Abstract
A dye-sensitized solar cell (DSSC) fabricated by using a novel silyl-anchor coumarin dye with alkyl-chain substitutes, a Br3−/Br− redox electrolyte solution containing water, and a Mg2+-doped anatase-TiO2 electrode with twofold surface modification by MgO and Al2O3 exhibited an open-circuit photovoltage over 1.4 V, demonstrating the possibility of DSSCs as practical photovoltaic devices.
Similar content being viewed by others
Introduction
Dye-sensitized solar cells (DSSCs, Figs S1 and S2) have been investigated actively as practical photovoltaic cells of the next generation, because of their ease of fabrication, shorter energy and CO2 payback times, possibly flexible and colorful characteristics, and fine photovoltaic properties especially in low-light intensities and under scattered lights such as indoor conditions1,2,3. The light-to-electric energy conversion efficiency of DSSCs has been improved continuously by the development of the constituents of the cell1,2,3,4,5,6,7,8, and so far the efficiency has reached higher than 14%9. When considering the practical application of the DSSCs, the photovoltage is also an important photovoltaic property and the improvement of the photovoltage would extend the applicability of DSSCs9,10,11,12,13,14. The expectable photovoltage (Vexp) in the DSSC depends on the energy gap between the quasi-Fermi level (approximately the energy level of the conduction-band edge, EC.B.) of the metal-oxide electrode and the redox potential of the redox mediator in the electrolyte (Fig. S2), and therefore typical DSSCs made of the anatase-TiO2 electrode and the I3−/I− redox mediator exhibit photovoltage lower than 0.9 V1,2,3,4.
Recently, we succeeded in obtaining a high open-circuit photovoltage (Voc) of 1.21 V in the DSSC by fabricating the cell with using a Mg2+-doped anatase-TiO2 (Mg-doped TiO2) electrode with negatively shifted EC.B. than the anatase-TiO2 electrode, a coumarin dye of SFD-5 (Fig. 1a) possessing an alkoxysilyl group as an anchor moiety for a chemisorption to the TiO2 electrode, and an electrolyte with the Br3−/Br− redox mediator which has more positive redox potential than the ordinary I3−/I− redox mediator and Co3+/Co2+ complexes (Fig. S3)13. However, the expectable photovoltage of the cell was estimated to be ~1.5 V and there is still room for improvement, which will allow the usage of DSSCs as an alternative to the conventional dry cells and as a charging device for the rechargeable nickel-metal hydride batteries. An introduction of alkyl-chain substituents near to the silyl-anchor moiety in the coumarin dye is expected to improve the photovoltage by preventing the back electron transfer from the electrode to the redox electrolyte15,16,17,18. The development of surface modifications of the TiO2 electrode using wide bandgap metal oxides or insulators would also bring higher photovoltage by obstructing the back electron transfer19,20. In addition, the EC.B. of the Mg-doped TiO2 can be raised more by increasing the amount of Mg doped in TiO2 from the Mg/Ti atomic ratio of 0.10 to higher21. Thus, we newly designed and synthesized an alkoxysilyl coumarin dye of ADEKA-3 (Fig. 1b), and succeeded in obtaining the photovoltage higher than 1.4 V by preparing Mg-doped TiO2 with larger Mg composition, applying twofold metal-oxide surface modification to the Mg-doped TiO2 electrodes by MgO and Al2O3, and adding water to the electrolyte solution of Br3−/Br− redox mediator with using the advantage of the durability of the alkoxysilyl-dye adsorbed electrodes to water.
