Abstract
The common polymorphic variant in the 5′ untranslated region of the excision repair cross-complementation group 5 (ERCC5) gene was described to generate an upstream open reading frame that regulates both the basal ERCC5 expression and its ability to be synthesized following DNA damage. This variant was reported to affect response to platinum therapy in a cohort of patients with pediatric ependymoma. The role of this variant was investigated in two cohorts of cancer patients, specifically in non-small-cell lung cancer (NSCLC) patients (N = 137) and in epithelial ovarian carcinoma (EOC) patients (N = 240), treated in first-line with platinum-based compounds. Differently from what reported for pediatric ependymoma, the analysis of the polymorphism in NSCLC patients cohort was not able to detect any difference among patients harboring different genotypes both in progression free survival (HR = 0.93; 95%CI 0.64–1.33; p-value = 0.678) and overall survival (HR = 0.90; 95%CI 0.62–1.33; p-value = 0.625). These data were corroborated in a EOC patients cohort, where similar progression free survival (HR = 0.91; 95% CI 0.67–1.24; p-value = 0.561) and overall survival (HR = 0.98; 95% CI 0.71–1.35; p-value = 0.912) were found for the different genotypes. These data, obtained in appropriately sized populations, indicate that the effect of this ERCC5 polymorphism is likely to be relevant only in specific tumors.
Similar content being viewed by others
Introduction
Platinum-based drugs induce damages that are mainly recognized and repaired by the nucleotide excision repair (NER)1. Functional NER has been described to have a critical role in determining the efficacy of the platinum-based therapy in preclinical models. Cancer cells harboring a proficient NER system are more prone to repair DNA lesions and survive when treated with platinum-based compounds2,3.
Excision repair cross-complementation group 5 (ERCC5), also known as Xeroderma pigmentosum group G (XPG), is a gene belonging to NER that encodes a single-strand specific endonuclease that makes the 3′ incision in the DNA repair process following damage. Modulation of this gene was reported to affect NER activity4.
An increasing amount of evidence indicates that genetic variants could be important in identifying patients with a high/low probability of response to specific treatments. Genetic variants in genes belonging to the DNA repair pathways have been reported to be important determinants of platinum response5,6.
The common polymorphic variant in the ERCC5 5′ untranslated region (rs751402) was described to generate an upstream Open Reading Frame (uORF) that affects both the basal ERCC5 expression and its ability to be synthesized following DNA damage. This variant was reported to affect the response to platinum therapy in a cohort of patients with pediatric ependymoma. Indeed, pediatric ependymoma patients harboring the uORF (genotypes AA and AG) have a marked resistance to platinum-based therapy evaluated as a shorter progression free survival (PFS) than patients harboring the G allele7. The same ERCC5 polymorphism was studied in 228 advanced chinese non-small-cell lung cancer (NSCLC) patients treated with platinum-based chemotherapy. It was shown that the AA genotype was associated with a better treatment response than the AG/GG genotypes and this was more evident in the subgroup of patients with squamous cell carcinoma8. In order to clarify the role of this polymorphism in affecting the response to a platinum-based therapy, the polymorphic variant in the ERCC5 5′ untranslated region was studied and correlated with therapeutic outcomes in two abundantly occurring malignancies, NSCLC and epithelial ovarian cancer (EOC), treated in first-line with platinum based compounds.
Results
NSCLC population
Between October 2007 and March 2012, patients receiving a first-line containing platinum compounds were enrolled in the TAILOR trial9. Of these, 137 patients were eligible for the present study. Ninety (65.7%) had GG genotype in the rs751402 locus, 43 (31.4%) harboured an AG variant, whereas four (2.9%) patients had AA polymorphism (hereafter included in the AG patients group). The minor allele prevalence was 18.6%, consistent with available data at NCBI database10.
For the GG population, the median age at diagnosis was 64.3 years (interquartile range (IQR): 57.3–69.9 years) whereas it was 65.5 years (IQR: 58.5–71.2 years) for the AG/AA population. The GG group was characterized by predominantly stage IIIBwet/IV (82.2%), adenocarcinoma histology (70.0%), former or never smoking habit (67.8%), ECOG-PS of 0 (58.9%) and a wild-type status of KRAS (75.5%). Similarly, the AG/AA patients were predominantly high stage (80.8%), with adenocarcinoma histology (70.2%), former or never smoking habit (66.0%), ECOG-PS of 0 (51.1%) and a wild-type status of KRAS (80.8%). None of the characteristics considered was associated with the different genotypes present in the polymorphic site rs751402 (Table 1).
