Abstract
As a vital beverage crop, tea has been extensively planted in tropical and subtropical regions. Nitrogen (N) levels and forms are closely related to tea quality. Based on different N levels and forms, we studied changes in NO3− and NH4+ fluxes in tea roots utilizing scanning ion-selective electrode technique. Our results showed that under both single and mixed N forms, influx rates of NO3− were much lower than those of NH4+, suggesting a preference for NH4+ in tea. With the increase in N concentration, the influx rate of NO3− increased more than that of NH4+. The NH4+ influx rates in a solution without NO3− were much higher than those in a solution with NO3−, while the NO3− influx rates in a solution without NH4+ were much lower than those in a solution with NH4+. We concluded that (1) tea roots showed a preference for NH4+, (2) presence of NO3− had a negative effect on NH4+ influx, and (3) NH4+ had a positive effect on NO3− influx. Our findings not only may help advance hydroponic tea experiments but also may be used to develop efficient fertilization protocols for soil-grown tea in the future.
Similar content being viewed by others
Introduction
As a crucial component of chlorophylls, nucleic acids, proteins and a great number of secondary plant metabolites, nitrogen (N) is essential for the growth of plants. Nitrate (NO3−) and ammonium (NH4+) are two major inorganic N forms for plants in soils. Due to various factors (e.g., root interference, soil moisture, soil microorganisms, etc.), the reciprocal transformation between ammonium and nitrate is very common in soils1. Thus, roots are always ready to absorb both forms of nitrogen in soils. Both ions can be absorbed and used by plants because root cells possess transport systems such as nitrate and ammonium transporters2. NO3− and NH4+ have different biochemical and energetic features for assimilation, leading to various net fluxes of NO3−/NH4+ and ion preferences of plants, although both ions can be used by plants3.
Comparative studies on net fluxes of NH4+ and NO3− have been conducted in different plants, and the preference for NH4+ or NO3− is usually associated with the physiological needs of plants in various ecosystems4. Tea is an important beverage crop that has been extensively planted in tropical and subtropical regions. In tea plants, N levels and forms, especially in young shoots, are associated with the quality of tea. Previous research has demonstrated that tea plants have a higher absorption of NH4+ compared to NO3− 5. However, high concentrations of NH4+ are toxic in a majority of plants, including woody plants. If only NO3− or both ions are provided, no detrimental influences can be detected in plants6,7. Little information can be found on the interactions between NH4+ and NO3− fluxes in tea roots, although the uptake of NH4+ and NO3− in tea has been explored extensively4,5,8. Moreover, most previous studies on N uptake were carried out using an 15N labeling method, which was unable to interpret the dynamic processes of NH4+ and NO3− fluxes9,10.
Taking an electrophysiological approach, scanning ion-selective electrode technique (SIET) can evaluate ion/molecule-specific activities non-invasively11. To date, NH4+, NO3−, H+, Cd2+, Ca2+, Mg2+, Na+, Cl−, K+, O2 and Al3+ have been identified utilizing SIET; however, the application of SIET for the examinations of net NH4+ and NO3− fluxes in tea roots has not yet been reported.
In this study, the fluxes of net NH4+ and NO3− in absorbing tea roots exposed to various N forms were evaluated with SIET non-invasively. This research had the following objectivities: (1) to monitor any alterations in net NH4+ and NO3− fluxes in tea roots under different N forms, and (2) to assess the interaction between fluxes of NH4+ and NO3− in tea roots. This research is the first attempt to identify fluxes of net NH4+ and NO3− in the presence of different N forms and interactions between NH4+ and NO3− fluxes in tea roots utilizing SIET. Our findings may help advance hydroponic tea experiments and effective fertilization protocols for future soil-grown tea plants.
