Abstract
Delay in cortical vein filling during the late-venous phase (delayed-LCVF) is characterized by opacification of cerebral veins despite contrast clearance from contralateral veins on dynamic computed tomography angiography (dCTA) in acute ischemic stroke (AIS) patients. The aim of the study was to investigate the associations of delayed-LCVF with clot location, reperfusion status at 24 hours, and 90-days functional outcome in AIS patients who received reperfusion therapy. A prospective cohort of AIS patients treated with intravenous thrombolysis was studied. Groupwise comparison, univariate, and multivariate regression analyses were used to study the association of delayed-LCVF with clot location and clinical outcomes. Of 93 patients (mean age = 72 ± 12 years) with hemispheric AIS included in the study, 46 (49%) demonstrated delayed-LCVF. Patients with delayed-LCVF demonstrated a significantly higher proportion of proximal occlusion (72% vs 13%, P =< 0.0001), and poor reperfusion at 24 hours (41% vs 11%, P = 0.001). The proportion of poor functional outcome at 90 days was not significantly different (22/56 (48%) vs 17/61 (36%), P = 0.297). The appearance of delayed-LCVF on baseline dCTA may be a surrogate for large vessel occlusion, and an early marker for poor 24-hour angiographic reperfusion.
Similar content being viewed by others
Introduction
Identification of patients who are most likely to benefit from reperfusion therapy using clinical and imaging markers is important in the quest for a more tailored approach to treatment in acute ischemic stroke (AIS). We recently reported a novel CT angiographic (CTA) finding on the presence of delayed cortical vein filling in late-venous phase in AIS patients1. Delayed late-phase cortical vein filling (delayed-LCVF) is characterised by late-venous phase opacification of cortical veins despite contrast clearance from contralateral cortical veins on four-dimensional (4D) dynamic time-resolved CTA (dCTA). We found that delayed-LCVF is independently associated with poor baseline collateral status. Currently, assessment of arterial leptomeningeal collateral status is indirect and performed qualitatively through visual examination of the extent and rate of backfilling of pial arteries that are fed by collateral vessels. Therefore, delayed-LCVF, a more ‘direct’ and reproducible measure, may prove to be very helpful to assess collateral status. There is growing interest in the role of leptomeningeal collaterals in AIS1,2,3,4,5,6,7,8. The presence of baseline arterial collaterals is emerging as an important parameter in the evaluation and treatment of cerebral ischemia9, and is linked to infarct core volume10, and functional outcomes6,11,12. Good collaterals are associated with good clinical outcomes; conversely, poor collaterals are linked to infarct growth3. Studies on the role of cortical veins in stroke pathophysiology and prognosis are limited1,2,12,13,14,15,16,17.
Location and the volume of the thrombus are also important factors in prognostication of AIS18,19,20,21,22,23,24,25,26,27. Proximal, high volume clots have poor clinical outcomes, while, low-volume, distal thrombus is associated with good clinical outcomes. Moreover, the size of thrombus and the anatomical differentiation between a proximal and a distal occlusion also influences the effectiveness of intra-venous thrombolysis (IVT) with recombinant tissue plasminogen activator (rtPA)18,19,20,21,22,23,24,25,26. IVT is more efficient in the dissolution of distal clots in comparison to proximal ones. Therefore, further studies on the association of thrombus location with delayed-LCVF, in AIS patients who received IVT, are important.
In this study, we prospectively studied the association of late stage cortical vein drainage in a group of AIS patients treated with IVT, with tissue at risk, clot location, and clinical outcome. The specific objectives of the study were:
-
1
To study the association of delayed-LCVF with ischemic infarct core and tissue at risk.
-
2
To study the association of delayed-LCVF with clot location.
-
3
To investigate if delayed-LCVF is associated with clinical outcomes including reperfusion status at 24 hours, and 90-day functional outcome.
We hypothesise that patients with delayed-LCVF will have smaller penumbra, higher infarct core volumes, and worse outcomes at 90 days. We also hypothesise that the AIS patients with proximal occlusion (M1 proximal (M1P) or ICA) will demonstrate a higher proportion of delayed-LCVF patterns in comparison to the patients with distal occlusion (M1 distal (M1D) or M2 or M3). We discuss the implications of the location of the clot in evaluating the clinical outcomes post IVT in the AIS cohort.
Results
Case Presentation
A case study showing the acute CTP and dCTA at baseline is shown in Fig. 1. Figure 2 depicts the follow-up CT & MR imaging findings at 24 hours.
Case Study 1.
Acute stroke imaging. Top Panel: Axial CTP demonstrated the presence of an occlusion in the left MCA. The red arrow points to the presence of a left MCA occlusion. CTP (coronal 5 mm average registered) shows the presence of penumbra and demonstrated region of reduced cerebral blood flow (CBF) and blood volume (CBV), as well as increased mean transit time (MTT) and time to peak (TTP) in the left MCA cortex. 2D MIP spiral dynamic CT angiography (CTA) images formatted in coronal (middle panel) (a–e), and sagittal (bottom panel) planes (f–j); CTA Right-Left view and CTA posteroanterior view. The Early phase is characterized by early filling of venous sinuses (a), followed by mid-venous phase (b–d). Late venous phase is depicted in (e) (blue-arrow). The presence of left-sided late-venous phase opacification of cortical veins (blue arrow) on left side despite contrast clearance from contralateral (right side) cortical veins can be seen on dynamic CTA image, (e,j). Background: A 72-year-old female with a history of hypertension, diabetes, hyperlipidaemia, and atrial fibrillation presented with right-sided facial droop and right-sided hemiparesis with NIHSS score of 21 on admission. Acute CTP demonstrated acute right middle cerebral artery (MCA) ischemia with evidence of a large penumbra. The patient demonstrated delayed-LCVF on baseline dCTA. The patient received intravenous rt-PA therapy at 90 minutes’ post stroke onset.