Results and Discussion
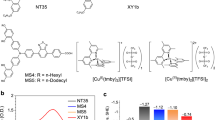
In ADEKA-3, the introduction of alkyl-chain substituents was performed by linking hexyl-thiophene rings to the coumarin moiety. A methyl group was also added to the coumarin moiety to prevent the co-planar arrangement of the coumarin moiety and the thiophene ring, which will produce a heightening of the HOMO level of the dye through the extension of the coumarin π system to the thiophene ring. The alkoxysilyl coumarin dye of ADEKA-3 exhibited similar UV-visible absorption spectra to SFD-5 in solutions, and a major absorption band of ADEKA-3 solution assignable to the π-π* transition was observed in visible region between 350 and 500 nm. The maximum molar absorption coefficient (εmax) at λmax was evaluated to be 48,700 at 415 nm (Fig. S4). The energy levels of HOMO and LUMO were estimated to be 1.18 V and −1.12 V vs. NHE, respectively, for ADEKA-3 (Table S1). The HOMO level is more positive than the redox potential of ~0.9 V vs. NHE of the Br3−/Br− redox10,13,22, thus providing thermodynamic driving force for the dye regeneration reaction by the electron transfer from the Br3−/Br− redox mediator to the oxidized dye formed through the light-excited-electron injection to the TiO2 electrode. The reliability of the relative positions of HOMO and LUMO levels were supported by the molecular orbital calculations for SFD-5 and ADEKA-3 (Figs S7 and S8).
The Mg-doped TiO2 crystalline nanoparticles with an increased Mg/Ti atomic ratio to 0.20 were synthesized by the solvothermal method13,21. As the reference to the Mg-doped TiO2, anatase-TiO2 nanoparticles without Mg-doping (undoped-TiO2) were also synthesized by the same method. The single phase of anatase structure was confirmed for the Mg-doped TiO2 crystalline nanoparticles by X-ray diffraction (XRD) experiments, and the particle size was estimated to be ~25 nm by using the Scherrer equation (Figs S9 and S10). The band gap of the Mg-doped TiO2 was evaluated to be 3.4 eV by a Tauc plot of the diffuse reflectance spectrum (Figs S11 and S12), which is 0.2 eV larger than that of the undoped-TiO2 consistently with the negative shift of the EC.B. by the Mg-doping21,23. Energy levels of the Mg-doped TiO2, ADEKA-3, and the Br3−/Br− redox mediator are drawn schematically in Fig. 2, which shows the suitability of ADEKA-3 as the sensitizing dye in the cell system with the Mg-doped TiO2 and the Br3−/Br− redox mediator.
The results of J-V measurements performed in this work are listed in Table 1 and shown in Fig. S13. The measurements were performed under AM-1.5G one sun illumination (100 mW cm−2). To check the performance of ADEKA-3 as a photosensitizer, J-V measurements were carried out for the cells sensitized by SFD-5 and ADEKA-3 as Entry 1 and 2, respectively, at 25 °C with using the TiO2 electrode without Mg-doping and a Br3−/Br− redox electrolyte solution (Electrolyte A: See Methods for the compositions of electrolytes used in this work.). The cell sensitized by ADEKA-3 exhibited 0.1 V higher Voc and smaller short-circuit photocurrent density (Jsc) than the cell sensitized by SFD-5, and light-to-electric energy conversion efficiencies (η) of these cells were almost the same. Since the dark current was smaller in the ADEKA-3-sensitized cell than the SFD-5-sensitized cell (Fig. S14), the increase of Voc in the ADEKA-3-sensitized cell is considered to be brought by the hexyl-chain substituents introduced in ADEKA-3, which are working as the suppressor for preventing the back electron transfer from the TiO2 electrode to the Br3−/Br− redox electrolyte by covering the naked surface of the TiO2 electrode between the adsorbed dye molecules15,16,17,18. In ADEKA-3, the HOMO level is higher in energy than that of SFD-5 by 0.21 eV, and thus the energy gap between the HOMO level and the redox potential of the Br3−/Br− redox mediator is smaller than that for SFD-5 (Fig. 2 and Table S1). The incident monochromatic photon-to-current conversion efficiencies (IPCE) were observed to tend to be lower in the ADEKA-3-sensitized cell than the SFD-5-sensitized cell (Fig. S15), and thus the smallness of the energy gap is considered as the reason for the lower Jsc value in the ADEKA-3-sensitized cell, which produced the delay of the dye regeneration reaction proceeding through the electron transfer from the redox mediator in the electrolyte solution to the dye in the oxidized state10,22.