Results
Net fluxes of NO3− and NH4+ under different N forms and levels
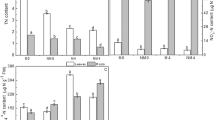
Tea roots were immersed in measuring solutions with different N forms (1 mM NH4NO3, 2 mM KNO3 or 1 mM (NH4)2SO4) to monitor the net fluxes of NO3− and NH4+ under various N forms. Net flux curves of NH4+ and NO3− are shown in Fig. 1. After the 7 d N starvation treatment, both NH4+ and NO3− presented influx states on the root surface when different N forms were given. In addition, the influx rates of NO3− and NH4+ improved gradually. In comparison to the treatments of a single N form, the influx rates of NH4+ and NO3− under the NH4NO3 treatment were more stable (Fig. 1a,b). The influx rates of NO3− were lower than those of NH4+ under both single and mixed N form treatments, which suggested that tea roots had a preference for NH4+ (Fig. 1a,b). When N levels were the same, the total N influx rate of the NH4NO3 treatment was considerably higher than that of the single N form treatments (Fig. 1c).
Net fluxes of NO3− and NH4+ on surfaces of tea roots under different N forms.
Net fluxes of NO3− and NH4+ on tea root surfaces under single (a) and mixed N forms (b). Total N influx rates under the different N forms (c). The mean ± SE (n = 6) is shown in the data. In order to eliminate the “noise” caused by the oscillation, not only 6 biological repetitions, but also 70 measurement time points in each repetition were considered. Thus, SE = SD/√420. The different letters indicate differences between means at P < 0.05.
The influx rates of NO3− and NH4+ under various proportions of N sources are shown in Fig. 2. With an increase in the NH4+ concentration, the influx rates of NH4+ first increased and then decreased under the same concentration of NO3− (Fig. 2). The highest influx rate of NH4+ appeared when the ratio of NH4+:NO3− was 1:1. For the influx rates of NO3−, the highest influx rate of NO3− appeared when the ratio of NO3−:NH4+ was 1.2:1. This suggested that NH4+:NO3− at 1:1 was the critical point and that the absorption rate of NH4+ might not improve with an increase of NH4+; meanwhile, the variation of the NO3− influx rate was extremely different.
The influx rates of NH4+ and NO3− under different N levels are shown in Fig. 3. The influx rates of NH4+ were 6.69 and 1.87 times higher compared to NO3− at 0.2 and 1.2 mM N levels, respectively. With increasing N concentration, the influx rates of NO3− and NH4+ improved significantly. In addition, the influx rate of NO3− improved more than NH4+ with the increase in N concentration. Although a high concentration of ammonium N is toxic for a majority of plants (including woody plants), the high concentration of NH4+ (1.2 mM) did not affect tea tree growth in this study (Supplementary Figures S1 and 2). From phenotyping data, biomass and N contents of root, stem and leaf were higher with the supply of NH4+-N compared to the supply of NO3−-N. Thus, tea trees had better growth with the supply of NH4+-N compared to the supply of NO3−-N (Supplementary Figure S1). In addition, the content of total free amino acid in the supply of NH4+-N was higher compared to the supply of NO3−-N (Supplementary Figure S2). The main amino acids of the tea tree (such as aspartic acid, glutamic acid and theanine) were higher with the supply of NH4+-N compared to the supply of NO3−-N (Supplementary Figure S2). This suggested that tea roots had a stronger NH4+ uptake ability, especially under low N conditions.
Interactions between NH4+ and NO3− fluxes in tea roots
Changes in NH4+ flux are shown in Fig. 4 after adding NH4+ to the bathing solution either with or without NO3−. NH4+ presented influx states on the root surface regardless of whether the bathing solution had NO3−. The NH4+ influx rates in the bathing solution without NO3− were much higher compared with those in a solution with NO3−. In plants without NO3− supply, the influx rates of NH4+ increased and peaked approximately 200 s after NH4+ addition (T1 stage), suggesting a vibrant status of the influx system without the NO3– supply and the ability to retain cytoplasmic NH4+ to a specific degree in tea roots. The influx rates of NH4+ remained stable (T2 stage), demonstrating that the NH4+ influx and efflux systems had reached a balance and that NH4+ influx was dominant. In plants with the NO3− supply, the influx rates of NH4+ did not increase; however, they stabilized quickly and maintained the rate (t1 stage). Approximately 800 s after NH4+ addition, the influx rates of NH4+ began to decrease (t2 stage). The NH4+ influx rates in the bathing solution with or without K+ are shown in Supplementary Figure S3. There was little difference between the NH4+ influx rates in the bathing solution with or without K+, indicating that adding K+ had little effect on the net flux of NH4+ in this study.