After a median follow-up of 59 months (IQR: 42–65), 128 patients progressed or died and 117 died. Median overall survival (OS) was 16.7 months (IQR 8.39–30.1 months) in the GG group and 13.2 months (IQR 7.24–27.3 months) in the AG/AA group (unadjusted hazard ratios (HR)(GG vs AG/AA) = 1.10, 95% confidence intervals (CI) 0.75–1.61, p = 0.625; adjusted HR(GG vs AG/AA) = 1.03, 95%CI 0.70–1.52, p = 0.892). Figure 1A shows the OS curves according to the rs751402 polymorphism. An ECOG-PS of 2 (HR = 1.56, 95%CI 1.15–2.13, p = 0.004), a IIIb wet/IV stage (HR = 1.79, 95%CI 1.09–2.03, p = 0.021) and a mutated KRAS (HR = 2.27, 95%CI 1.47–3.50, p < 0.001) were associated to a shorter OS (Table 2).
Median PFS was 7.2 months (IQR 3.88–13.8 months) in the GG group and 6.9 months (IQR 3.5–12.0 months) in the AG/AA group (unadjusted HR(GG vs AG/AA) = 1.08, 95%CI 0.75–1.56, p = 0.678; adjusted HR(GG vs AG/AA) = 1.00, 95%CI 0.69–1.45, p = 0.989). Figure 1B shows the PFS curves according to the rs751402 polymorphism. An ECOG-PS of 2 (HR = 1.36, 95%CI 1.03–1.80, p = 0.030), a IIIbwet/IV stage (HR = 1.83, 95%CI 1.13–2.97, p = 0.015) and a mutated KRAS (HR = 1.66, 95%CI 1.10–2.51, p = 0.016) were associated to a shorter PFS, while a squamous histotype (HR = 0.60, 95%CI 0.39–0.92, p = 0.019) conferred a longer PFS (Table 2).
EOC population
Between September 1979 and December 2004, blood samples were collected for 240 patients diagnosed for advanced (stage III/IV) EOC. Of these, 152 (66.4%) had GG genotype in the rs751402 locus, 70 (30.6%) harboured an AG variant whereas 7 (3.0%) patients had AA polymorphism (hereafter included in the AG patients group). For 11 patients we were unable to characterize the rs751402 locus. The minor allele prevalence was 18.3%, again consistent with available data at NCBI database10.
For the GG population the median age at diagnosis was 55.4 years (IQR: 46.8–65.8) whereas it was 51.7 years (IQR: 45.2–61.0) for the AG/AA population. The GG group was characterized by predominantly high grade (91.5%), serous histology (77.6%) and a residual tumor after surgery >10 cm (27.0%). Similarly, the AG/AA patients were predominantly high grade (90.9%), serous histology (76.6%) and with residual tumor after surgery >10 cm (27.3%). None of the characteristics considered was associated with the different genotypes present in the polymorphic site rs751402 (Table 3).
After a median follow-up of 11.3 years (IQR: 9.5–12.8), 186 patients progressed or died and 178 died.
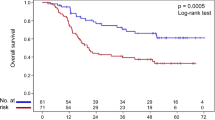
Median OS was 3.9 years in the GG group and 3.8 years in the AG/AA group (unadjusted HR(GG vs AG/AA) = 0.97, 95%CI 0.71–1.34, p = 0.868; adjusted HR(GG vs AG/AA) = 0.88, 95%CI 0.63–1.22, p = 0.429). Figure 2A shows the OS curves according to the rs751402 polymorphism. A tumor grade of 2/3 (HR = 3.69, 95%CI 1.73–7.87, p = 0.001), a residual tumor >2 cm (HR = 2.41, 95%CI 1.72–3.38, p < 0.001) and an older age (HR = 1.03, 95%CI 1.01–1.04, p < 0.001) were the characteristics associated to a shorter OS (Table 4).
