larvae in each dietary group were tested for their sensitivity of a gustatory receptor neuron to the sucrose concentration series, myo-inositol or sinigrin. Amplified signals were digitized by an A/D-interface (IDAC-4, Syntech) and sampled and stored on a personal computer. Electrophysiological responses were quantified by counting the number of spikes in the first second after the start of stimulation.
Spikes were analyzed and counted visually by the experimenter with the aid of Autospike version 3.7 software (Syntech), running the sub-routine “Spike Conversion”, by which all spikes in a recording can be classified according to their amplitude but also their waveform (shape). We then, in general, applied an amplitude threshold and the scale bar to select the target spike groups originating from the “sucrose-best neuron” or other neurons tested. In general, this medium-sized spike type but not the “largest” spike type was identified from the “sucrose-best” neuron because these spikes had two distinct features: (1) it always exhibited the most regular waveform; (2) it had an obvious phasic temporal response pattern. Applying these criteria, amplitude, uniform waveform and phasic temporal pattern resulted into the counts we reported. We then verified the counts arrived at by visual inspection and adjusted the count when the software seemed to have unduly skipped or included a waveform. Such adjustments were within 5% of the count reported by the Autospike software.
Data analysis
The mean response frequency, i.e. the number of spikes fired by the sucrose-best neuron in the lateral sensillum in the first second (spk.s−1) was calculated. For statistical analysis, the original number of spikes per second in response to each stimulus was square-root transformed. The univariate ANOVA with Student-Newman-Keuls (SNK) Post-hoc test was used to compare: (1) the mean response frequency of the sucrose-best neuron in caterpillars exposed to one type of artificial diet over eight successive generations in response to different concentrations of sucrose; (2) the mean response frequency of the sucrose-best neuron to concentrations of sucrose in caterpillars exposed to one type of artificial diet in the F8 and F9 generation, and caterpillars exposed to other two artificial diets in F9 generation, i.e. caterpillars in F8-HS, F9-HS, F8-HS + F9-LS and F8-HS + F9-MS. An one-way ANOVA followed by Post-hoc SNK test was used to compare: (1) the mean response frequency of the sucrose-best neuron in caterpillars with different feeding experiences in response to 1 mM sucrose; (2) the mean response frequency of the myo-inositol-sensitive neuron or the sinigrin-sensitive neuron in the medial sensillum of caterpillars from Wild, F1-HS, F9-HS, F9-LS in response to 1 mM myo-inositol or 1 mM sinigrin. The significance level was set at P < 0.05 or P < 0.01. All statistical analyses were conducted using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Ma, Y. et al. Trans-generational desensitization and within-generational resensitization of a sucrose-best neuron in the polyphagous herbivore Helicoverpa armigera (Lepidoptera: Noctuidae). Sci. Rep. 6, 39358; doi: 10.1038/srep39358 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kinnamon, S. C. & Cummings, T. A. Chemosensory transduction mechanisms in taste. Annu. Rev. Physiol. 54, 715–731 (1992).
Hallem, E. A., Dahanukar, A. & Carlson, J. R. Insect odor and taste receptors. Annu. Rev. Entomol. 51, 113–135 (2006).
Liman, E. R., Zhang, Y. V. & Montell, C. Peripheral coding of taste. Neuron 81, 984–1000 (2014).
Dukas, R. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347–374 (2004).
Mitchell, B. K., Itagaki, H. & Rivet, M. P. Peripheral and central structures involved in insect gustation. Microsc. Res. Techniq. 47, 401–415 (1999).
Chapman, R. F. Contact chemoreception in feeding by phytophagous insects. Annu. Rev. Entomol. 48, 455–484 (2003).
Halpern, B. P. & Tapper, D. N. Taste stimuli: quality coding time. Science 171, 1256–1258 (1971).
Aoki, K. et al. Possible peripheral mechanism for taste disorder in rats administered S-1. Int. J. Clin. Oncol. 19, 549–556 (2014).
McCaughey, S. A. & Glendinning, J. I. Experience with sugar modifies behavioral but not taste-evoked medullary responses to sweeteners in mice. Chem. Senses 38, 793–802 (2013).
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Zhang, Y. et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003).
van Giesen, L. et al. Multimodal stimulus coding by a gustatory sensory neuron in Drosophila larvae. Nat. Commun. 7, 10687; 10.1038/ncomms10687 (2016).
Jermy, T. The role of experience in the host selection of phytophagous insects In Perspectives in Chemoreception and Behavior (eds R. F. Chapman, E. A. Bernays & J. G. Stoffolano Jr. ) Ch. 9, 143–157 (Springer: New York, 1987).
Bernays, E. A., Oppenheim, S., Chapman, R. F., Kwon, H. & Gould, F. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: a behavioral test of the hypothesis with two closely related caterpillars. J. Chem. Ecol. 26, 547–563 (2000).