Case Study 1.
Follow-up imaging at 24 hours. Non-contrast CT (NCCT) at 24 hours shows low attenuation within the head of the left caudate nucleus, anterior limb of the left internal capsule and lentiform nucleus, as well as patchy low attenuation in the white matter in the corona radiata and temporal lobe, consistent with the known left middle cerebral artery (MCA) infarct. No evidence of haemorrhagic transformation of areas of parenchymal abnormality was seen. DWI-MRI showed patchy infarcts in left MCA territory. T2-FLAIR also confirmed the presence of patchy infarcts in the left hemisphere. Magnetic resonance angiography (MRA) shows that the left MCA has reanalysed however, there remains some poor flow into the branches with the suggestion of some stenosis at the bifurcation. 24 hour CTA showed evidence of partial recanalization. Poor flow at the bifurcation possibly due to stenosis. Background: Despite thrombolysis, the patient showed only partial recanalization/reperfusion. On 24 hour CTA, poor flow into the branches was observed suggestive of stenosis at the bifurcation despite recanalization. There is evidence of infarction involving the basal ganglia and some patchy changes in the left MCA territory. Long-term prognosis of the patient was poor (mRS = 4 at 90 days).
Baseline characteristics
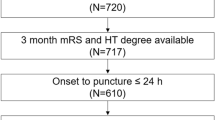
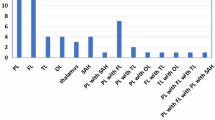
Of the 154 patients, 93 (60.4%) patients (mean age = 71.6 ± 12.4 years; the number of females = 49 (52.7%)) with acute anterior circulation vessel occlusion who received IVT met the inclusion criteria (Table 1). Figure 3 shows the distribution of patients for different clot locations, reperfusion status at 24 hours and functional outcome at 90 days. Out of 93 patients with hemispheric ischaemic stroke included in the study, 46 (49.5%) patients showed delayed-LCVF. The median NIHSS score at admission and 24 hours were 14 (IQR = 8) and 7 (IQR = 9), respectively. Fifty-four (58%) patients demonstrated good functional outcome (mRS 0–2) at 90 days and 52.7% of patients demonstrated major reperfusion at 24 hours. The average OTT for this AIS cohort was 162.6 ( ± 83.9) minutes. The summary of clinical outcomes for all patients is shown in Table 2.
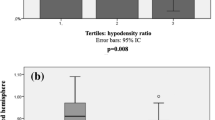
Delayed-LCVF vs No delayed-LCVF
There were no significant differences between the age, sex, NIHSS at admission and at 24 hours, and other clinical risk factors among patients with, and without, delayed LCVF. Patients with delayed-LCVF demonstrated a significant association with poor baseline collaterals (85% vs 21%, P =< 0.001; OR = 20.6; 95% CI = [7, 60]; P=< 0.001), and longer time to peak of maximum arterial enhancement (TPME) (median TPME (in seconds) = 8 vs 6, P =< 0.001; OR = 3.3; 95% CI = [2, 5.4]; P =< 0.001) (Table 1). There were no significant differences in onset to treatment time (OTT) between the delayed vs non-delayed LCVF groups (P = 0.215). We also observed no significant association of OTT with 90 days’ functional outcome (P = 0.2090) which is in agreement with previous study27.
Occlusion of the internal carotid artery (ICA) (32.6% vs 6.4%, P = 0.002), and M1 proximal (M1P) (29% vs 6.4%, P =< 0.001) was significantly higher in the delayed-LCVF group in comparison to non-delayed-LCVF. Conversely, distal M1 (M1D) (15% vs 36%, P = 0.03), and M2 and/or M3 (13% vs 51%, P =< 0.001) occlusions were significantly lower in delayed-LCVF group. When pooled, proximal occlusion (ICA and/or M1P) was commonly seen in delayed-LCVF (72% vs 13%, P =< 0.001) group, and conversely, occurrence of distal occlusion (M1D and/or M2 and/or M3) was significantly lower in delayed-LCVF group (28% vs 87%, P =< 0.001), versus the non-delayed-LCVF group.
Association of delayed-LCVF with infarct core and tissue at risk
Acute core volumes were not significantly different between delayed-LCVF vs non-delayed LCVF groups (18.85 mL vs 13.15 mL, P = 0.731) (Table 1). Univariate analysis revealed no significant association of the acute core volume and presence of delayed-LCVF (OR = 1; 95% CI = [0.99, 1.02]; P = 0.494). In terms of penumbra volume, no significant difference was found between the two groups, delayed-LCVF vs non-delayed LCVF (61.05 mL vs 64.45 mL, P = 0.574). Moreover, delayed-LCVF was not associated with penumbra volume (OR = 1.01; 95% CI = [0.99, 1.01]; P = 0.732). No significant association was found between the delayed-LCVF and 24-hour core volume (18 mL vs 12.5 mL, P = 0.37; OR = 1, 95% CI = [1, 1.01], P = 0.247) (Table 2). Moreover, delayed-LCVF was also not associated with penumbral salvage (24 mL vs 43 mL, P = 0.31; OR = 1, 95% CI = [0.99, 1], P = 0.272).