Median PFS was 2.3 years in both the GG and in the AG/AA group (unadjusted HR(GG vs AG/AA) = 0.90, 95%CI 0.66–1.23, p = 0.525; adjusted HR(GG vs AG/AA) = 0.83, 95%CI 0.61–1.14, p = 0.252). Figure 2B shows the PFS curves according to the rs751402 polymorphism. Tumor grade of 2/3 (HR = 3.03, 95%CI 1.55–5.92, p = 0.001), residual tumor >2 cm (HR = 2.18, 95%CI 1.57–3.02, p < 0.001) and an older age (HR = 1.02, 95%CI 1.01–1.04, p < 0.001) were the characteristics associated to a shorter PFS (Table 4).
Discussion
The identification of factors able to select patients who would better benefit from therapy is one of the big challenge in oncology. It is well known that some somatic mutations or rearrangements such as gene duplication or deletion in the tumor define subgroup of patients more responsive to chemotherapy11,12. In addition to genetic abnormalities of the tumor, the genotype of the patient could play an important role given that polymorphic variants in particular genes have been shown to influence the response to treatments13,14,15,16.
The response of cells to platinum-based compounds is strictly influenced by their ability to manage the DNA lesions induced by drug treatments17. The NER pathway is one of the major DNA repair pathway in mammalian cells and has been shown to have a key role in the removal of platinum-based DNA adducts18. Dysfunctions in NER pathway have been associated to a different sensitivity of cells to platinum-based drugs19 and preclinical evidence suggests that cells with high expression of NER genes are less sensitive to platinum-based compounds20,21. On the other hand, the translation of these data in the clinic setting has been more complex and conflicting data have been reported22,23,24.
The ERCC5 gene, also known as XPG, is one of the essential DNA repair enzymes of the NER pathway4. XPG expression has been reported to be a determinant of platinum-based molecules activity in preclinical studies25. Its role as a predictive biomarker of response to platinum-based treatment in the clinic is conflicting. Indeed, while a positive correlation between XPG protein expression and response to chemotherapy was reported26 when the XPG mRNA levels were considered, the correlation was not found27.
The heterogeneity in the treatment response might be partially explained by the inter-individual genomic heterogeneity due to polymorphic sites. In this regard, the polymorphisms in the promoter region of the genes are of particular interest.
Recently, a common polymorphic variant in the ERCC5 5′ untranslated region was reported. Somers and colleagues proposed that the presence of the A allele in the position −420 upstream of the physiological AUG start codon generates a new open reading frame in addition to the canonical ERCC5 ORF sited at position −177 from AUG. The presence of the A allele both in homozygosity or heterozygosity was shown to affect the basal protein expression and its ability to be synthesized following DNA damage. In fact, after DNA damage induced by cisplatin treatment, a global reduction of protein synthesis was demonstrated, but cells harboring the A allele in rs751402 site showed a maintenance of ERCC5 protein expression. The clinical corroboration of this finding was that pediatric ependymoma patients harboring the uORF (A allele), have a marked resistance to platinum-based therapy as shown by the reduced PFS as compared to patients with the G allele7. These data, however, contrasted with the ones reported by He and colleagues who investigated the same genetic variant in a cohort of Asiatic population (N = 228) affected by advanced NSCLC treated with platinum-based compounds. They reported that the A allele homozygous patients had a better response to cisplatin treatment than the AG and GG genotype patients8. It has to be stressed that this conflicting results in NSCLC have been obtained considering the AA allele different from AG while is known that AA and AG both affect ERCC5 protein expression and function7.
To better define the role of this polymorphic site in the response to a platinum based therapy, we studied the same polymorphism in two well characterized cohorts of cancer patients affected by two of the most common malignancies worldwide, NSCLC and EOC. The patients analyzed in this study have been treated with platinum-based chemotherapy in first-line and have long term clinical follow-up, allowing the investigation of the impact of the genomic variant on clinical outcomes. Although we analyzed a quite large number of patients, our study was unable to detect any role for the polymorphic variant in the ERCC5 5′ untranslated region in affecting outcomes in both the EOC and NSCLC patients. To note, the HRs obtained in both populations were very close to 1 supporting the hypothesis that the variant has no role on the prognosis of these patients. Based on presented data, obtained in appropriately sized populations, we can conclude that the effect of the ERCC5 polymorphism in the 5′ untraslated region has no effects on response to therapy of NSCLC and EOC Caucasian patients. Peculiar type of tumor and ethnicity (considering comparable histopathological characteristics) could be responsible for the different results obtained in pediatric ependymoma and NSCLC population respectively.