Renwick, J. A. & Huang, X. P. Rejection of host plant by larvae of cabbage butterfly: Diet-dependent sensitivity to an antifeedant. J. Chem. Ecol. 21, 465–475 (1995).
Dethier, V. G. & Crnjar, R. M. Candidate codes in the gustatory system of caterpillars. J. Gen. Physiol. 79, 549–569 (1982).
Wada-Katsumata, A., Ozaki, M., Yokohari, F., Nishikawa, M. & Nishida, R. Behavioral and electrophysiological studies on the sexually biased synergism between oligosaccharides and phospholipids in gustatory perception of nuptial secretion by the German cockroach. J. Insect Physiol. 55, 742–750 (2009).
Ramirez, G. P., Martinez, A. S., Fernandez, V. M., Corti Bielsa, G. & Farina, W. M. The influence of gustatory and olfactory experiences on responsiveness to reward in the honeybee. PLoS One 5, e13498; 10.1371/journal.pone.0013498 (2010).
Werner-Reiss, U., Galun, R., Crnjar, R. & Liscia, A. Sensitivity of the mosquito Aedes aegypti (Culicidae) labral apical chemoreceptors to phagostimulants. J. Insect Physiol. 45, 629–636 (1999).
Mitchell, B. K. & McCashin, B. G. Tasting green leaf volatiles by larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata. J. Chem. Ecol. 20, 153–769 (1994).
Hill, D. L. Neural plasticity in the gustatory system. Nutr. Rev. 62, S208–S217 (2004).
Pontes, G., Minoli, S., Insaurralde, I. O., de Brito Sanchez, M. G. & Barrozo, R. B. Bitter stimuli modulate the feeding decision of a blood-sucking insect via two sensory inputs. J. Exp. Biol. 217, 3708–3717 (2014).
Wada-Katsumata, A., Silverman, J. & Schal, C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science 340, 972–975 (2013).
Tang, Q. B. et al. Inheritance of electrophysiological responses to leaf saps of host- and nonhost plants in two Helicoverpa species and their hybrids. Arch. Insect Biochem. Physiol. 86, 19–32 (2014).
del Campo, M. L. & Miles, C. I. Chemosensory tuning to a host recognition cue in the facultative specialist larvae of the moth Manduca sexta. J. Exp. Biol. 206, 3979–3990 (2003).
Contreras, R. J. & Frank, M. Sodium deprivation alters neural responses to gustatory stimuli. J. Gen. Physiol. 73, 569–594 (1979).
Zhou, D., van Loon, J. J. & Wang, C. Z. Experience-based behavioral and chemosensory changes in the generalist insect herbivore Helicoverpa armigera exposed to two deterrent plant chemicals. J. Comp. Physiol. A 196, 791–799 (2010).
Zhou, D. S., Wang, C. Z. & van Loon, J. J. Chemosensory basis of behavioural plasticity in response to deterrent plant chemicals in the larva of the Small Cabbage White butterfly Pieris rapae. J. Insect Physiol. 55, 788–792 (2009).
Glendinning, J. I., Ensslen, S., Eisenberg, M. E. & Weiskopf, P. Diet-induced plasticity in the taste system of an insect: localization to a single transduction pathway in an identified taste cell. J. Exp. Biol. 202, 2091–2102 (1999).
Schoonhoven, L. M. What makes a caterpillar eat? In Advances in Chemoreception and Behavior (eds R. F. Chapman, E. A. Bernays & J. G. Stoffolano ) 69–97 (Springer-Verlag, 1987).
Jermy, T., Hanson, F. E. & Dethier, V. G. Induction of specific food preference in Lepidopterous larvae. Entomol. Exp. Appl. 11, 211–230 (1968).
Glendinning, J. I. & Gonzalez, N. A. Gustatory habituation to deterrent allelochemicals in a herbivore: concentration and compound specificity. Anim. Behav. 50, 915–927 (1995).
Renwick, J. A. A. & Huang, X. P. Development of sensitivity to feeding deterrents in larvae of Pieris rapae. Entomol. Exp. Appl. 80, 90–92 (1996).
Glendinning, J. I. et al. A peripheral mechanism for behavioral adaptation to specific “bitter” taste stimuli in an insect. J. Neurosci. 21, 3688–3696 (2001).
Glendinning, J. I. & Hills, T. T. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J. Neurophysiol. 78, 734–745 (1997).
Glendinning, J. I., Domdom, S. & Long, E. Selective adaptation to noxious foods by a herbivorous insect. J. Exp. Biol. 204, 3355–3367 (2001).
Tang, D. L., Wang, C. Z., Luo, L. E. & Qin, J. D. Comparative study on the responses of maxillary sensilla styloconica of cotton bollworm Helicoverpa armigera and oriental tobacco budworm H. assulta larvae to phytochemicals. Sci. China. Ser. C 43, 606–612 (2000).