Since ADEKA-3 was ascertained as an effective dye for producing high photovoltage, the Mg-doped TiO2 electrode was applied to the cell sensitized by the dye as Entry 3. The cell exhibited the Voc of 1.23 V, which is about 20% higher than that of the cell using the TiO2 electrode without Mg-doping. The increase of the Voc is considered to be due to the higher EC.B. of the Mg-doped TiO2 than that of the TiO2 without Mg-doping. For a further increment of the photovoltage in the cell, we examined surface modifications of the Mg-doped TiO2 electrode by wide bandgap metal oxides of MgO and by Al2O3 following the MgO modification (MgO + Al2O3) as Entries 4 and 5, respectively. The surface modification by MgO was confirmed to be effective also in the present cell system in improving the photovoltage, and the improvement is understood as the result of the negative shift of the EC.B. of the Mg-doped TiO2 by the MgO modification (Fig. S16)20. More efficient improvement was observed by the twofold surface modification with MgO and Al2O3. The Al2O3 modification was confirmed not to affect the EC.B. of the MgO-modified Mg-doped TiO2 by the UV-visible spectra (Fig. S16), and the modification is thought to form a blocking layer on the surface of the Mg-doped TiO2 electrode suppressing the back electron transfer from the electrode to the redox mediator in the electrolyte solution19,20.
In DSSCs, photovoltage is known to be increased by the addition of compounds having coordination ability to the surface of TiO2 electrodes, such as 4-tert-butylpyridine (TBP), to electrolyte solutions which shift the EC.B. negatively through the coordination. We examined the addition of 4-methylpyridine (MP) and 4-trimethylsilylpyridine (TMSP) to the Br3−/Br− redox electrolyte solution24, and prepared Electrolyte B with an experimentally optimized composition for high photovoltage. By using the electrolyte solution as Entry 6, the Voc was increased slightly and reached to 1.39 V. As an additive to the electrolyte solution for the improvement of the photovoltage, water is expected to be effective because of its high coordination ability owing to lone pairs on the oxygen atom and small molecular size25,26,27. However, the addition of water to electrolyte solutions causes the elimination of sensitizing dyes from the TiO2 electrodes generally in the case of conventional carboxy-anchor dyes, and the application of electrolyte solutions containing water has been rather limited28. On the other hand, alkoxysilyl dyes chemisorb the TiO2 electrodes by forming Si-O-Ti bonds through the condensation reaction between the alkoxysilyl groups and the hydroxy groups on the TiO2 surface, and the dye adsorbed electrodes have quite high durability to solvents, e.g. nitrile, water, and mixture of them7,29,30,31. Thus, we attempted to use a Br3−/Br− redox electrolyte solution containing water with the concentration of 0.10 M (Electrolyte C) as Entry 7. By the addition of water to the electrolyte, the Voc was improved actually to 1.45 V. The addition of water also brought about a decrement of the photocurrent to the cell, but the η was still to be ~4% (Tables 1 and S2, and Figs 3 and S13). And further, the Voc reached 1.50 V by lowering the cell temperature to 5 °C (Entry 8) as the result of a possible rise of the EC.B. and a deceleration of the back electron transfer reaction32. To the best of our knowledge, the observed Voc of 1.45 V at an ordinary temperature is the highest ever reported for DSSCs with a single-cell structure (Table S3)7,8,9,10,11,13,14,17,21,33,34.
Conclusions
We succeeded in producing the photovoltage over 1.4 V with a reasonably high conversion efficiency close to 4% in the DSSC by using the alkoxysilyl-anchor coumarin dye of ADEKA-3, the Mg-doped TiO2 electrode with the twofold surface modification by MgO and Al2O3, and the Br3−/Br− redox electrolyte solution containing water. The observed Voc is higher than those of other types of solar cells (Table S4), and is comparable to that of a conventional dry cell5. The achievement of such a high photovoltage, which is owing to the surpassing property of a silyl-anchor dye as a sensitizing dye for DSSCs, demonstrates the possibility of DSSCs as practical photovoltaic devices.