Methods
NSCLC patients
Participating centers registered all consecutive patients with NSCLC before or during first-line chemotherapy to participate to TAILOR trial9. All patients received platinum-based chemotherapy in combination with either vinorelbine, gemcitabine or pemetrexed according to the physician’s choice. Patients with EGFR mutations, early stages patients and patients receiving the adjuvant therapy were excluded from this analysis. Other inclusion or exclusion criteria have been published elsewhere9,28. Research protocol was approved by the Ethics Committee of Ospedale Fatebenefratelli e Oftalmico, Milan (03 October 2007) and the study has been carried out following the principles of the Declaration of Helsinki. All patients who were eligible for participation provided written informed consent with all applicable governing regulations before undergoing any study procedure.
EOC patients
From September 1979 to December 2004 San Gerardo Hospital (Monza, Italy) EOC patients with biological material available were consecutively enrolled in the present study. All patients received platinum-based chemotherapy as first-line. The study has been carried out following the principles of the Declaration of Helsinki and the Ethics Committee of San Gerardo Hospital, Monza, Italy approved the collection and usage of blood samples. Written informed consent was obtained from all patients before undergoing any study procedure.
Samples collection and genotyping
Blood samples were collected in tubes containing K2EDTA and stored at −20 °C. DNA was extracted from blood samples using Maxwell 16 Blood DNA Purification Kit (Promega, Milan, Italy). The rs751402 polymorphism was genotyped using a TaqMan SNP Genotyping assay (Life Technologies, Monza, Italy), based on Real Time PCR technique (ABI 7900, Life Technologies). The PCR was carried out in 384-wells plates with a reaction volume of 5 μL containing TaqMan Genotyping Master Mix (Life Technologies), MGB probes and primers and 10 ng of genomic DNA. Primers and probes sequences are property of Life Technologies. Completed PCR plates were analysed using the TaqMan Genotyper Software (Life Technologies).
Statistical methods
Baseline covariate distributions were summarised using descriptive statistics (median and interquartile range) for continuous variables; absolute frequencies and percentages for categorical variables). Wilcoxon-Mann-Whitney test for continuous covariates and Chi-square test for categorical covariates were used to detect statistical association. Progression free survival was defined as the time from the day of first-line treatment start up (NSCLC analysis) or the time from the date of diagnosis (EOC analysis) to the date of first progression or death from any cause, whichever came first. Overall survival was defined as as the time from the day of first-line treatment start (NSCLC analysis) or from the date of diagnosis (EOC analysis) to the date of death from any cause. Patients alive and without disease progression at the date of study cut-off were censored at the last available information on status. Survival curves were estimated with the Kaplan-Meier method. Cox proportional hazards models were used for univariate and multivariate (adjusted for ECOG-PS, histology and KRAS status for NSCLC analysis; adjusted for age, tumor grade and residual tumor for EOC analysis) analysis to estimate the association between polymorphism and progression free survival or overall survival. Results were expressed as hazard ratios and their 95% confidence intervals. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
Additional Information
How to cite this article: Rulli, E. et al. The 5′UTR variant of ERCC5 fails to influence outcomes in ovarian and lung cancer patients undergoing treatment with platinum-based drugs. Sci. Rep. 6, 39217; doi: 10.1038/srep39217 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Marteijn, J. A., Lans, H., Vermeulen, W. & Hoeijmakers, J. H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol 15, 465–481 (2014).
Zeng-Rong, N. et al. Elevated DNA repair capacity is associated with intrinsic resistance of lung cancer to chemotherapy. Cancer Res 55, 4760–4764 (1995).
Selvakumaran, M., Pisarcik, D. A., Bao, R., Yeung, A. T. & Hamilton, T. C. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res 63, 1311–1316 (2003).
Scharer, O. D. XPG: its products and biological roles. Adv Exp Med Biol 637, 83–92 (2008).
Caiola, E. et al. DNA-damage response gene polymorphisms and therapeutic outcomes in ovarian cancer. Pharmacogenomics J 13, 159–172 (2013).
D’Antonio, C. et al. Pharmacogenomics in lung cancer chemotherapy: a review of what the oncologist should know. Anticancer Res 34, 5241–5250 (2014).
