Abstract
In a medical context, decision-making is associated with complicated assessment of gains, losses and uncertainty of outcomes. We here provide novel evidence about the brain mechanisms underlying decision-making of analgesic treatment. Thirty-six healthy participants were recruited and completed the Analgesic Decision-making Task (ADT), which quantified individual tendency of risk-taking (RPI), as the frequency of choosing a riskier option to relieve pain. All the participants received resting-state (rs) functional magnetic resonance imaging (MRI) and structural MRI. On rs-functional connectome, degree centrality (DC) of the bilateral anterior insula (aINS) was positively correlated with the RPI. The functional connectivity between the aINS, the nucleus accumbens and multiple brain regions, predominantly the medial frontal cortex, was positively correlated with the RPI. On structural signatures, the RPI was positively correlated with grey matter volume at the right aINS, and such an association was mediated by DC of the left aINS. Regression analyses revealed that both DC of the left aINS and participants’ imagined pain relief, as the utility of pain reduction, could predict the individual RPI. The findings suggest that the functional and structural brain signature of the aINS is associated with the individual differences of risk-taking tendency in the context of analgesic decision-making.
Similar content being viewed by others
Introduction
When making a medical decision – either for choosing over-the-counter medicines or for shared decision-making between patients and clinicians – one needs to carefully balance between both gains (e.g., therapeutic potency) and losses (e.g., the adverse effect)1,2. Behavioral findings have revealed that the choice about an analgesic treatment, a very common scenario of medical decision-making3,4, is influenced by multiple treatment-related attributes, including the potency in pain reduction, the probability that the treatment would work successfully, the probability that an adverse effect would occur, and the time course of the therapeutic effect5,6. These studies adopted the Analgesic Decision-making Task (ADT), which was designed to mimic the clinical scenarios where one needs to choose between a conservative or ‘riskless’ option was less potent, with a higher probability to successfully relieve pain, and a radical or ‘riskier’ option was more potent, with a lower probability to successfully relieve pain (Fig. 1A). The findings suggested that making a medical decision is associated with complicated assessment of risk, which relates to the unpredictability of an outcome7. However, these aspects of medical decision-making have not been systematically investigated.
The Analgesic Decision-making Task (ADT).
In the Analgesic Effect sub-task, the participant needs to choose between the two treatment options, which are opposite in pain-relieving potency and the probability that the treatment would successfully work. In the Adverse Effect sub-task, the participant needs to choose between the options that are opposite in pain-relieving potency and the probability to have an adverse effect. In the Time-course Effect sub-task, the participant needs to choose between the options that are opposite in pain-relieving potency and the time for the treatment to reach its maximal effect (Panel A). The difference in pain experience across the three categories of clinical pain was statistically insignificant (Friedman test, χ2(2) = 0.55, P = 0.76) (Panel B). The difference in imagined pain relief across the three figurative conditions was statistically significant (Friedman test, χ2(2) = 71.51, P < 0.001). The subsequent pairwise comparison showed that the imagined pain relief in Δ9 → 0 was significantly higher than those in Δ9 → 3 (P < 0.01), and the imagined pain relief in Δ9 → 3 was significantly higher than those in Δ9 → 6 (P < 0.01) (Panel C). The difference in RPI across the three sub-tasks of the ADT was statistically insignificant (Friedman test, χ2(2) = 4.46, P = 0.11) (Panel D). In Panel B–D, the bar denotes the median and the horizontal lines denote the first and the third quartiles. The correlation was statistically significant between imagined pain relief Δ9 → 6 and the RPI calculated from all task scenarios (RPITOTAL) (Panel E), RPIANE (Panel F) and RPITE (Panel G).
Evidence from functional magnetic resonance imaging (MRI) studies has revealed that when an individual is assessing the gains and losses for a risky financial decision, the anterior insula (aINS) and the nucleus accumbens (NAc), as the core components of the risk-related network, were frequently activated8,9. The aINS activation is closely associated with anticipation of aversive stimulus10, and its functional connectivity with the dorsal anterior cingulate cortex (dACC) would reflect a heightened salience about pain11. The aINS activation may represent the degree of uncertainty of an outcome12 and play a critical role in the aversion of losses13,14. In contrast, the NAc activation is frequently reported in the scenario when an individual pursued gains14,15, echoing its role in the mesolimbic dopaminergic system16. Activation of the mesolimbic system is associated with pain relief, a desirable status that can be considered as a reward17,18. The functional roles of the aINS and the NAc are parallel to the processing of pain and pleasure17,19, which are major motivators for medical care-seeking. Furthermore, the variation in intrinsic brain signatures, including resting-state (rs) functional connectivity (FC) and grey matter volume (GMV), is associated with the individual differences in risk-taking tendency20,21,22. The findings imply that the variation in intrinsic brain signatures, of the aINS and the NAc may account for the individual differences in risk-taking tendency.
We here adopted the ADT for assessing the risk-taking tendency regarding the choice of analgesic treatment, which was quantified as the risk preference index (RPI). We analyzed the structural (GMV) and functional (rs-FC connectome) signatures of a risk-related network composed of 26 brain regions. We hypothesized that at the aINS and the NAc, network degree centrality (DC), FC and GMV, would be correlated with the individual differences in RPI.