Influence of NO3− on NH4+ net fluxes on tea root surfaces.
After adding (NH4)2SO4 to the bathing solution without or with 1 mM KNO3, the changes in tea root NH4+ net fluxes (averaged over 49 s) are presented. The mean ± SE of NH4+ influxes during the measurement period are shown (n = 6). (NH4)2SO4 was added at the vertical arrows.
Changes in NO3− flux are shown in Fig. 5 after adding NO3− to the bathing solution without or with NH4+. NO3− presented influx states on the root surface regardless of whether the bathing solution had NH4+. The NO3− influx rates in the bathing solution without NH4+ were much lower compared to those in a solution with NO3−, which were just the opposite of the NH4+ influxes above. In plants without the NH4+ supply, the influx rates of NO3− remained stable. However, with the NH4+ supply, the influx rates of NO3− could be divided into three stages: (1) a decrease with the lowest point approximately 400 s after NH4+ addition (T1 stage); (2) a spiral increase from approximately 400 to 1200 s after NH4+ addition (T2 stage); and (3) a slope decrease (T3 stage). The influx rates of NO3− were more unstable compared to NH4+, especially in the bathing solution with NH4+.
Influence of NH4+ on NO3− net fluxes on tea root surfaces.
Variations of NO3− net fluxes in tea roots (averaged over 49 s) after adding KNO3 to the bathing solution without or with 0.5 mM (NH4)2SO4 are presented. The mean ± SE of NO3− influxes during the measurement period are shown (n = 6). KNO3 was added at the vertical arrows.
Discussion
In all treatments, tea roots showed absorption states of NH4+ and NO3−, showing that the thresholds for plant development were higher than the cytosolic concentrations of NH4+ and NO3 after a 7 d N deprivation. Tea roots needed to maintain a certain level of NH4+ or NO3− in the cytoplasm7. Additionally, tea roots showed a preference for NH4+ when NH4+ and NO3− existed at the same time. Greater net uptake of NH4+ compared to net uptake of NO3− were reported in maize, rice and wheat roots when NH4+ and NO3− were supplied simultaneously12,13,14. In comparison to NO3− influx, there were some possible reasons for the observed preference for NH4+ influx. As various root tissues needed various amounts of NH4+ and NO3−, one reason might involve root morphology. A higher concentration of NH4+ was needed for protein synthesis in the meristem zone12. In addition, NH4+ absorbed by plants was transformed to amino acids directly in the roots, which required less energy and reducing equivalents for assimilation and transportation in most species15,16. For tea plants, ammonia supplied to the tea roots was quickly stored as theanine, glutamine and arginine in the roots and leaves before the sprouting new shoots17. Previous research18 has reported that after tea plants were fed with 15N-NO3− and 15N-NH4+, the amount of total amino acid in the xylem sap significantly increased, and those fed with 15N-NH4+ had a greater increase compared to those fed 15N-NO3−. Different from other plants, tea can turn redundant glutamic acid into theanine, which was a peculiar amino acid in tea19. Moreover, NH4+ was more readily assimilated than NO3− into theanine20. This process might have eliminated NH4+ toxicity in tea roots and have created a NH4+ preference in tea5.