Somers, J. et al. A common polymorphism in the 5′ UTR of ERCC5 creates an upstream ORF that confers resistance to platinum-based chemotherapy. Genes Dev 29, 1891–1896 (2015).
He, C., Duan, Z., Li, P., Xu, Q. & Yuan, Y. Role of ERCC5 promoter polymorphisms in response to platinum-based chemotherapy in patients with advanced non-small-cell lung cancer. Anticancer Drugs 24, 300–305 (2013).
Garassino, M. C. et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 14, 981–988 (2013).
NCBI. http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=751402.
Yang, W. et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res 41, D955–961 (2013).
Gonzalez de Castro, D., Clarke, P. A., Al-Lazikani, B. & Workman, P. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clin Pharmacol Ther 93, 252–259 (2013).
Wang, L., McLeod, H. L. & Weinshilboum, R. M. Genomics and drug response. N Engl J Med 364, 1144–1153 (2011).
Tomalik-Scharte, D., Lazar, A., Fuhr, U. & Kirchheiner, J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J 8, 4–15 (2008).
Robert, J., Morvan, V. L., Smith, D., Pourquier, P. & Bonnet, J. Predicting drug response and toxicity based on gene polymorphisms. Crit Rev Oncol Hematol 54, 171–196 (2005).
Caiola, E., Broggini, M. & Marabese, M. Genetic markers for prediction of treatment outcomes in ovarian cancer. Pharmacogenomics J 14, 401–410 (2014).
Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7, 573–584 (2007).
Damia, G., Imperatori, L., Stefanini, M. & D’Incalci, M. Sensitivity of CHO mutant cell lines with specific defects in nucleotide excision repair to different anti-cancer agents. Int J Cancer 66, 779–783 (1996).
Scharer, O. D. Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol 5, a012609 (2013).
Fautrel, A. et al. Overexpression of the two nucleotide excision repair genes ERCC1 and XPC in human hepatocellular carcinoma. J Hepatol 43, 288–293 (2005).
Steffensen, K. D., Waldstrom, M. & Jakobsen, A. The relationship of platinum resistance and ERCC1 protein expression in epithelial ovarian cancer. Int J Gynecol Cancer 19, 820–825 (2009).
Kap, E. J., Popanda, O. & Chang-Claude, J. Nucleotide excision repair and response and survival to chemotherapy in colorectal cancer patients. Pharmacogenomics 17, 755–794 (2016).
Macerelli, M. et al. Can the response to a platinum-based therapy be predicted by the DNA repair status in non-small cell lung cancer? Cancer Treat Rev 48, 8–19 (2016).
Smith, S. et al. ERCC1 genotype and phenotype in epithelial ovarian cancer identify patients likely to benefit from paclitaxel treatment in addition to platinum-based therapy. J Clin Oncol 25, 5172–5179 (2007).
Farrell, N. P. Preclinical perspectives on the use of platinum compounds in cancer chemotherapy. Semin Oncol 31, 1–9 (2004).
Walsh, C. S. et al. ERCC5 is a novel biomarker of ovarian cancer prognosis. J Clin Oncol 26, 2952–2958 (2008).
Ganzinelli, M. et al. Expression of DNA repair genes in ovarian cancer samples: biological and clinical considerations. Eur J Cancer 47, 1086–1094 (2011).
Marabese, M. et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget 6, 34014–34022 (2015).
Acknowledgements
This work was partially supported from AIRC (IG12915 to MB and IG14536 to GD), TRANSCAN ERA-NET BIORARE (MCG) and AIFA (MCG). EC is recipient of a Fondazione Italiana Ricerca contro il Cancro (FIRC) fellowship.
Author information
Authors and Affiliations
Contributions
M.C.G., M.B. and M.M. conceptualized the study. M.C.G. and L.C. collected data. E.R. did the statistical analysis. F.G., M.G. and E.C. did the molecular analyses. M.M. and G.D. wrote the paper. All authors reviewed and approved the submitted report.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rulli, E., Guffanti, F., Caiola, E. et al. The 5′UTR variant of ERCC5 fails to influence outcomes in ovarian and lung cancer patients undergoing treatment with platinum-based drugs. Sci Rep 6, 39217 (2016). https://doi.org/10.1038/srep39217
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39217
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.