Methods
Participants
The current observational study adopted a cross-sectional design. Thirty-six participants (18 females) between ages of 21 and 46 years (M = 28.1; SD = 5.3) were recruited in at the university campus (see Table 1 for the demographic and clinical profiles of the participants). The sample size was decided based on power analysis, using G*Power 3.1.9.223, for a two-tailed bivariate correlation analysis with alpha = 0.05, power = 0.8, and an medium effect size 0.45. All the participants were recruited via posted advertisement. None of the participants had reported a history of chronic pain or had been previously diagnosed with a psychiatric disorder (see Table 1 for the detailed demographic and behavioral results).
Research Ethics
The study protocol and the relevant methods were approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB code: 2013–080021BCY). All methods were performed in accordance with the relevant guidelines and regulations. All of the participants provided written informed consent before participating in this study.
Assessment of Prior Pain Experience and Imagined Pain Relief
Previous studies have shown that one’s prior experience of clinical pain and subjective satisfaction (i.e., utility) of pain reduction may play a key role in the choice of analgesic treatment24,25. We first assessed the participants’ prior experience of clinical pain, including toothache, headache and stomach ache, and their imagined pain relief in three conditions: from 9 to 0 (Δ9 → 0), 9 to 3 (Δ9 → 3) and 9 to 6 (Δ9 → 6), based on an 11-point numerical rating scale.
Before performing the ADT, the participants were asked to rate their most intense prior experience of clinical pain, respectively for toothaches, headaches and stomach aches, using a 0–100 scale (0 = no pain, 100 = worst possible pain). Subsequently, we assessed the utility (i.e., subjective satisfaction ref. 26) of pain reduction, which was quantified as imagined pain relief5, the degree of satisfaction about pain reduction. The participants were asked to imagine that they were experiencing an acute pain (toothache, headache and stomach ache), with an intensity of 9, based on an 11-point numerical rating scale (NRS) (0 = non-painful and 10 = extremely painful). Subsequently, they were asked to rate imagined pain relief for the following figurative conditions of pain reduction (1) from 9 to 0 (ΔP9 → 0), (2) from 9 to 6 (ΔP9 → 6), and (3) from 9 to 3 (ΔP9 → 3). Imagined pain relief was rated based on a 0–100 numerical scale (0 = no relief and 100 = the strongest relief).
Analgesic Decision-making Task
We assessed the participants’ preferences of analgesic treatment in 22 figurative scenarios where they imagined that they were in pain5,6. The task is implemented as a pencil-and-paper questionnaire that consisted of three sub-tasks: the ‘Analgesic Effect (ANE) task (8 scenarios), the ‘Adverse Effect’ (ADE) task (8 scenarios), and the ‘Time-course Effect’ (TE) task (6 scenarios), presented in a counterbalanced order. The participants needed to imagine that they were experiencing pain at 9, based on the same NRS used in the assessment of prior pain experience, and they would make a choice between two figurative analgesic treatments to reduce the pain. The design of each sub-task was based on our previous studies5,6: (i) in the ANE task, the riskier (radical) treatment was always more potent but less likely to work successfully, and the riskless (conservative) treatment always was less potent but more likely to work successfully. (ii) In the ADE task, the riskier treatment was always more potent but more likely to induce an adverse effect, and the riskless treatment was less potent but less likely to induce an adverse effect. (iii) In the TE task, the riskier treatment was always more potent over the long run but was slower to reduce pain, and the riskless treatment was less potent over the long run but was quicker to reduce pain. The potency of pain reduction and other attributes (i.e., the probabilities that a treatment will work and an adverse effect will occur, and the time delayed for maximal effect) were parametrized across the 22 scenarios. The participants were asked to weigh the potency of pain reduction and the other attributes, when making their decisions. Detailed parameters of the experimental design are illustrated in Supporting Information and our previous studies5,6.
Quantification of Individual Risk-taking Tendency
We quantified the tendency of risk-taking as the frequency to choose the ‘riskier’ option in the ADT. For all the 22 scenarios (8 for the ANE task, 8 for the ADE task and 6 for the TE task), we calculated the Risk Preference Index (RPI) as follows:

N is the frequency that a participant chose the riskier option. A higher RPI indicated a stronger tendency that the participant would choose the riskier treatment, i.e., the treatment that has a stronger pain-relieving effect, regardless of the influence of probability, adverse effect or time-course of therapeutic effect. To further investigate risk-taking tendency in each sub-task, we analyzed the frequency that a participant chose the riskier option in the ANE task (NANE), the ADE task (NADE) and the TE task (NTE). The RPI for each task was calculated as a proportion (RPIANE = NANE/8, RPIADE = NADE/8, and RPITE = NTE/6).