Bernays, E. A., Chapman, R. F. & Singer, M. S. Changes in taste receptor cell sensitivity in a polyphagous caterpillar reflect carbohydrate but not protein imbalance. J. Comp. Physiol. A 190, 39–48 (2004).
Ishikawa, S. Responses of maxillary chemoreceptors in the larva of the silkworm, Bombyx mori, to stimulation by carbohydrates. J. Cell. Comp. Physiol. 61, 99–107 (1963).
Den Otter, C. J. Responses of the African Armyworm and three species of borers to carbohydrates and phenolic substances: an electro- and behavioural physiological study. Entomol. Exp. Appl. 63, 27–37 (1992).
Bernays, E. A. & Chapman, R. F. Taste cell responses in the polyphagous arctiid, Grammia geneura: towards a general pattern for caterpillars. J Insect Physiol 47, 1029–1043 (2001).
Albert, P. J. & Parisella, S. Physiology of a sucrose-sensitive cell on the galea of the eastern spruce budworm larva, Choristoneura fumiferana. Physiol. Entomol. 13, 243–247 (1988).
Omand, E. A peripheral sensory basis for behavioral regulation. Comp. Biochem. Physiol. A 38, 265–278 (1971).
Simmonds, M. S., Simpson, S. J. & Blaney, W. M. Dietary selection Behaviour in Spodoptera Littoralis: The effects of conditioning diet and conditioning period on neural responsiveness and selection behaviour. J. Exp. Biol. 162, 73–90 (1992).
Fitt, G. P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 34, 17–52 (1989).
Mitter, C., Poole, R. W. & Matthews, M. Biosystematics of the Heliothinae (Lepidoptera: Noctuidae). Annu. Rev. Entomol. 38, 207–225 (1993).
Zalucki, M., Daglish, G., Firempong, S. & Twine, P. The biology and ecology of Heliothis armigera (Hubner) and Heliothis punctigera Wallengren (Lepidoptera, Noctuidae) in Australia–what do we know. Aust. J. Zool. 34, 779–814 (1986).
Zhang, H. J. et al. Comparisons of contact chemoreception and food acceptance by larvae of polyphagous Helicoverpa armigera and oligophagous Bombyx mori. J. Chem. Ecol. 39, 1070–1080 (2013).
Abbaszadeh, G., Srivastava, C. & Walia, S. Insecticidal and antifeedant activities of clerodane diterpenoids isolated from the Indian bhant tree, Clerodendron infortunatum, against the cotton bollworm, Helicoverpa armigera. J. Insect Sci. 14, 29 (2014).
Singh, G., Rup, P. J. & Koul, O. Acute, sublethal and combination effects of azadirachtin and Bacillus thuringiensis toxins on Helicoverpa armigera (Lepidoptera: Noctuidae) larvae. Bull. Entomol. Res. 97, 351–357 (2007).
Kent, L. B. & Robertson, H. M. Evolution of the sugar receptors in insects. BMC Evol. Biol. 9, 41; 10.1186/1471-2148-9-41 (2009).
Leffingwell, J. C. Leaf chemistry In Tobacco: Production, Chemistry and Technology (eds D. L. Davis & M. T. Nielsen ) Ch. 8, 267 (Blackwell Science, 1999).
Huang, F. & Huang, Y. F. Determination of major soluble sugars in tobacco and tobacco products by high performance liquid chromatography. Chin. Tob. Sci. 33, 47–51 (2012).
Huang, Y. F. Determination of fructose, glucose and sucrose in tobacco using high performance liquid chromatography. Tob. Sci. &Tech. 40–43 (2010).
Bian, Y. L., Du, K., Wang, Y. J. & Deng, D. X. Distribution of sugar content in corn stalk. Acta Agron. Sin. 35, 2252–2257 (2009).
Qi, H. Y., Li, T. L. & Zhou, L. N. Changes of composition and content of carbohydrate during tomato fruit development. J. Shengyang Agric. Univ. 32, 346–348 (2001).
Tarpley, L. & Sassenrath, G. F. Carbohydrate profiles during cotton floral bud (Square) development. J. Agron. Crop Sci. 192, 363–372 (2006).
Frazier, C. R., Mason, P., Zhuang, X. & Beeler, J. A. Sucrose exposure in early life alters adult motivation and weight gain. PLoS One 3, e3221 (2008).
Dias, B. G. & Ressler, K. J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17, 89–96 (2014).
Simpson, S. J., James, S., Simmonds, M. S. & Blaney, W. M. Variation in chemosensitivity and the control of dietary selection behaviour in the locust. Appetite 17, 141–154 (1991).