Associations with clot location
Results of univariate logistic regression analysis for association with proximal clot is shown in Supplementary Table 2. Independent variables (with P ≤ 0.1) and other important covariates (NIHSS at admission) were used for stepwise backward multivariate logistic regression analysis to study the association with incidence of the proximal clot (see Table 3; Model 3A). Finally, TPME, dyslipidemia, and delayed-LCVF were retained in the final multivariate logistic regression model and treated as potential confounders (Table 3; Model 3C). Higher rates of delayed-LCVF (OR = 106.62; 95% CI = [15, 756]; P =< 0.0001) and dyslipidemia (OR = 5.8; 95% OR = [1.6, 21]; P = 0.007) were positively associated with incidence of proximal clot. Interestingly, each unit increase in TPME was negatively associated with presence of proximal clot (OR = 0.56; 95% CI = [0.35, 0.9]; P = 0.02). The model showed good discrimination ability with an area under the receiver operating characteristic (ROC) curve of 0.89 (sensitivity = 82%, specificity = 81.5%). We also compared the reduced models with and without the inclusion of delayed-LCVF (Model 3C (reduced model with delayed-LCVF) vs Model 3B (reduced model without delayed-LCVF)). We found that the addition of delayed-LCVF significantly increased the discrimination accuracy of the model (BICModel3C vs BICModel3B = 95 vs 131; ROCModel3C vs ROCModel3B = 0.89 vs 0.66; SensitivityModel3C vs SensitivityModel3B = 82% vs 36%; SpecificityModel3C vs SpecificityModel3B = 81.5% vs 81.5%; PPVModel3C vs PPVModel3B = 76.19% vs 58.3%).
Delayed-LCVF association with reperfusion status at 24 hours, and functional outcome at 90 days
Patients with delayed-LCVF demonstrated a significantly higher proportion of poor angiographic reperfusion at 24 hours (68% vs 31.8%, P = 0.001) (Table 2). Moreover, bivariate logistic regression analysis also revealed a significant association of delayed-LCVF with overall angiographic reperfusion status at 24 hours (P =< 0.001). Delayed-LCVF was positively associated with poor reperfusion at 24 hours (OR = 4.4; 95% CI = [1.8, 10.6]; P = 0.001). Independent variables (with P ≤ 0.1; age, NIHSS at admission, acute core volume, collateral status, delayed-LCVF, clot location and hypertension) (see Supplementary Table 3) were used for stepwise backward multivariate logistic regression analysis to study the association with poor angiographic reperfusion (see Table 4; Model 4 A). In the reduced model (Table 4; Model 4 C), delayed-LCVF, clot location, and baseline core volume were retained. Higher rates of delayed-LCVF (OR = 3.7; 95% CI = [1.2, 11.28]; P = 0.021), and increasing acute core volume (OR = 1.02; 95% OR = [1, 1.04]; P = 0.021) were significantly associated with poor angiographic reperfusion at 24 hours. Comparison between the reduced models with and without inclusion of delayed-LCVF (Model 4C (reduced model with delayed-LCVF) vs Model 4B (reduced model without delayed-LCVF)) revealed that the addition of delayed-LCVF to the model revealed no added advantage on discriminative accuracy: BIC (BICModel4C vs BICModel4B = 127 vs 128), sensitivity (SensitivityModel4C vs SensitivityModel4B = 70.45% vs 70.45%), or discrimination accuracy (ROCModel4C vs ROCModel4B = 0.74 vs 0.70; SpecificityModel4C vs SpecificityModel4B = 68.75% vs 64.58%; PPVModel4C vs PPVModel4B = 67.4% vs 64.58%) (Table 4). In the model without delayed-LCVF (Model 4B), proximal clot (OR = 2.9; 95% CI = [1.2, 7.17]; P = 0.017) and acute core volume (OR = 1.02; 95% CI = [1, 1.04]; P = 0.015) were significantly associated with poor angiographic reperfusion status at 24 hours.
Delayed-LCVF was not significantly associated with penumbral salvage (Median Penumbral Salvage, in mL = 24.15 vs 43.45, P = 0.31; median penumbral salvage, in percentage = 52.9% vs 84.2%, P = 0.1) or functional outcome at 90 days (47.8% vs 36%, P = 0.297) (Table 2). To study the association with functional outcome at 90 days using backward stepwise multivariate regression analyses, independent variables (with P ≤ 0.1; NIHSS at admission, baseline core volume, penumbra, dyslipidaemia, and reperfusion status at 24 hours (see Supplementary Table 1) and other important covariates (clot location, delayed-LCVF) were included in the final multivariate regression model (Table 5; Model 5A). In the reduced multivariate regression model (Model 5C), delayed-LCVF was not significantly associated with functional outcome at 90 days, when adjusted for NIHSS at admission, acute core volume, clot location, and 24-hour reperfusion status (Table 3). Increasing acute core volume and reperfusion status at 24 hours were significantly associated with poor functional outcome at 90 days. Comparison of model characteristics between the two models, with and without delayed-LCVF, revealed addition of delayed-LCVF significantly improved the discriminative accuracy: BIC (BICModel5C vs BICModel5B = 92.5 vs 116), sensitivity (SensitivityModel5C vs SensitivityModel5B = 82% vs 69%), or discrimination accuracy (ROCModel5C vs ROCModel5B = 0.93 vs 0.80; SpecificityModel5C vs SpecificityModel5B = 88.7% vs 79.25%; PPVModel4C vs PPVModel4B = 84.21% vs 71%) (Table 5).