Acquisition and Pre-processing of Imaging Data
Resting-state functional MRI (rs-fMRI) and T1-weighted magnetic resonance imaging (T1-MRI) were performed at the 3 T MRI Laboratory of National Yang-Ming University, using a 3 Tesla Siemens MRI scanner (Siemens Magnetom Tim Trio, Erlangen, Germany). The rs-fMRI images were acquired with the following parameters: gradient echo planar imaging (EPI) with T2* weighted sequence ([TR] = 2000 ms, [TE] = 20 ms, matrix size = 64 × 64 × 40, voxel size = 3.4 × 3.4 × 3.4 mm3, and 183 volumes in total). The T1-MRI images were acquired with the following parameters: a high-resolution sequence ([TR] = 2530 ms, [TE] = 3.02 ms, matrix size = 256 × 256 × 192, voxel size = 1 × 1 × 1 mm3). During rs-fMRI, the participants were instructed to be relaxed, remain awake, and keep their eyes open and fix on a cross symbol on the screen.
Pre-processing of the rs-fMRI data was performed using the Data Processing Assistant for Resting-State fMRI27 and the Resting-State fMRI Data Analysis Toolkit28. The first three scans were discarded for magnetic saturation effects. The imaging data were slice-timing corrected, realigned for head motion, normalized to the Montreal Neurological Institute (MNI) template, and smoothed with an 8 mm Gaussian kernel. The time series was de-trended and band-pass filtered (0.01–0.08 Hz) to extract the low-frequency oscillating components contributing to intrinsic functional connectivity. A regression model was used to remove the spurious or non-specific effects from the following covariates: (a) the parameters of translational and rotational motion, obtained from realignment, (b) the mean signal within the lateral ventricles, and (c) the mean signal within the deep white matter. The regression of whole-brain global signal was not performed, due to the debate on its effect29.
Analysis of Behavioral Data
The difference in prior experience of clinical pain (i.e., toothache, headache and stomach ache), the difference in imagined pain relief (i.e., ΔP9 → 0, ΔP9 → 3 and ΔP9 → 6), and the difference in RPI in all three sub-tasks (i.e., ANE, ADE and TE), were respectively investigated, using the Friedman test. Post-hoc pairwise comparisons were performed using the Wilcoxon-signed rank test, with Bonferroni correction for multiple comparison. We adopted the non-parametric methods due to the non-normality of data (Kolmogorov-Smirnov test, P < 0.1, see Table 1). We first compared the pain ratings from the three categories of clinical pain. A statistically significant difference of the pain ratings would suggest that some of the clinical pain may have a stronger impact on one’s prior pain experience, compared to the others. Based on our previous findings5,6, we expected that the imagined pain relief would be significantly higher in ΔP9 → 3, compared to ΔP9 → 6. To characterize the association between imagined pain relief and the RPIs, we performed partial correlation analyses between the variables, controlled for sex and age. Based on our previous findings6, we expected that imagined pain relief from 9 to 6 (i.e., Δ9 → 6), which represents the individual utility of pain reduction, was correlated with the RPI.
Analysis of Imaging Data
Acquisition and pre-processing of the imaging data were documented in SI Methods. Our general hypothesis focused on the association between risk-taking tendency and functional/structural signatures of the aINS and the NAc. To test the hypothesis, we performed the following three correlation-based analyses:
-
i
On the pattern of rsFC connectome, we performed partial correlation analyses to investigate the association between DC of the bilateral aINS and NAc and RPI, controlled for sex and age. The analyses were performed, respectively for RPITOTAL, RPIANE, RPIADE and RPITE. We hypothesized that degree centrality (DC) of the aINS (DCaINS_L/DCaINS_R) and the NAc (DCNAc_L/DCNAc_R) is associated with the individual RPI. To test the hypothesis, we constructed a risk-related network, primarily based on imaging meta-analysis (see Fig. 2A, also see Supporting Information for detailed procedures). The network consisted of 26 bilateral brain regions (i.e., network nodes) (see Fig. 2B for the regions, and also Table 2 for their definition). A higher DC indicates that a node is more connected with the other nodes, i.e., playing an important role in the network.
Table 2 Selection of the Nodes and Definition of Brain Regions. Figure 2 The risk-related network derived from an imaging meta-analysis implemented using Neurosynth (see SI Methods for detailed procedures).
The network composed of anterior insula, nucleus accumbens, the orbitofrontal cortex, the anterior cingulate gyrus, and the lateral and medial prefrontal cortex (Panel A). The brain regions (i.e., the nodes) of the risk-related network in the rsFC connectome analysis (Panel B).
-
ii
On the aINS/NAc functional connectivity, to understand the pattern of their connections with the other brain regions, we further performed exploratory whole-brain seed-based FC analyses, using the bilateral aINS and the NAc, respectively, as the seeds. We tested a multiple regression model that modeled participants’ sex, age as nuisance regressors and RPI (separately for RPITOTAL, RPIANE, RPIADE, and RPITE) as the predictors, and the seed-based functional connectivity as the dependent variable. The bilateral aINS and the NAc were respectively used as the seed. The analyses would reveal the brain cluster which connectivity was positively correlated with RPI. A cluster would be considered statistically significant with a threshold of intensity (uncorrected P [Punc] < 0.005) and a threshold of cluster size (familywise-error corrected P [PFWE] < 0.05).