Previous studies demonstrated that crop growth and yield were significantly improved when two forms of nitrogen were supplied at the same time. In this study, the highest total N influx rates were observed with the NH4NO3 treatment when N levels were the same, which suggested that tea roots had the highest nitrogen absorption efficiency when two forms of nitrogen were supplied simultaneously. This result was consistent with other plants reported14,21,22. For most plants, roots released H+ after absorption of NH4+, leading to a decreased pH in the growth medium, while roots released OH− after absorption of NO3−, leading to the increased pH in the growth medium23,24,25. A mixed application of NO3− and NH4+ at a 1:1 ratio encouraged higher foliar N content and glutamine synthetase (GS) and glutamate synthase (GOGAT) activity in tea26. Although tea roots preferred NH4+, a single application of ammonium nitrogen could aggravate soil acidification in tea gardens27, and could significantly raise the amount of aluminum taken up by tea plants, leading to a decrease in tea quality28. Therefore, to get a higher N absorption efficiency in tea and reduce soil acidification in tea gardens, two forms of nitrogen should be supplied simultaneously.
With the increase in N concentration, the influx rate of NO3− was improved more than NH4+, which might be contributed to differences in activities and expressions of the transport systems between the two ions. Net NO3− and NH4+ absorption can be regulated by low-affinity (LATS) and high-affinity transporters (HATS). When the exterior NH4+ concentration was below 1 mM, HATS played a leading role in the uptake of NH4+ absorption, and when the exterior NH4+ concentration was above 1 mM, LATS were activated29. While HATS played a main role in regulating NO3− uptake when the external NO3− concentration was below 1 mM, LATS started to work when the external NO3− concentration was above 0.5 mM30. According to previous studies, with the increase in N concentration, the LATS for NO3− were stimulated much earlier than the LATS for NH4+. In addition, Glass et al.31 and Britto et al.32 reported that transport through the low-affinity systems were poorly regulated when the high-affinity NH4+ fluxes were effectively regulated. This might lead to the massive vain cycling of NH4+ across the plasma membrane and toxic effects of superfluous NH4+ accumulation. Thus, the influx rate of NO3− was improved more than NH4+ with the increase in N concentration. The present data showed that the influx rate of NO3− was significantly lower than the influx rate of NH4+ under low N conditions (0.2 mM N), which might be contributed to a lower energy cost for both transport and assimilation of NH4+ 16.
The present data demonstrates that the presence of NO3− had a negative effect on net NH4+ uptake. Before being assimilated by plants, NO3− was restored as NH4+, leading to the increase NH4+ concentration in the cytoplasm. The HATS of NH4+ were influenced by the negative feedback regulations and an increased cytosolic NH4+ concentration suppressed the root influx of NH4+ 31. As widely acknowledged, NO3− is a mobile ion and can be restored both in the roots and leaf. Nitrate in tea roots can be directly transported to the xylem sap and then to the leaf18. When NO3− was restored as NH4+ in the leaf, the concentration of NH4+ in the leaf would increase. A shoot-to-root signal might be regarded as the effect of the local N status that controls the influx of NH4+ 33. In addition, NO3− had an inhibitory influence on GS enzyme activity, which might also a reason for the negative effect of NO3− on NH4+ influx34,35 (Fig. 6).
Proposed mechanisms of interaction between NH4+ and NO3− fluxes in tea roots.
The absorption and transformation processes of NH4+ and NO3− are shown. Some influence factors of NH4+ and NO3− absorption are listed to explain possible mechanisms of interaction between the NH4+ and NO3− fluxes in tea roots.
In contrast, the presence of NH4+ had a positive effect on net NO3− uptake, which was consistent with previous studies performed in other species14,33,36. There were several reasons to support this result. First, NH4+ has been reported to increase the respiration rate of plants, which can provide energy for NO3− uptake37. Second, the balance of H+, NH4+ and NO3− could be used to explain this result. Tea roots took up a large amount of NH4+ during growth and later released H+ to maintain the charge balance in the plant body38. According to our current understanding of NO3− transportation, NO3− influx occurs with one H+ symport, and two possible H+ ions promote the inward transportation of one NO3− ion, while the efflux of H+ is meant to balance the influx of NH4+. According to previous studies, due to NH4+ stimulation of H+ efflux, a stimulation of NO3− absorption by NH4+ might increase the availability of H+ for co-transport39,40. Moreover, NH4+ could significantly increase the activities of the GS and GOGAT enzymes, which plays an important role in nitrate reduction and nitrogen assimilation, providing material bases for NO3− absorption34. In our study, the NO3− influx rates were more irregular in the various treatments. This was because NO3− could develop the functions of a mobile ion and an osmoticum14 (Fig. 6).