Discussion
In this study, we sought to investigate associations of novel cortical vein filling pattern, observed during the late venous phase on time-resolved dCTA, with the acute core, tissue at risk, clot location, reperfusion status at 24 hours, and long-term functional outcomes in a cohort of AIS patients treated with IVT. In the current study, we found that the patients with proximal (thrombus in ICA and/or M1P) occlusion are at significantly higher risk of showing delayed-LCVF. We also noted a strong association of delayed-LCVF with poor reperfusion status at 24 hours. We could not demonstrate that the impact of delayed-LCVF on these acute outcomes translated into poor functional outcomes at 90 days, but this may be due to our small sample size and the fact that functional outcome is quite distal in the causal chain we are investigating. The inclusion of delayed-LCVF to the multivariate model significantly improved the predictive accuracy of poor functional outcome. We found no association of delayed-LCVF with ischemic infarct core or tissue at risk. Previously, we reported the presence of delayed cortical vein filling pattern in late-venous phase on dCTA in a cohort of AIS patients, where delayed-LCVF was found to be independently associated with poor baseline arterial collaterals and delay in maximised collateral enhancement1. There are limited studies on the role of cortical veins in stroke pathophysiology, and their associations with thrombus location and clinical outcome13,17,28,29,30,31.
Our findings indicate that delayed-LCVF was significantly more common in patients with M1P and/or ICA occlusion. Delayed-LCVF showed significant improvement in discriminative accuracy when it was added to the multivariate regression model. As such, the appearance of delayed-LCVF on dCTA is a surrogate for proximal large vessel occlusion which is known to be associated with poor outcome. Previous studies have reported that AIS patients with thrombus in M1D, M2, and M3 segments are more likely to undergo recanalization than those with M1P and ICA occlusions18,27. Large vessel occlusions are less likely to be recanalised after IVT and are more likely to have poor clinical outcomes. The fact that delayed-LCVF is strongly associated with proximal thrombus may be used as an important parameter towards stroke prognostication and selection of patients for IVT. We postulate that proximal large vessel occlusions may lead to delayed-late cortical vein filling.
In this study, we found that patients who showed delayed-LCVF on baseline dCTA demonstrated the significantly higher rate of poor angiographic reperfusion at 24 hours. This is clinically relevant suggesting that patients with the delayed-LCVF pattern on baseline dCTA will be poorly reperfused despite IVT. However, the addition of delayed-LCVF to multivariate regression model didn’t improve the predictive accuracy of the model over and above clot location. Our results show that delayed-LCVF is a statistically significant prognostic indicator of early angiographic reperfusion (at 24 hours). From the current literature, we know that reperfusion status is a significant predictor of long-term (90 days) outcome32,33. In a multivariate logistic regression analysis, delayed-LCVF was not a significant covariate for predicting 90 days’ functional outcome. Although, our point estimate supports an effect on 90 days mRS (OR = 1.6), we do not have sufficient power to demonstrate this at a statistically significant level. We hypothesize that patients with the delayed-LCVF pattern on baseline dCTA may show an unfavourable trajectory. Interestingly, the addition of delayed-LCVF significantly improved the predictive accuracy of functional outcome at 90 days. The presence of delayed-LCVF may aid in identifying patients at risk of 24-hour poor angiographic reperfusion. Other studies have also shown significant association of cerebral-venous flow with prognosis in stroke in both animals and humans13,17,34. Interestingly, animal studies focussing primarily on early and mid-venous phase have shown that the presence of cortical vein filling after ischaemic stroke was associated with decreased severity of hemiparesis and lower infarct volumes. This led to the hypothesis that the cortical venous flow may produce favourable outcomes as it would be more commonly prevalent in strokes with good collaterals34. Another study on humans also found that the cortical venous drainage was associated with good clinical outcomes17.
Imaging biomarkers towards the identification of patients who might benefit from early reperfusion therapy and guiding early intervention options to limit or even freeze infarct progression is crucial for strategies in acute stroke treatment35,36. The advent of cutting-edge next generation 320-detector row 640-slice multi-detector CT (MDCT) scanners have facilitated the acquisition of whole-brain, sub-second, and volumetric acquisition of 4D-dCTA data1,37,38. CTP/CTA is not an invasive procedure compared to digital subtraction angiography (DSA), and is routinely obtained during clinical care of stroke patients8,32,33,39, has proven to be of added clinical utility in the early evaluation of stroke, facilitating precise localization of site of occlusion40, and identification of hypoperfused territory at risk of infarction41. The CTP was acquired simultaneously with the CTA with the use of same contrast bolus42. Dynamic CTA allows evaluation of intracranial vasculature and visualisation of contrast flow from its arterial to venous phases. Using appropriate reconstructions of dCTA using MIP algorithm, we investigated various stages of venous drainage and downstream venous dynamics; including the assessment of delayed-LCVF appearance in late venous phase. Assessment of impaired cortical venous drainage may provide valuable information over and above arterial collateral assessment, and the presence of delayed-LCVF could have a role in making informed decisions on patient management and prognosis.
We understand that our study has several limitations, including small sample size and the variability in the cortical venous structures. Since the publication of MR CLEAN, REVASCAT43,44, EXTEND-IA, ESCAPE, and SWIFT PRIME, the standard practice now includes intravenous thrombolysis when possible, complementing thrombectomy43,44,45,46. However, endovascular treatment was not available at our centre at the time of the study. We tried to account for small sample size by using the Wilcoxon-Rank test, which would be conservative in this case. We acknowledge that additional occlusions distal to the M1 segment and variations in the prominences of M2, M3, and M4 trunks may have an impact on the assessment of delay in maximised enhancement. Moreover, it may also be influenced by the differences in the filling time of collaterals in different areas of the MCA territory.