-
iii
On the structural signature, we first performed a region-of-interest (ROI)-based analysis, focusing on the bilateral aINS and NAc. We tested a multiple regression model that modeled the sex, age, total brain volume, all as nuisance regressors, and RPI (separately for RPITOTAL, RPIANE, RPIADE, and RPITE) as the predictors, and grey matter volume (GMV) as the dependent variable. Because we focused on the role of the aINS and the NAc, we performed an ROI-based analysis30, using the masks of bilateral aINS and NAc as the ROIs (see Table 2 for ROI definition). A cluster would be considered statistically significant with a threshold of intensity (uncorrected P [Punc]<0.001) and cluster size (familywise-error corrected P [PFWE]<0.05, corrected for small volume).
Analysis of the Mediating Effect of the aINS Connectome
Findings from the previous analyses revealed that RPITOTAL was positively correlated with both the functional signatures (i.e., DCaINS_L/DCaINS_R) and structural signatures (i.e., GMVaINS_R) (see Results). To further clarify the association between these variables, we performed a mediation analysis31 to investigate if the association between GMVaINS_R and RPI was mediated by DCaINS_L/DCaINS_R (i.e., the mediator variable). We assigned GMV as the independent variable, RPI as the dependent variable and DC as the mediator. DC of the right and the left aINS was used, separately, as the mediator. Sobel test was performed to examine the significance of the ‘indirect model’, which represents that a mediational effect of DC.
Predicting Risk-taking Preference from Behavioral and Brain Signatures
The abovementioned results pointed to the conclusion that functional connectome of the left aINS plays a key role in the individual differences of risk-taking tendency. In addition, imagined pain relief and prior pain experience may also guide one’s analgesic decisions. Therefore, we performed a multiple regression analysis for testing the ad hoc hypothesis that functional connectome of the aINS would predict one’s risk-taking tendency in medical decision-making. We modeled RPITOTAL, as the dependent variable, and the following variables as the predictors: (a) imagined pain relief ΔP9 → 6, (b) prior pain experience, and (c) DCaINS_L, which was a significant mediator of RPI, according to the results from the mediational analysis (see Results). The variables ΔP9 → 6 and prior pain experience were log-transformed for normality. We first investigated the covariation between the predictors. The three predictors were not significantly correlated with each other. Secondly, we performed a regression analysis, using the input model, to investigate the predictors which predicted RPITOTAL with a statistically significance. Thirdly, we performed an analysis, using the stepwise method, to investigate the relative effect of prediction from the behavioral and rsFC-connectome predictors.
Results
Behavioral Findings
Prior clinical pain did not significantly differ between the three categories of clinic pain (Fig. 1B), suggesting a homogeneous experience across different clinical pain. Imagined pain relief increased across the three conditions (Δ9 → 6 < Δ9 → 3 < Δ9 → 0, Fig. 1C). The findings indicated that the participants were sensitive to the changes in the utility of pain reduction5,6. Across the three ADT sub-tasks – the Analgesic Effect task (ANE), the Adverse Effect task (ADE) and the Time-course Effect task (TE) – we did not find significant difference in the RPI (Fig. 1D). Further investigation revealed that the correlation was statistically significant between imagined pain relief Δ9 → 6 and the RPI calculated from all task scenarios (RPITOTAL) (r = −0.41, P = 0.017, Fig. 1E), RPIANE (r = −0.38, P = 0.025, Fig. 1F) and RPITE (r = −0.35, P = 0.042, Fig. 1G), but not RPIADE (r = −0.07, P = 0.7). The results confirmed our previous findings that risk-taking tendency is associated with subjective utility of pain reduction (see Fig. 3 and SI Methods for detailed results from the ADT).
Results of the Analgesic Decision-making Task.
The color image presents the frequency that a participant would choose the riskier treatment option. The value ranges from 0–8 in the Analgesic Effect Task (ANE) and the Adverse Effect task (ADE), which both consisted of 8 scenarios. The value ranges from 0–6 in the Time-course Effect Task (TE), which consists of 6 scenarios (Panel A). The change of risk-taking preference in different conditions of the sub-tasks. In the ANE task, the risk-taking preference of the group (i.e., group RPI) increases as the overall probability to have an analgesic effect decreases. In the ADE task, group RPI increases as the overall probability to have an adverse effect decreases. In the TE task, group RPI increases as the time delayed to reach maximal effect decreases (Panel B).
Analysis of rsFC Connectome
In general, partial correlation analyses revealed that RPITOTAL was positively correlated with both DCaINS_L (r = 0.49, P = 0.002) and DCaINS_R (r = 0.41, P = 0.014), controlled for participants’ sex and age (Fig. 4A). In terms of each sub-task, RPIANE was positively correlated with bilateral DCaINS and bilateral DCNAc (Fig. 4B), confirming our hypothesis. In contrast, RPIADE and RPITE did not show significant correlation with DCaINS or DCNAc.
Results of the rsFC connectome analysis.