In conclusion, the elucidation of the mechanisms related to N transport is difficult when assessing net N flux. Net N flux is established as the total of N efflux and influx. Additionally, net N flux is affected by transportation and assimilation rates. The findings showed that tea roots presented influx states of NH4+ and NO3− after a 7 d N-starvation. The uptake rates of NH4+ in tea plants were higher than those of NO3−. NH4+-N can make tea trees grow better when only one single N source can be provided. Furthermore, the presence of NO3− had a negative effect on net NH4+ influx, while NH4+ had a positive influence on net NO3− influx. These findings may not only help guide further hydroponic experiments with tea but also help in developing efficient fertilization protocols for field-grown tea.
Methods
Plant materials and cultivation
Camellia sinensis var. Longjing 43 was used in this study. Annual cutting seedlings of Longjing 43 were transplanted to a full-strength nutrient solution for 75 d. The full-strength nutrient solution contained macronutrients (mmol L−1) NH4NO3 (1), KH2PO4 (0.07), K2SO4 (0.3), MgSO4·7H2O (0.67), CaCl2·2H2O (0.53), and Al2(SO4)3·18H2O (0.035) and micronutrients (μmol L−1) H3BO4 (7), MnSO4·H2O (1), ZnSO4·7H2O (0.67), CuSO4·5H2O (0.13), (NH4)6Mo7O24·4H2O (0.047) and EDTA-Fe (4.2) at pH 5.0. The nutrient solution was circulated by pumps for 24 h every day and replaced every 3 days. Next, an N starvation treatment was carried out for 7 d. The N starvation treatment was conducted using the following nutrient solution which contained macronutrients (mmol L−1) KH2PO4 (0.07), K2SO4 (0.3), MgSO4·7H2O (0.67), CaCl2·2H2O (0.53), and Al2(SO4)3·18H2O (0.035) and micronutrients (μmol L−1) H3BO4 (7), MnSO4·H2O (1), ZnSO4·7H2O (0.67), CuSO4·5H2O (0.13), (NH4)6Mo7O24·4H2O (0.047) and EDTA-Fe (4.2) at pH 5.020. The nutrient solution was circulated by pumps for 24 h every day and replaced every three days. After the 7 d N starvation treatment, the seedlings were harvested to measure ion fluxes.
Determinations of NO3− and NH4+ fluxes at the root surface
The absorbing tea roots were chosen and cut off from the root system of every plant in every treatment group to evaluate the net fluxes of NO3− and NH4+ in tea roots under various N forms. For the different nitrogen form treatments, tea roots were immersed in measuring solutions with different N forms (NH4NO3−N: 0.1 mM CaSO4, 1 mM NH4NO3 and 0.3 mM MES; NH4+-N: 0.1 mM CaSO4, 1 mM (NH4)2SO4, and 0.3 mM MES; and NO3−-N: 0.1 mM CaSO4, 2 mM KNO3 and 0.3 mM MES). MES is 2-(N-morpholino)ethanesulfonic acid hydrate buffer. For different N level treatments, tea roots were soaked in measuring solutions with different N levels (0.2 mM NH4+-N: 0.3 mM MES, 0.1 mM CaSO4 and 0.1 mM (NH4)2SO4; 1.2 mM NH4+-N: 0.3 mM MES, 0.1 mM CaSO4 and 0.6 mM (NH4)2SO4; 0.2 mM NO3−-N: 0.3 mM MES, 0.1 mM CaSO4 and 0.2 mM KNO3; and 1.2 mM NO3−-N: 0.3 mM MES, 0.1 mM CaSO4 and 1.2 mM KNO3). Before analysis, tea roots were transferred to Petri dishes containing 10 mL of measuring solution and equilibrated for 10 min to reduce possible transition effects due to changes in the environmental conditions. Next, the equilibrated root was moved to another Petri dish containing fresh measuring solution and either NH4+ or NO3− flux was measured utilizing the SIET technique. The coefficient of variation under different balance times were shown in Supplementary Figure S4. When the pretreatment time was 10 min, the coefficient of variations under the different treatments was lower and more stable. Therefore, a 10-min pretreatment time was enough and suitable for