To conclude, in this study, we sought to study the associations of delayed-LCVF with core volume, tissue at risk, clot location, and clinical outcome (vis a vis reperfusion at 24 hours, functional outcome (in terms of modified Rankin score (mRS)) at 90 days) in a prospective cohort of AIS patients who received intravenous thrombolytic therapy. Endovascular procedures or mechanical thrombectomy was not available at our centre at the time of the study. Delayed-LCVF patterns were more commonly seen in proximal thrombus occlusion in M1P and/or ICA. Based on these findings, the appearance of delayed-LCVF on dCTA can be used as a surrogate for proximal thrombus or large vessel occlusion. It may also be useful in identifying patients at risk of poor angiographic reperfusion at 24 hours. Moreover, given the propensity of proximal thrombus towards poor clinical outcome after IVT47, and significant association of delayed-LCVF with both proximal thrombus and poor reperfusion at 24 hours, we postulate that the AIS patients with delayed-LCVF may progress unfavourably, and therefore alternate revascularisation strategies may be considered. We also found that addition of delayed-LCVF significantly improves predictive accuracy of 90 days’ functional outcome. However, in the present cohort, we acknowledge that delayed-LCVF was not found to be a determinant factor in predicting functional outcome at 90 days. In light of the paucity of literature on the cerebral venous system and their role in stroke prognostication, we believe this study may be of clinical relevance towards understanding the role of cerebro-venous system, in particular, cortical vein, in the prognosis of AIS patients. We propose that delayed-LCVF is a marker that will allow clinicians to extract more prognostic information from imaging that is already routinely acquired. Also, in combination with NCCT, CTA/CTP can be rapidly obtained with minimal delay in treatment, and is widely available in emergency departments, and is well tolerated41. Dynamic CTA is a promising technique for the dynamic assessment of the cerebral vasculature. We caution that these results must be understood as preliminary and within the context of the study design. Further prospective studies are recommended to study the role of cortical veins in stroke prognostication.
Materials and Methods
Study design and patient selection
Consecutive acute ischaemic stroke patients admitted to the comprehensive stroke unit, Department of Neurology at our academic medical centre were prospectively studied provided they satisfied the following inclusion criteria: (a) aged 18 and above years, (b) acute anterior circulation vessel occlusion followed by IVT, (c) hemispheric stroke, and (d) dCTA data available at baseline and 24 hours. Patients without identifiable thrombus on the baseline dCTA were excluded. Patients received 0.9 mg/kg intravenous recombinant tissue plasminogen activator (rtPA). Baseline clinical characteristics included age, sex, and clinical risk factors (hypertension, diabetes, dyslipidaemia, history of smoking (past/present), atrial fibrillation (AF), depression and history of stroke and/or transient ischemic attack (TIA)). Clinical data were procured from the patient records. National Institutes of Health Stroke Scale (NIHSS) scores at the time of initiation of the rtPA and at 24 hours were obtained. The time delay between stroke onset and administration of tPA (time to tPA) or onset to treatment (OTT) was also recorded. Management of patients was in accordance with local guidelines and as per the discretion of the treating stroke physician. This study was approved by the Hunter New England Human Research Ethics Committee (HNEHREC, Newcastle, NSW) in accordance with the National Statement on Ethical Conduct in Human Research 2007. All methods were carried out in accordance with the approved guidelines. Informed consent was obtained from the patient in accordance with the Declaration of Helsinki.
All the patients underwent non-contrast CT (NCCT), CT Perfusion (CTP) and CT angiography (CTA) at baseline and follow-up (24 h) NCCT, CTA, and magnetic resonance imaging (MRI), following our routine stroke imaging protocol1. Volumes of the acute perfusion lesion (relative delay time (DT) ≥ 3 seconds) and acute infarct core (relative CBF ≤ 30%) were calculated using previously validated thresholds8,48. Penumbra volume was defined as the volume of the perfusion lesion (DT threshold ≥ 3 seconds) minus the volume of the infarct core (relative CBF threshold < 30% within the DT ≥ 3 sec lesion). The threshold of DT ≥ 3 seconds was based on previous studies48,49.
Maximum intensity projection (MIP) and multiplanar reformat (MPR) reconstructions in coronal and sagittal planes of baseline axial CTA were obtained on the imaging workstation (Vitrea® fX, Version 1.0, Vital Images, Minnetonka, MN, USA). These images were reviewed by consensus by two experienced readers (SB & CL). Three-dimensional volume rendering was applied to obtain the optimized spatial orientation and precise localisation of ischemic lesion. The determination of the location of the clot was based on the most proximal position of the occlusion. Clot location was divided into two groups: (a) proximal clot: any thrombus/occlusion in the M1 proximal (M1P) or ICA, and (b) distal clot: any thrombus/occlusion in M1 distal (M1D), M2 or M350. The exact location of the thrombus or clot M1 proximal (M1P), M1 distal (M1D), M2, M3, or internal carotid artery (ICA)) was determined. ICA occlusion was determined based on the presence of a clot in ICA terminus. The M1 segment of the MCA was divided into two parts of equal length, namely the proximal (M1P) and the distal half (M1D)27. M1 MCA was defined as a vessel extending from the ICA bifurcation to the origin of the first major branch in the Sylvian sulcus. Delayed-LCVF was identified by late venous phase opacification of cortical veins despite contrast clearance from contralateral cortical veins on maximum intensity projection (MIP) images from dCTA. The time to peak of maximum arterial enhancement (TPME) was also recorded1. Collateral grading was done to assess the morphological status using dCTA data based on the degree of reconstitution of the MCA up to the distal end of its occlusion. Collateral grading was classified as ‘good’, ‘reduced’ or ‘poor’ using the Miteff scale6,51. Good collateral grading was assigned if the entire MCA distal to the occluded segment was reconstituted, i.e., if collaterals reconstituted vessels in the: (a) distal portion of the occluded vessel, or (b) proximal portion of the segment adjacent to the occluded vessel (e.g., if there was proximal M1 occlusion, the distal M1 or proximal M2 segments reconstituted)6,51. Collateral grading was assigned “poor” status if the reconstitution of the distal MCA was only partial, i.e., if collaterals reconstituted vessels in the: (a) distal portion of the segment adjacent to the occluded vessel, or (b) two segments distal to the occluded vessel, or (c) little or no significant reconstitution of the territory of the occluded vessel6,51.