DC of the bilateral aINS is positively correlated with RPITOTAL, controlled for participants’ sex and age. The lower panel revealed that a participant with a higher RPITOTAL showed denser FC at the bilateral aINS, compared to a participant with a lower RPITOTAL (Panel A). DC of the bilateral aINS and the NAc is positively correlated with RPIANE, but not RPIADE or RPITE, controlled for participants’ sex and age (Panel B).
Analysis of aINS/NAc Functional Connectivity
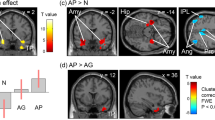
We found that the individual RPI was significantly positively correlated with the FC between the bilateral aINS/NAc and multiple brain regions (Table 3). Notably, RPITOTAL was positively correlated with the FC between the left aINS and the bilateral dorsal anterior cingulate cortex (dACC)/the right pallidum and putamen/the right aINS (Fig. 5A) and the FC between the right NAc and the right paracingulate cortex (PAC)/frontal pole. RPIANE was positively correlated with the FC between the left aINS and the bilateral PAC/dACC, the FC between the left NAc and the pre-Supplementary Motor Area (pre-SMA)/the right inferior frontal gyrus (IFG) (Fig. 5B), and the FC between the right NAc and the right dmPFC (Fig. 5C). RPITE was positively correlated with the FC between the left aINS and the dorsomedial prefrontal cortex (dmPFC)/the right IFG/premotor cortex.
Results of the analyses of aINS/NAc FC.
RPITOTAL is positively correlated with the rsFC between left aINS and multiple brain regions including the right pallidum and putamen, the right dACC and the right aINS (Panel A). RPIANE is positively correlated with the rsFC between the left NAc and the right pre-SMA/IFG (Panel B). RPIANE is positively correlated with the rsFC between the right Nac and the right dmPFC (Panel C). In all panels, the clusters larger than 350 voxels were visualized.
Analysis of Grey Matter Volume
We found that RPITOTAL was positively correlated with the GMV at the right aINS (GMVaINS_R) ([x,y,z] = [32,14,−12], Z = 3.64, cluster-based PFWE = 0.011, corrected for small volume) (Fig. 6A). In contrast, RPIANE, RPIADE and RPITE were not significantly correlated with either GMVaINS or GMVNAc. Additionally, we performed a whole-brain exploratory analysis to investigate the brain region that showed revealed significantly positive correlation between the GMV and RPITOTAL, RPIANE, RPIADE and RPITE. A stringent statistical threshold (family-wise error-corrected, P = 0.05) was adopted in the analysis. The only above-threshold cluster in the whole-brain analysis is the right prefrontal cortex, which GMV showed positive correlation with RPITE ([x,y,z] = [30,60,9], Z = 4.82, PFWE = 0.027) (Fig. 6B).
Results of the structural signatures and mediation analysis.
GMV of the right aINS is positively correlated with RPITOTAL (Panel A). GMV of the right frontal pole is positively correlated with RPITE (Panel B). The mediation analysis revealed that the association between right aINS GMV and RPITOTAL is mediated by DC of the left aINS. The number denotes the non-standardized coefficient from the regression model that predicts the relationship between the IV, DV and mediator. All models are controlled for sex and age (Panel C).
Analysis of the Mediating Effect of the aINS Connectome
We found that the association between GMVaINS_R was significantly mediated by the DCaINS_L (Sobel test, T = 0.21, P = 0.035) (Fig. 6C, also see Table 4 for detailed results). The findings suggested that the association between brain structure and RPI could be mediated by the functional connectome of the aINS.
Predicting Risk-taking Preference from Behavioral and Brain Signatures
We first investigated the covariation between the predictors. The three predictors were not significantly correlated with each other. Secondly, we performed a regression analysis using the input model. The result showed that all the three predictors explained 38.5% of the variation of RPITOTAL (adjusted R2 = 0.385, P = 0.001). The significant predictors were DC of the left aINS (beta = 0.471, P = 0.001) and imagined pain relief (beta = −0.398, P = 0.007). In contrast, prior experience of pain was not a significant predictor. Thirdly, we performed an analysis using the stepwise method. The results showed that DC of the left aINS was the predominant predictor, which explained 26.1% of the variation of RPITOTAL (ΔR = 0.26, P = 0.001). Additionally, imagined pain relief explained 12% of the variation (ΔR = 0.12, P = 0.016).
Discussion
Summary of the Major Findings
In a medical context, decision-making is associated complicated assessment of gains, losses and uncertainty of outcomes5,6. Previous studies mostly focused on the behavioral and brain mechanisms underlying financial decision-making14,32. We here provide novel evidence about the brain mechanisms underlying decision-making in a medical context. Confirming our hypothesis, we found the aINS, a ‘core component’ of the risk-related network9 is associated with the individual differences in risk-taking tendency, assessed by the ADT. Specifically, we found that, on rs-FC connectome, the higher DC of the bilateral aINS and NAc, the stronger tendency or higher frequency (i.e., a higher RPI) that the participants would choose a riskier treatment to relieve pain (Fig. 4). FC between the aINS, the NAc and multiple brain regions, predominantly the SFG, the dACC and the medial frontal cortex, was positively correlated with RPI (Fig. 5). On structural signatures, we found that the RPI was positively correlated with the GMV at the right aINS (Fig. 6) and such an association was mediated by DCaINS_L. Finally, the regression model revealed that both the functional connectome (i.e., DCaINS_L) and psychological utility (i.e., imagined pain relief) can predict risk-taking tendency. Altogether, the findings suggested that the functional and structural brain signatures of the aINS are associated with the individual differences of risk-taking tendency in the context of medical decision-making.