our study. Six repetitions were established for each treatment. In order to determine the area along the root axis corresponding with maximal net NH4+ and NO3− influx, the net fluxes of both ions were measured along the root tips to an area located 40 mm from the apex (Figure S5). The maximum net NH4+ and NO3− influxes occurred in area between 15 and 25 mm from the root apex. Thus, we chose area between 15 and 25 mm from the root apex as the measurement site. The measuring time of each root was 10 min. The SIET technique was used to measure the net ion flux (NMT-NRP-00A00 system, Younger USA Science and Technology Corporation). The SIET system and the corresponding application process have been previously described in detail for ion flux detection14. Briefly, ion-selective microelectrodes designed with 2–4 μm apertures were manufactured and silanized (for the NH4+ electrode, 100 mM NH4Cl was used as a backfilling solution, followed by a NH4+ selective liquid ion exchange cocktail (#09879, Sigma); for the NO3− electrode, 10 mM KNO3 was used as the backfilling solution, followed by a NO3− selective liquid ion exchange cocktail (#72549, Sigma)). Prior to performing the flux measurements, the microelectrodes were calibrated14.
The absorbing roots of tea were soaked in a test solution and excised from the root system to evaluate the effect of NO3− on NH4+ flux (D (with NO3−): 0.3 mM MES, 0.1 mM CaSO4, 0.1 mM (NH4)2SO4, and 1 mM KNO3; E (without NO3−): 0.3 mM MES, 0.1 mM CaSO4 and 0.1 mM (NH4)2SO4). NH4+ flux was measured utilizing the SIET technique for 5 min after a 10-min balance in the measuring solution. Next, 0.5 mM (NH4)2SO4 was added to the measuring solution. After each addition of (NH4)2SO4, during the first 1–2 min, the measuring solution was mixed thoroughly by expelling and sucking it into a pipette 10 times. NH4+ flux was measured using the SIET technique for another 25 min. The unstable data during the early stage were removed to gain ion flux curves.
The absorbing roots of tea were immersed in a measuring solution and excised from the root system to study the effect of NH4+ on NO3− flux (F (with NH4+): 0.3 mM MES, 0.1 mM CaSO4, 0.2 mM KNO3, and 0.5 mM (NH4)2SO4; G (without NH4+): 0.3 mM MES, 0.1 mM CaSO4 and 0.2 mM KNO3). NO3− flux was measured utilizing the SIET technique for 5 min after a 10-min balance in the measuring solution. Next, 1.0 mM KNO3 was added to the measuring solution. The test process was the same as above.
Statistical analysis
To verify the importance of differences between treatments, one-way ANOVA was performed. Microsoft Excel (Microsoft Corporation, USA) and SPSS Window version 17 (SPSS Incorporation, Chicago, USA) were used to analyze data. To draw figures for the data, OriginPro 8.1 (Origin Incorporation, Chicago, USA) was utilized.
Additional Information
How to cite this article: Ruan, L. et al. Characteristics of NH4+ and NO3− fluxes in tea (Camellia sinensis) roots measured by scanning ion-selective electrode technique. Sci. Rep. 6, 38370; doi: 10.1038/srep38370 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Romero, C. M. et al. Microbial immobilization of nitrogen-15 labelled ammonium and nitrate in an agricultural soil. Soil Sci. Soc. Am. J. 79, 595–602 (2015).
Jackson, L. E., Burger, M. & Cavagnaro, T. R. Roots nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 59, 341–363 (2008).
Patterson, K. et al. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 33, 1486–1501 (2010).
Yang, Y. Y. et al. Characterization of ammonium and nitrate uptake and assimilation in roots of tea plants. Russ. J. Plant Physl. 60, 91–99 (2013).