Outcome measures
The modified Rankin Scale (mRS) was used to assess clinical outcome in terms of functional status at 3 months. Patient outcomes were dichotomized into good (mRS 0–2) versus poor/bad (mRS 3–6). Angiographic assessment of the degree of reperfusion was done by an independent blinded reviewer on a repeat CTA acquired at 24 hours using modified thrombolysis in cerebral infarction (mTICI) score52. An mTICI grade of 2a, defined as tissue reperfusion in <50% of the occluded artery territory, was identified as partial reperfusion. Major reperfusion corresponded to tissue reperfusion in ≥50% of the occluded artery territory with grades of 2b or 3 on the mTICI scale. All patients with partial or nil angiographic reperfusion at 24 hours were lumped together into “poor reperfusion” category. Penumbral salvage was defined as the difference between the acute CTP lesion volume (PWI lesion) and the 24-hour DWI lesion volume53,54. We identified penumbral salvage in patients where 24-hour DWI lesion volume was smaller than the acute perfusion lesion volume. Percentage of penumbra salvaged was defined as (penumbral salvage volume/penumbra volume) × 10055.
Statistical analysis
All the statistical analyses were performed using STATA (Version 10, 2001; College Station, TX, USA). Numerical values given are the means (±standard deviation) or medians (interquartile range) for age, core and penumbra volumes, mRS scores, NIHSS at admission, NIHSS at 24 hours, and change in NIHSS scores as appropriate. For ordinal or continuous data, Mann-Whitney (Wilcoxon rank-sum) test was used. Nominal data were analysed with the Pearson’s chi-squared (χ2) and the 2-tailed Fisher exact test. Groupwise comparison was made between the patients with and without delayed-LCVF. Group differences were considered significant at values of P < 0.05. To test the independent association of significant variables with delayed-LCVF, logistic regression models were fitted. Baseline infarct lesion volume and penumbra volume was dichotomized into small (≤25 mL) or large (>25 mL) pertinent to the findings on 25 mL threshold of core volume that accurately predicted the presence of penumbra (tissue at risk), response to thrombolysis, and excellent outcome56,57. A stepwise backwards multivariate logistic regression analyses was used to study the association of delayed-LCVF with clot location, 24-hour angiographic reperfusion status, and 90 days functional outcome. Independent variables with P ≤ 0.1 (on univariate regression) and other important clinical covariates were included in the multivariate logistic regression model. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the overall rate of correct classification for the multivariate models were estimated. Finally, the receiver operating characteristic (ROC) curve for the regression model was plotted, and the area under the curve was computed to evaluate the discriminative ability.
Additional Information
How to cite this article: Bhaskar, S. et al. Association of Cortical Vein Filling with Clot Location and Clinical Outcomes in Acute Ischaemic Stroke Patients. Sci. Rep. 6, 38525; doi: 10.1038/srep38525 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bhaskar, S. et al. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: Associations with collateral status. Journal of Cerebral Blood Flow & Metabolism, doi: 10.1177/0271678x16637611 (2016).
Beyer, S. E. et al. Predictive value of the velocity of collateral filling in patients with acute ischemic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 35, 206–212, doi: 10.1038/jcbfm.2014.182 (2015).
Campbell, B. C. V. et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 33, 1168–1172, doi: 10.1038/jcbfm.2013.77 (2013).
Lima, F. O. et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke; a journal of cerebral circulation 41, 2316–2322, doi: 10.1161/strokeaha.110.592303 (2010).
Menon, B. K. et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 33, 365–371, doi: 10.1038/jcbfm.2012.171 (2013).
Miteff, F. et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain: a journal of neurology 132, 2231–2238, doi: 10.1093/brain/awp155 (2009).
Yeo, L. L. et al. Assessment of intracranial collaterals on CT angiography in anterior circulation acute ischemic stroke. AJNR. American journal of neuroradiology 36, 289–294, doi: 10.3174/ajnr.A4117 (2015).
Bhaskar, S. et al. Baseline collateral status and infarct topography in post-ischaemic perilesional hyperperfusion: An arterial spin labelling study. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism, doi: 10.1177/0271678x16653133 (2016).
Liebeskind, D. S. Stroke: the currency of collateral circulation in acute ischemic stroke. Nature reviews. Neurology 5, 645–646, doi: 10.1038/nrneurol.2009.193 (2009).
Bang, O. Y. et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry 79, 625–629, doi: 10.1136/jnnp.2007.132100 (2008).
Menon, B. K. et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR. American journal of neuroradiology 32, 1640–1645, doi: 10.3174/ajnr.A2564 (2011).