Neural Correlates of Risk-tendency of Analgesic Decision-making
Our findings fit into the proposal that during financial decision-making, risk assessment is associated with the activation of a ‘risk matrix’9,33. In such a network, the aINS plays a key role during the anticipatory phase of decision-making, when the participants need to assess the risk related to outcomes. Consistently, we found a significant role about the aINS in medical decision-making. The aINS is associated with not just the anticipation of incoming painful stimuli11, but also the emotional experience of recalled/imagined pain34. Therefore, a denser connection of the aINS (i.e., a higher DCaINS) during the resting status may reflect a heightened salience about the current status, which would direct the individual towards future pain-related experience. Consistently, the seed-based FC analysis showed that the aINS-dACC connectivity, i.e., the main component of the salience network35, and the connectivity between the right and the left aINS, were correlated with RPITOTAL (Fig. 5A). Such an increased saliency about pain would motivate the individual to take a riskier option, which would deliver a stronger pain-relieving effect. Consistent with this view, we found that functional connectome and FC of the NAc was associated with RPI, especially in the ANE task, the sub-task that required a participant to consider the probability to have their pain relieved (Figs 4B and 5B). In accordance with our results, evidence from financial decision-making tasks has revealed that the NAc activation was associated with the anticipation preceding the selection of a risky option, which would receive more gains, compared to a riskless option14,15. Notably, the patients with chronic pain, compared to healthy controls, showed a higher impulsivity to gain in a gambling task, and this impulsivity was associated with the changes in NAc connectivity36. As part of the mesolimbic dopaminergic system, the NAc plays a key role in processing of the pleasure from a reward17. Altogether, the findings suggested that a stronger tendency of choosing the riskier analgesic option may be associated with a higher salience about pain and a greater expected reward for pain relief.
We noted that the findings were not all consistent through the three sub-tasks. In the ADE tasks, neither DCaINS nor DCNAc was associated with RPI. Consistently, FC of these regions was not significantly correlated with RPI (Table 3). The contrasting findings from the ANE and the ADE tasks may suggest distinct the behavioral and brain mechanisms underlying the risk decision-making for gains (i.e., pursing analgesic effect) and losses (i.e., avoiding adverse effect). In the ANE task, the decision is oriented to pursue a reward, and the NAc would play a dominant role. The RPI was positively correlated with FC between the bilateral NAc and the brainstem as well as the hippocampus (Table 3). The NAc-hippocampus connectivity may reflect the effect of motivational significance on memory formation, bridging past experience with future decisions37. In contrast, in the ADE task, the decision was oriented to avoid harm, rather than to pursue a reward. Notably, our behavioral results showed that the behavioral index RPIANE, but not RPIADE, is associated with imagined pain relief (Fig. 1). The behavioral patterns were consistent with the imaging findings, showing a distinct mechanism underlying medical decisions of different orientations. Finally, in the TE task, we noted that FC between the left aINS and multiple regions in the dorsolateral prefrontal cortex (DLPFC), including the SFG, the MFG and the premotor cortex, was positively correlated with the RPI (Table 3). In the delayed-discounting task, the DLPFC regions composed of the time network, which reflected the sensitivity to time delay21. The DLPFC is associated with deliberation and planning38, and plays a key role in inhibiting an impulsive decision, i.e., seeking a quick but smaller reward39. In our TE task, increased FC of these regions may reflect the tendency that a participant would deliberate the future benefits of pain relief (i.e., preferring a riskier option) and inhibit the impulsivity for a quick but lesser relief.
Limitations of the Study and Further Considerations
Our findings need to be interpreted based on the following limitations of study design. First, the ADT paradigm is designed based on the hypothetical choice paradigm, which has been widely used in assessing individual preference of monetary decision-making32. Such a paradigm would be, ecologically, more consistent with the scenario of making medical decisions, i.e., the situation when a patient needs to make a decision before she/he actually perceives the treatment effect. It should be noted that the results from a hypothetical choice paradigm can be different from an experience-based paradigm40. In the latter paradigm, decision makers receive feedback, such as the pain-relieving effect, from their decisions. Secondly, though the ADT was specifically designed to simulate a clinical pain-relieving scenario, it was simplified in many aspects. For example, we did not investigate the influence of financial costs about the treatment and assumed it is the same across all the task scenarios. The simplification may compromise the ecological validity of the ADT. Thirdly, the participants included in the study all received a higher degree of education. The homogeneity in education level suggested that the participants were able to understand the decision-making scenarios, which require basic literacy and numeracy. Our conclusion may not be generalized to the illiterate or innumerate people. Finally, we aimed to investigate the association between functional/structural signatures and individual differences in risk-taking. Such a correlation-based observation cannot elucidate the causal relationship between brain signatures and behavioral variations. Our findings may imply that a greater GMV and stronger functional connection at the aINS would predispose the tendency of risk-taking in making medical decisions. Conversely, it may imply that a long-term experience about making riskier decisions may, in a long run, reshape the functional and structural signatures, as an effect of brain plasticity.