Methods
Device fabrication
Preparation procedures of the dye (SFD-5 or ADEKA-3)-adsorbed TiO2 electrodes used in the cells were described in Supplementary Information. When applying the MgO surface modification to the Mg-doped TiO2 electrodes, the electrodes before the dye adsorption were immersed into a 50 mM 2-propanol solution of Mg(OC2H5)2 at 25 °C for 1 h, rinsed in ethanol, and then calcined in air at 490 °C for 30 min20. In the twofold surface modification with MgO and Al2O3, the MgO-modified Mg-doped TiO2 electrodes were immersed into a 30 mM 2-propanol solution of Al[OCH(CH3)2]3 at 25 °C for 45 min, rinsed in ethanol, and then calcined in air at 490 °C for 1 h20. We used electrochemical cells of an open sandwich type through this work for photovoltaic measurements. A Pt-treated FTO-coated glass plate which was prepared by the rf magnetron sputtering of Pt and the reported H2PtCl6 treatment35 was employed as the counter electrode. Three Br3−/Br− redox electrolyte solutions were used as the electrolytes: Electrolyte A) 0.03 M Br2 + 0.65 M 1-n-butyl-3-methylimidazolium bromide (BMImBr) + 0.20 M tetra-n-pentylammonium bromide (TPABr) + 0.07 M 4-tert-butylpyridine (TBP) + 0.07 M guanidinium thiocyanate (GuSCN) in acetonitrile (AN)/valeronitrile (VN)/ethylene carbonate (EC)/tetrahydrofuran (THF) (4:3:2:1 in volume)13, Electrolyte B) 0.03 M Br2 + 0.65 M BMImBr + 0.20 M TPABr + 0.05 M TBP + 0.01 M 4-methylpyridine (MP) + 0.02 M 4-trimethylsilylpyridine (TMSP) + 0.07 M GuSCN in AN/VN/EC/THF (4:3:2:1 in volume), and Electrolyte C) 0.03 M Br2 + 0.65 M BMImBr + 0.20 M TPABr + 0.05 M TBP + 0.01 M MP + 0.02 M TMSP + 0.07 M GuSCN + 0.10 M H2O in AN/VN/EC/THF (4:3:2:1 in volume) (Fig. S17). The Mg-doped TiO2 or TiO2 electrode sensitized by SFD-5 or ADEKA-3, the counter electrode, and a polyethylene film spacer of 30 μm thick were assembled, and one of the Br3−/Br− redox electrolyte solutions was injected into the space between the electrodes (Fig. S18).
Photovoltaic measurements
The photovoltaic performances of the fabricated DSSCs were assessed from the IPCE spectra and the J-V properties of the cells with maintaining the aperture area of the cells to be 1.00 × 1.00 cm2 by the use of a square black shade mask. The IPCE spectra were obtained by using a monochromatic light source of SM-25 (Bunkoukeiki) and an electrometer of R8240 (Advantest) at 25 °C. The J-V properties were measured by using a solar simulator with Class AAA of OTENTO-SUN III (Bunkoukeiki) and a source meter of R6240A (Advantest) under the simulated sunlight irradiation of AM-1.5G one sun condition (100 mW cm−2) at 25 or 5 °C. The details were described in Supplementary Information.
Molecular orbital calculation
We optimized the molecular structures and calculated the energy levels of frontier orbitals and others for the alkoxysilyl-anchor coumarin dyes on the Gaussian 09 program package by using a density functional theory (DFT)36. The details were described in Supplementary Information.
Additional Information
How to cite this article: Kakiage, K. et al. Achievement of over 1.4 V photovoltage in a dye-sensitized solar cell by the application of a silyl-anchor coumarin dye. Sci. Rep. 6, 35888; doi: 10.1038/srep35888 (2016).
References
Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 42, 1788–1798 (2009).
Hagfeldt, A. et al. Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010).
Kalyanasundaram, K. Ed. Dye-Sensitized Solar Cells (EPFL Press, Lausanne, 2010).
Hardin, B. E., Snaith, H. J. & McGehee, M. D. The renaissance of dye-sensitized solar cells. Nat. Photonics 6, 162–169 (2012).
Green, M. A. et al. Solar cell efficiency tables (version 46). Prog. Photovolt: Res. Appl. 23, 805–812 (2015).