Simmonds, M. S. J., Blaney, W. M. & Schoonhoven, L. M. Effects of larval diet and larval age on the responsiveness of taste neurones of Spodoptera littoralis to sucrose. J. Insect Physiol. 38, 249–257 (1992).
Roeder, K. A. & Behmer, S. T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 28, 1135–1143 (2014).
Deans, C. A., Sword, G. A. & Behmer, S. T. Revisiting macronutrient regulation in the polyphagous herbivore Helicoverpa zea (Lepidoptera: Noctuidae): New insights via nutritional geometry. J. Insect Physiol. 81, 21–27 (2015).
Bernays, E. A. & Chapman, R. F. A neurophysiological study of sensitivity to a feeding deterrent in two sister species of Heliothis with different diet breadths. J. Insect Physiol. 46, 905–912 (2000).
Blom, F. Sensory activity and food intake: a study of input-output relationships in two phytophagous insects. Neth. J. Zool. 28, 277–340 (1978).
Shields, V. D. C. & Mitchell, B. K. Responses of maxillary styloconic receptors to stimulation by sinigrin, sucrose and inositol in two crucifer-feeding, polyphagous lepidopterous species. Philos. Trans. R. Soc. Lond. B 347, 447–457 (1995).
Lefkowitz, R. J. et al. G-protein-coupled receptors: regulatory role of receptor kinases and arrestin proteins. Cold Spring Harbor Symp. Quant. Biol. 57, 127–133 (1992).
Colbert, H. A. & Bargmann, C. I. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14, 803–812 (1995).
Fox, A. N., Pitts, R. J., Robertson, H. M., Carlson, J. R. & Zwiebel, L. J. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl. Acad. Sci. USA 98, 14693–14697 (2001).
Honjo, K. & Furukubo-Tokunaga, K. Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J. Neurosci. 25, 7905–7913 (2005).
Liu, D. & Liman, E. R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 100, 15160–15165 (2003).
Zhang, Y. V., Raghuwanshi, R. P., Shen, W. L. & Montell, C. Food experience-induced taste desensitization modulated by the Drosophila TRPL channel. Nat. Neurosci. 16, 1468–1476 (2013).
Dolzer, J., Krannich, S., Fischer, K. & Stengl, M. Oscillations of the transepithelial potential of moth olfactory sensilla are influenced by octopamine and serotonin. J. Exp. Biol. 204, 2781–2794 (2001).
Abisgold, J. D. & Simpson, S. J. The effect of dietary protein levels and haemolymph composition on the sensitivity of the maxillary palp chemoreceptors of locusts. J. Exp. Biol. 135, 215–229 (1988).
Amakawa, T. Effects of age and blood sugar levels on the proboscis extension of the blow fly Phormia regina. J. Insect Physiol. 47, 195–203 (2001).
Wu, K. J. & Gong, P. Y. A new and practical artificial diet for the cotton bollworm. Insect Sci. 277–282 (1997).
Tang, Q. B., Hong, Z. Z., Cao, H., Yan, F. M. & Zhao, X. C. Characteristics of morphology, electrophysiology, and central projections of two sensilla styloconica in Helicoverpa assulta larvae. Neuroreport 26, 703–711 (2015).
Glendinning, J. I., Nelson, N. M. & Bernays, E. A. How do inositol and glucose modulate feeding in Manduca sexta caterpillars? J. Exp. Biol., 1299–1315 (2000).
Roessingh, P., Hora, K. H., van Loon, J. J. A. & Menken, S. B. J. Evolution of gustatory sensitivity in Yponomeuta caterpillars: sensitivity to the stereo-isomers dulcitol and sorbitol is localised in a single sensory cell. J. Comp. Physiol. A 184, 119–126 (1999).
van Loon, J. J. A. Chemoreception of phenolic acids and flavonoids in larvae of two species of Pieris. J. Comp. Physiol. A 166, 889–899 (1990).
Acknowledgements
We thanks for Ms. Juan Qu for the rearing culture of the insects. We also thank Mr. Huan Zhan and Mr. Huan Cao for their assistance in electrophysiology. The work was supported by National Natural Science Foundation of China (Grant No. 31272373 and 31672367) and Special Fund for Agro-scientific Researches of the Public Interests of China (No. 201203036).
Author information
Authors and Affiliations
Contributions
Qingbo Tang, Fengming Yan and Joop J.A. van Loon designed the study. Ying Ma, Jingjing Li and Xuening Zhang carried out the experiments. Qingbo Tang also analysed the results. Xincheng Zhao assisted with the analysis of the results. Qingbo Tang and Joop J.A. van Loon wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ma, Y., Li, J., Tang, Q. et al. Trans-generational desensitization and within-generational resensitization of a sucrose-best neuron in the polyphagous herbivore Helicoverpa armigera (Lepidoptera: Noctuidae). Sci Rep 6, 39358 (2016). https://doi.org/10.1038/srep39358
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39358
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.