Clinical Implications
The current findings revealed that both structural and functional signatures of the aINS would reflect one’s risk-taking tendency. The clinical significance of this finding can be related to the role of the aINS in psychiatric disorders and chronic pain. Heighted activation of the aINS was associated with increased salience and perceived threat to pain11,41. It is noteworthy that the aINS plays a key role in anticipating aversive stimuli (ref. 11, especially in the anxiety-prone individuals10 Alterations in brain processing of anticipatory stimuli can be associated with the development of psychiatric disorders, such as generalized anxiety42. Consistently, in our study, the ADT requires the participants to anticipate experience of pain relief during decision-making. Therefore, changes in the aINS signatures implied that the individual suffer from psychiatric disorders or chronic pain may show a different risk-taking behavior, compared to healthy controls. The association between individual traits, their medical history and risk-taking tendency, would be clinically significant issue and require further investigation.
Conclusions
Our novel behavioral and neuroimaging evidence suggests that the functional and structural brain signatures of the aINS are associated with the individual differences of risk-taking tendency in the context of analgesic decision-making.
Additional Information
How to cite this article: Lin, C.-S. et al. Functional and Structural Signatures of the Anterior Insula are associated with Risk-taking Tendency of Analgesic Decision-making. Sci. Rep. 6, 37816; doi: 10.1038/srep37816 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Frosch, D. L. & Kaplan, R. M. Shared decision making in clinical medicine: past research and future directions. American journal of preventive medicine 17, 285–294 (1999).
McNutt, R. A. Shared medical decision making: problems, process, progress. Jama 292, 2516–2518, doi: 10.1001/jama.292.20.2516 (2004).
Dale, O. et al. Prevalence of use of non-prescription analgesics in the Norwegian HUNT3 population: Impact of gender, age, exercise and prescription of opioids. BMC public health 15, 461, doi: 10.1186/s12889-015-1774-6 (2015).
Sarganas, G. et al. Prevalence, trends, patterns and associations of analgesic use in Germany. BMC pharmacology & toxicology 16, 28, doi: 10.1186/s40360-015-0028-7 (2015).
Lin, C. Making the decision to stop pain: Probability and magnitude effects of expected pain relief on the choice of analgesics. Eur J Pain 17, 587–598, doi: 10.1002/j.1532-2149.2012.00214.x (2013).
Lin, C. S., Wu, S. Y. & Wu, L. T. Preferences for Analgesic Treatments Are Influenced by Probability of the Occurrence of Adverse Effects and the Time to Reach Maximal Therapeutic Effects. PloS one 10, e0130214, doi: 10.1371/journal.pone.0130214 (2015).
Rushworth, M. F. & Behrens, T. E. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature neuroscience 11, 389–397, doi: 10.1038/nn2066 (2008).
Levin, I. P. et al. A neuropsychological approach to understanding risk-taking for potential gains and losses. Frontiers in neuroscience 6, 15, doi: 10.3389/fnins.2012.00015 (2012).
Knutson, B. & Huettel, S. A. The risk matrix. Current Opinion in Behavioral Sciences 5, 141–146 (2015).
Simmons, A., Strigo, I., Matthews, S. C., Paulus, M. P. & Stein, M. B. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological psychiatry 60, 402–409, doi: 10.1016/j.biopsych.2006.04.038 (2006).
Wiech, K. et al. Anterior insula integrates information about salience into perceptual decisions about pain. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 16324–16331, doi: 10.1523/JNEUROSCI.2087-10.2010 (2010).
Singer, T., Critchley, H. D. & Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends in cognitive sciences 13, 334–340, doi: 10.1016/j.tics.2009.05.001 (2009).
Knutson, B., Rick, S., Wimmer, G. E., Prelec, D. & Loewenstein, G. Neural predictors of purchases. Neuron 53, 147–156, doi: 10.1016/j.neuron.2006.11.010 (2007).
Kuhnen, C. M. & Knutson, B. The neural basis of financial risk taking. Neuron 47, 763–770, doi: 10.1016/j.neuron.2005.08.008 (2005).
Matthews, S. C., Simmons, A. N., Lane, S. D. & Paulus, M. P. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport 15, 2123–2127 (2004).
Robbins, T. W. & Everitt, B. J. Neurobehavioural mechanisms of reward and motivation. Current opinion in neurobiology 6, 228–236 (1996).
Leknes, S. & Tracey, I. A common neurobiology for pain and pleasure. Nature reviews. Neuroscience 9, 314–320, doi: 10.1038/nrn2333 (2008).
Mitsi, V. & Zachariou, V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience, doi: 10.1016/j.neuroscience.2016.05.017 (2016).