Ruan, J. Y. et al. Effect of Nitrogen Form and Root Zone pH on Growth and Nitrogen Uptake of Tea (Camellia sinensis) Plants. Ann. Bot. 99, 301–310 (2007).
Ehlting, B. et al. Interaction of nitrogen nutrition and salinity in Grey poplar (Populus tremula × alba). Plant Cell Environ. 30, 796–811 (2007).
Babourina, O. et al. Nitrate supply affects ammonium transport in canola roots. J. Exp. Bot. 58, 651–658 (2007).
Morita, A., Ohta, M. & Yoneyama, T. Uptake, transport and assimilation of 15N nitrate and 15N ammonium in tea (Camellia sinensis L.) plants. Soil Sci. Plant Nutr. 44, 647–654 (1998).
Dijkstra, F. A. et al. Plant and microbial uptake of nitrogen and phosphorus affected by drought using 15N and 32P tracers. Soil Biol. Biochem. 82, 135–142 (2015).
Lee, B. R. et al. Genotypic variation in N uptake and assimilation estimated by 15N tracing in water deficit-stressed Brassica napus. Environ. Exp. Bot. 109, 73–79 (2015).
Xu, Y., Sun, T. & Yin, L. P. Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube. J. Integr. Plant Biol. 48, 823–831 (2006).
Colmer, T. D. & Bloom, A. J. A. Comparison of NH4+ and NO3− net fluxes along roots of rice and maize. Plant Cell Environ. 21, 240–246 (1998).
Taylor, A. R. & Bloom, A. J. Ammonium, nitrate, and proton fluxes along the maize root. Plant Cell Environ. 21, 1255–1263 (1998).
Zhong, Y. et al. Net ammonium and nitrate fluxes in wheat roots under different environmental conditions as assessed by scanning ion-selective electrode technique. Sci. Rep. 4, 7223 (2014).
Hachiya, T., Terashima, I. & Noguchi, K. Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia × hybrida petals. Plant Cell Environ. 30, 1269–1283 (2007).
Miller, A. & Cramer, M. Root nitrogen acquisition and assimilation. Plant Soil 274, 1–36 (2004).
Takeo, T. Ammonium-type nitrogen assimilation in tea plants. Biosci. Biotech. Bioch. 44, 2007–2012 (2014).
Oh, K., Kato, T. & Xu, H. L. Transport of nitrogen assimilation in xylem vessels of green tea plants fed with NH4−N and NO3−N. Pedosphere 18, 222–226 (2008).
Sari, F. & Velioglu, Y. S. Changes in theanine and caffeine contents of black tea with different rolling methods and processing stages. Eur. Food Res. Technol. 237, 229–236 (2013).
Ruan, J. Y. et al. Effect of root zone pH and form and concentration of nitrogen on accumulation of quality-related components in green tea. J. Sci. Food Agric. 87, 1505–1516 (2007).
De Bona, F. D., Schmidt, F. & Monteiro, F. A. Importance of the nitrogen source in the grass species Brachiaria brizantha responses to sulfur limitation. Plant Soil 373, 201–216 (2013).
Yin, S. et al. Influence of medium salt strength and nitrogen source on biomass and metabolite accumulation in adventitious root cultures of Pseudostellaria heterophylla. Acta Physiol. Plant 35, 2623–2628 (2013).
Hinsinger, P. et al. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 248, 43–59 (2003).
Tang, C. et al. Proton release of two genotypes of bean (Phaseolus vulgaris L.) as affected by N nutrition and P deficiency. Plant Soil 260, 59–68 (2004).
Bar-Yosef, B., Mattson, N. S. & Lieth, H. J. Effects of NH4:NO3:urea ratio on cut roses yield, leaf nutrients content and proton efflux by roots in closed hydroponic system. Sci. Hortic. 122, 610–619 (2009).
Du, X. H. et al. Inorganic nitrogen fertilizers induce changes in ammonium assimilation and gas exchange in Camellia sinensis L. Turk. J. Agric. For. 39, 28–38 (2015).