Parthasarathy, R. et al. A Combined Arterial and Venous Grading Scale to Predict Outcome in Anterior Circulation Ischemic Stroke. Journal of neuroimaging: official journal of the American Society of Neuroimaging, doi: 10.1111/jon.12260 (2015).
Abud, D. G. et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR. American journal of neuroradiology 26, 2602–2609 (2005).
Dorn, F. et al. Early venous drainage after successful endovascular recanalization in ischemic stroke – a predictor for final infarct volume? Neuroradiology 54, 745–751, doi: 10.1007/s00234-011-0966-8 (2012).
Jensen-Kondering, U. & Böhm, R. Asymmetrically hypointense veins on T2*w imaging and susceptibility-weighted imaging in ischemic stroke. World Journal of Radiology 5, 156–165, doi: 10.4329/wjr.v5.i4.156 (2013).
Krishnan, V., Searls, D. E., Haussen, D. C., Henninger, N. & Thomas, A. Venous ischemia secondary to drainage constriction in a carotid-cavernous arteriovenous fistula. Clinical neurology and neurosurgery 115, 1476–1478, doi: 10.1016/j.clineuro.2012.11.001 (2013).
Parthasarathy, R. et al. Prognostic evaluation based on cortical vein score difference in stroke. Stroke; a journal of cerebral circulation 44, 2748–2754, doi: 10.1161/strokeaha.113.001231 (2013).
Behme, D., Kowoll, A., Weber, W. & Mpotsaris, A. M1 is not M1 in ischemic stroke: the disability-free survival after mechanical thrombectomy differs significantly between proximal and distal occlusions of the middle cerebral artery M1 segment. Journal of neurointerventional surgery 7, 559–563, doi: 10.1136/neurintsurg-2014-011212 (2015).
Dorn, F. et al. Mechanical Thrombectomy of M2-Occlusion. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 24, 1465–1470, doi: 10.1016/j.jstrokecerebrovasdis.2015.04.013 (2015).
Kwak, H. S., Hwang, S. B., Jin, G. Y., Hippe, D. S. & Chung, G. H. Predictors of functional outcome after emergency carotid artery stenting and intra-arterial thrombolysis for treatment of acute stroke associated with obstruction of the proximal internal carotid artery and tandem downstream occlusion. AJNR. American journal of neuroradiology 34, 841–846, doi: 10.3174/ajnr.A3304 (2013).
Lemmens, R. et al. Effect of endovascular reperfusion in relation to site of arterial occlusion. Neurology 86, 762–770, doi: 10.1212/wnl.0000000000002399 (2016).
Lima, F. O. et al. Prognosis of untreated strokes due to anterior circulation proximal intracranial arterial occlusions detected by use of computed tomography angiography. JAMA neurology 71, 151–157, doi: 10.1001/jamaneurol.2013.5007 (2014).
Porelli, S. et al. CT angiography in an acute stroke protocol: correlation between occlusion site and outcome of intravenous thrombolysis. Interventional neuroradiology: journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences 19, 87–96 (2013).
Schwaiger, B. J., Gersing, A. S., Zimmer, C. & Prothmann, S. The Curved MCA: Influence of Vessel Anatomy on Recanalization Results of Mechanical Thrombectomy after Acute Ischemic Stroke. AJNR. American journal of neuroradiology 36, 971–976, doi: 10.3174/ajnr.A4222 (2015).
Shi, Z. S., Loh, Y., Walker, G. & Duckwiler, G. R. Clinical outcomes in middle cerebral artery trunk occlusions versus secondary division occlusions after mechanical thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke; a journal of cerebral circulation 41, 953–960, doi: 10.1161/strokeaha.109.571943 (2010).
Soize, S. et al. Outcome after mechanical thrombectomy using a stent retriever under conscious sedation: comparison between tandem and single occlusion of the anterior circulation. Journal of neuroradiology. Journal de neuroradiologie 41, 136–142, doi: 10.1016/j.neurad.2013.07.001 (2014).
Saarinen, J. T. et al. The mid-M1 segment of the middle cerebral artery is a cutoff clot location for good outcome in intravenous thrombolysis. European journal of neurology 19, 1121–1127, doi: 10.1111/j.1468-1331.2012.03689.x (2012).
Kim, Y. W., Kim, H. J., Choi, S. H. & Kim, D. C. Prominent hypointense veins on susceptibility weighted image in the cat brain with acute infarction: DWI, SWI, and PWI. Acta radiologica (Stockholm, Sweden: 1987) 55, 1008–1014, doi: 10.1177/0284185113508181 (2014).
Meoded, A., Poretti, A., Benson, J. E., Tekes, A. & Huisman, T. A. Evaluation of the ischemic penumbra focusing on the venous drainage: the role of susceptibility weighted imaging (SWI) in pediatric ischemic cerebral stroke. Journal of neuroradiology. Journal de neuroradiologie 41, 108–116, doi: 10.1016/j.neurad.2013.04.002 (2014).
Sorimachi, T., Morita, K., Sasaki, O., Koike, T. & Fujii, Y. Change in cortical vein appearance on susceptibility-weighted MR imaging before and after carotid artery stenting. Neurological research 33, 314–318, doi: 10.1179/016164110x12644252260510 (2011).
Verma, R. K. et al. Leptomeningeal collateralization in acute ischemic stroke: impact on prominent cortical veins in susceptibility-weighted imaging. European journal of radiology 83, 1448–1454, doi: 10.1016/j.ejrad.2014.05.001 (2014).
Eilaghi, A. et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology 269, 240–248, doi: 10.1148/radiol.13122327 (2013).