Baliki, M. N., Geha, P. Y., Fields, H. L. & Apkarian, A. V. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66, 149–160, doi: 10.1016/j.neuron.2010.03.002 (2010).
Cox, C. L. et al. Your resting brain CAREs about your risky behavior. PloS one 5, e12296, doi: 10.1371/journal.pone.0012296 (2010).
Li, N. et al. Resting-state functional connectivity predicts impulsivity in economic decision-making. The Journal of neuroscience: the official journal of the Society for Neuroscience 33, 4886–4895, doi: 10.1523/JNEUROSCI.1342-12.2013 (2013).
Zhou, Y. et al. The neural correlates of risk propensity in males and females using resting-state fMRI. Frontiers in behavioral neuroscience 8, 2, doi: 10.3389/fnbeh.2014.00002 (2014).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods 41, 1149–1160, doi: 10.3758/BRM.41.4.1149 (2009).
Leknes, S. et al. The importance of context: when relative relief renders pain pleasant. Pain 154, 402–410, doi: 10.1016/j.pain.2012.11.018 (2013).
Robinson, M. E. & Wise, E. A. Prior pain experience: influence on the observation of experimental pain in men and women. The journal of pain: official journal of the American Pain Society 5, 264–269, doi: 10.1016/j.jpain.2004.04.003 (2004).
Baron, J. Thinking and Deciding. 4th edn, (Cambridge University Press, 2007).
Chao-Gan, Y. & Yu-Feng, Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience 4, 13, doi: 10.3389/fnsys.2010.00013 (2010).
Song, X. W. et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one 6, e25031, doi: 10.1371/journal.pone.0025031 (2011).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage 44, 893–905, doi: 10.1016/j.neuroimage.2008.09.036 (2009).
Poldrack, R. A. Region of interest analysis for fMRI. Social cognitive and affective neuroscience 2, 67–70, doi: 10.1093/scan/nsm006 (2007).
MacKinnon, D. P., Fairchild, A. J. & Fritz, M. S. Mediation analysis. Annual review of psychology 58, 593–614, doi: 10.1146/annurev.psych.58.110405.085542 (2007).
Kahneman, D. & Tversky, A. Prospect theory: An analysis of decision under risk. Econometrica 47, 262–292 (1979).
Mohr, P. N., Biele, G. & Heekeren, H. R. Neural processing of risk. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 6613–6619, doi: 10.1523/JNEUROSCI.0003-10.2010 (2010).
Fairhurst, M., Fairhurst, K., Berna, C. & Tracey, I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PloS one 7, e48711, doi: 10.1371/journal.pone.0048711 (2012).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain structure & function 214, 655–667, doi: 10.1007/s00429-010-0262-0 (2010).
Berger, S. E. et al. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC research notes 7, 739, doi: 10.1186/1756-0500-7-739 (2014).
Kahn, I. & Shohamy, D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 23, 187–192, doi: 10.1002/hipo.22077 (2013).
Mushiake, H., Saito, N., Sakamoto, K., Itoyama, Y. & Tanji, J. Activity in the lateral prefrontal cortex reflects multiple steps of future events in action plans. Neuron 50, 631–641, doi: 10.1016/j.neuron.2006.03.045 (2006).
Kim, S. & Lee, D. Prefrontal cortex and impulsive decision making. Biological psychiatry 69, 1140–1146, doi: 10.1016/j.biopsych.2010.07.005 (2011).
Hertwig, R. & Erev, I. The description-experience gap in risky choice. Trends in cognitive sciences 13, 517–523, doi: 10.1016/j.tics.2009.09.004 (2009).
Ploner, M., Lee, M. C., Wiech, K., Bingel, U. & Tracey, I. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences of the United States of America 107, 355–360, doi: 10.1073/pnas.0906186106 (2010).
Paulus, M. P. & Stein, M. B. An insular view of anxiety. Biological psychiatry 60, 383–387, doi: 10.1016/j.biopsych.2006.03.042 (2006).
Brodersen, K. H. et al. Decoding the perception of pain from fMRI using multivariate pattern analysis. NeuroImage 63, 1162–1170, doi: 10.1016/j.neuroimage.2012.08.035 (2012).
Acknowledgements
C-S Lin was funded by Yen Tjing Ling Medical Foundation (CI-104-23) and Ministry of Science and Technology of Taiwan (103-2314-B-010-025-MY3). This work was supported in part by the 3 T MRI Core Facility in National Yang-Ming University.
Author information
Authors and Affiliations
Contributions
C.-S. Lin and S.-Y. Wu conceived and designed the study. C.-S. Lin collected the data. C.-S. Lin and H.-H. Lin analyzed the data. C.-S. Lin, H.-H. Lin and S.-Y. Wu wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, CS., Lin, HH. & Wu, SY. Functional and Structural Signatures of the Anterior Insula are associated with Risk-taking Tendency of Analgesic Decision-making. Sci Rep 6, 37816 (2016). https://doi.org/10.1038/srep37816
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37816
This article is cited by
-
Genetics of self-reported risk-taking behaviour, trans-ethnic consistency and relevance to brain gene expression
Translational Psychiatry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.