Ruan, J. Y., Zhang, F. S. & Mink, H. W. Effect of nitrogen form and phosphorus source on the growth, nutrient uptake and rhizosphere soil property of Camellia sinenisis L. Plant Soil 223, 63–71 (2000).
Ruan, J. Y. et al. Effects of litter incorporation and nitrogen fertilization on the contents of extractable aluminium in the rhizosphere soil of tea plant (Camallia sinensis (L.) O. Kuntze). Plant Soil 263, 283–296 (2004).
Wang, M. Y. et al. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol. 103, 1259–1267 (1993).
Crawford, N. M. & Glass, A. D. M. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3, 389–395 (1998).
Glass, A. D. et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 53, 855–864 (2002).
Britto, D. T. et al. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. P. Natl. Acad. Sci. USA 98, 4255–4258 (2001).
Loqué, D. & von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 55, 1293–1305 (2004).
Rana, N. K. et al. A CsGS is regulated at transcriptional level during developmental stages and nitrogen utilization in Camellia sinensis (L.) O. Kuntze. Mol. Biol. Rep. 37, 703–710 (2010).
Cramer, M. & Lewis, O. The influence of NO3− and NH4+ nutrition on the carbon and nitrogen partitioning characteristics of wheat (Triticum aestivum L.) and maize (Zea mays L.) plants. Plant Soil 154, 289–300 (1993).
Jampeetong, A., Brix, H. & Kantawanichkul, S. Effects of inorganic nitrogen form on growth, morphology, N uptake, and nutrient allocation in hybrid Napier grass (Pennisetum purpureum × Pennisetum americanum cv. Pakchong1). Ecol. Eng. 73, 653–658 (2014).
Hachiya, T. & Noguchi, K. Integrative response of plant mitochondrial electron transport chain to nitrogen source. Plant Cell Rep. 30, 195–204 (2011).
Wan, Q., Xu, R. K. & Li, X. H. Proton release by tea plant (Camellia sinensis L.) roots as affected by nutrient solution concentration and pH. Plant Soil Environ. 58, 429–434 (2012).
Smart, D. R. & Bloom, A. J. Investigations of ion absorption during NH4+ exposure. I-relationship between H+ efflux and NO3− absorption. J. Exp. Bot. 49, 95–100 (1998).
Garnett, T. P. et al. Kinetics of ammonium and nitrate uptake by eucalypt roots and associated proton fluxes measured using ion selective microelectrodes. Funct. Plant Biol. 30, 1165–1176 (2003).
Acknowledgements
The study was supported by the Open Foundation of State Key Laboratory of Soil and Sustainable Agriculture (Y20160011), the Earmarked Fund for China Agriculture Research System (CARS-23), and the National Natural Science Foundation of China (31570695, 41301228 and 41601329).
Author information
Authors and Affiliations
Contributions
L.Y.W. and H.C. designed the experiment. L.R. conducted the measurements, data analysis and wrote the manuscript. K.W., F.Z., L.Y.W. and P.X.B. assisted with the data analysis. C.C.Z. assisted with the experiment. These authors reviewed the manuscript before the submission.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ruan, L., Wei, K., Wang, L. et al. Characteristics of NH4+ and NO3− fluxes in tea (Camellia sinensis) roots measured by scanning ion-selective electrode technique. Sci Rep 6, 38370 (2016). https://doi.org/10.1038/srep38370
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38370
This article is cited by
-
Genome-wide identification, expression profiling, and protein interaction analysis of the CCoAOMT gene family in the tea plant (Camellia sinensis)
BMC Genomics (2024)
-
Transcriptomic and metabolomic profiling of Camellia oleifera seedling roots treated with different nitrogen forms
Plant Growth Regulation (2023)
-
The GC-TOF/MS-based Metabolomic analysis reveals altered metabolic profiles in nitrogen-deficient leaves and roots of tea plants (Camellia sinensis)
BMC Plant Biology (2021)
-
The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis
BMC Plant Biology (2021)
-
A novel insight into nitrogen and auxin signaling in lateral root formation in tea plant [Camellia sinensis (L.) O. Kuntze]
BMC Plant Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.