Soares, B. P. et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke; a journal of cerebral circulation 41, e34–40, doi: 10.1161/strokeaha.109.568766 (2010).
Sasaki, M., Honmou, O., Radtke, C. & Kocsis, J. D. Development of a middle cerebral artery occlusion model in the nonhuman primate and a safety study of i.v. infusion of human mesenchymal stem cells. PloS one 6, e26577, doi: 10.1371/journal.pone.0026577 (2011).
Heiss, W.-D. Ischemic Penumbra: Evidence From Functional Imaging in Man. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 20, 1276–1293 (2000).
Donnan, G. A., Baron, J.-C., Davis, S. M. & Sharp, F. R. In The Ischemic Penumbra 7–20 (2007).
Inoue, S. et al. Diagnostic imaging of cerebrovascular disease on multi-detector row computed tomography (MDCT). Brain and nerve = Shinkei kenkyu no shinpo 63, 923–932 (2011).
Snyder, K. V., Mokin, M. & Bates, V. E. Neurologic applications of whole-brain volumetric multidetector computed tomography. Neurologic clinics 32, 237–251, doi: 10.1016/j.ncl.2013.08.001 (2014).
Bhaskar, S. et al. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: Associations with collateral status. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism, doi: 10.1177/0271678x16637611 (2016).
Lev, M. H. et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. Journal of computer assisted tomography 25, 520–528 (2001).
Ezzeddine, M. A. et al. CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke; a journal of cerebral circulation 33, 959–966 (2002).
Lev, M. H. et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke; a journal of cerebral circulation 32, 2021–2028 (2001).
Goyal, M. et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 387, 1723–1731, doi: 10.1016/s0140-6736(16)00163-x (2016).
Jovin, T. G. et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. The New England journal of medicine 372, 2296–2306, doi: 10.1056/NEJMoa1503780 (2015).
Broderick, J. P. et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. The New England journal of medicine 368, 893–903, doi: 10.1056/NEJMoa1214300 (2013).
Campbell, B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 372, 1009–1018, doi: 10.1056/NEJMoa1414792 (2015).
Barreto, A. D. et al. Thrombus burden is associated with clinical outcome after intra-arterial therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation 39, 3231–3235, doi: 10.1161/strokeaha.108.521054 (2008).
Yu, Y. et al. Defining Core and Penumbra in Ischemic Stroke: A Voxel- and Volume-Based Analysis of Whole Brain CT Perfusion. Scientific reports 6, 20932, doi: 10.1038/srep20932 (2016).
Lin, L., Bivard, A., Levi, C. R. & Parsons, M. W. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: back-to-back quantitative analysis. Stroke; a journal of cerebral circulation 45, 1727–1732, doi: 10.1161/strokeaha.114.005419 (2014).
Sillanpaa, N. et al. Location of the clot and outcome of perfusion defects in acute anterior circulation stroke treated with intravenous thrombolysis. AJNR. American journal of neuroradiology 34, 100–106, doi: 10.3174/ajnr.A3149 (2013).
Christoforidis, G. A., Mohammad, Y., Kehagias, D., Avutu, B. & Slivka, A. P. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR. American journal of neuroradiology 26, 1789–1797 (2005).
Suh, S. H. et al. Clarifying differences among thrombolysis in cerebral infarction scale variants: is the artery half open or half closed? Stroke; a journal of cerebral circulation 44, 1166–1168, doi: 10.1161/strokeaha.111.000399 (2013).
Bivard, A., Spratt, N., Levi, C. & Parsons, M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain: a journal of neurology 134, 3408–3416, doi: 10.1093/brain/awr257 (2011).
Bivard, A., Stanwell, P., Levi, C. & Parsons, M. Arterial spin labeling identifies tissue salvage and good clinical recovery after acute ischemic stroke. Journal of neuroimaging: official journal of the American Society of Neuroimaging 23, 391–396, doi: 10.1111/j.1552-6569.2012.00728.x (2013).
Huang, X. et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. The Lancet Neurology 14, 368–376, doi: 10.1016/S1474-4422(15)70017-7.
Davalos, A. et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 62, 2187–2192 (2004).
Parsons, M. W. et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 30, 1214–1225, doi: 10.1038/jcbfm.2010.3 (2010).
Acknowledgements
The authors would like to acknowledge the financial support from Hunter Medical Research Institute (HMRI), Hunter New England Health (HNE Health, NSW) and University of Newcastle. We would also like to thank the patients and carers, and the physicians, administrators, radiographers, stroke clinical audit and programming staff who contributed to the collection of data. We are especially grateful for administrative support from Louise-Anne Jordan, Malcolm Evans, Michelle K. Wyborn and Kristy Morris.
Author information
Authors and Affiliations
Contributions
Contributors S.B. and C.L. conceived and designed the study. S.B., M.P., A.B., and C.L. collected the data. S.B., J.A., P.S., and C.L. analysed the data. S.B. and C.L. drafted the article and all authors contributed to its revision.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bhaskar, S., Bivard, A., Stanwell, P. et al. Association of Cortical Vein Filling with Clot Location and Clinical Outcomes in Acute Ischaemic Stroke Patients. Sci Rep 6, 38525 (2016). https://doi.org/10.1038/srep38525
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38525
This article is cited by
-
Evaluation of extent vs velocity of cortical venous filing in stroke outcome after endovascular thrombectomy
Neuroradiology (2023)
-
Prognostic capacity of hyperdense middle cerebral artery sign in anterior circulation acute ischaemic stroke patients receiving reperfusion therapy: a systematic review and meta-analysis
Acta Neurologica Belgica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.