Mathew, S. et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242–247 (2014).
Kakiage, K. et al. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 50, 6379–6381 (2014).
Kakiage, K. et al. Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem. Commun. 51, 6315–6317 (2015).
Kakiage, K. et al. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 51, 15894–15897 (2015).
Teng, C. et al. Tuning the HOMO energy levels of organic dyes for dye-sensitized solar cells based on Br−/Br3− electrolytes. Chem. Eur. J 16, 13127–13138 (2010).
Feldt, S. M., Wang, G., Boschloo, G. & Hagfeldt, A. Effects of driving forces for recombination and regeneration on the photovoltaic performance of dye-sensitized solar cells using cobalt polypyridine redox couples. J. Phys. Chem. C 115, 21500–21507 (2011).
Wang, M., Grätzel, C., Zakeeruddin, S. M. & Grätzel, M. Recent developments in redox electrolytes for dye-sensitized solar cells. Energy Environ. Sci. 5, 9394–9405 (2012).
Kakiage, K. et al. Fabrication of a dye-sensitized solar cell containing a Mg-doped TiO2 electrode and a Br3−/Br− redox mediator with a high open-circuit photovoltage of 1.21 V. Chem. Commun. 49, 179–180 (2013).
Ishii, A. & Miyasaka, T. A metallocene molecular complex as visible-light absorber for high-voltage organic-inorganic hybrid photovoltaic cells. ChemPhysChem 15, 1028–1032 (2014).
Ellis, H. et al. Linker unit modification of triphenylamine-based organic dyes for efficient cobalt mediated dye-sensitized solar cells. J. Phys. Chem. C 117, 21029–21036 (2013).
Tian, H. & Sun, L. Iodine-free redox couples for dye-sensitized solar cells. J. Mater. Chem. 21, 10592–10601 (2011).
Feldt, S. M. et al. Regeneration and recombination kinetics in cobalt polypyridine based dye-sensitized solar cells, explained using Marcus theory. Phys. Chem. Chem. Phys. 15, 7087–7097 (2013).
Liang, M. & Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 42, 3453–3488 (2013).
Xia, J. & Yanagida, S. Strategy to improve the performance of dye-sensitized solar cells: Interface engineering principle. Solar Energy 85, 3143–3159 (2011).
Ozawa, H., Okuyama, Y. & Arakawa, H. Effective enhancement of the performance of black dye based dye-sensitized solar cells by metal oxide surface modification of the TiO2 photoelectrode. Dalton Trans. 41, 5137–5139 (2012).
Iwamoto, S. et al. Fabrication of dye-sensitized solar cells with an open-circuit photovoltage of 1 V. ChemSusChem 1, 401–403 (2008).
Wang, Z.-S., Sayama, K. & Sugihara, H. Efficient eosin Y dye-sensitized solar cell containing Br−/Br3− electrolyte. J. Phys. Chem. B 109, 22449–22455 (2005).
Zhang, C. et al. Charge recombination and band-edge shift in the dye-sensitized Mg2+-doped TiO2 solar cells. J. Phys. Chem. C 115, 16418–16424 (2011).
Kakiage, K. et al. Significant improvement of photovoltaic performance of dye-sensitized solar cells by using 4-trimethylsilylpyridine as organic additive to electrolyte solution. Chem. Lett. 41, 895–896 (2012).
Liu, Y., Hagfeldt, A., Xiao, X.-R. & Lindquist, S.-E. Investigation of influence of redox species on the interfacial energetics of a dye-sensitized nanoporous TiO2 solar cell. Sol. Energy Mater. Sol. Cells 55, 267–281 (1998).
Mikoshiba, S. et al. Ionic liquid type dye-sensitized solar cells: increases in photovoltaic performances by adding a small amount of water. Curr. Appl. Phys. 5, 152–158 (2005).
Manzhos, S., Segawa, H. & Yamashita, K. The effect of ligand substitution and water co-adsorption on the adsorption dynamics and energy level matching of amino-phenyl acid dyes on TiO2 . Phys. Chem. Chem. Phys. 14, 1749–1755 (2012).
Law, C., Moudam, O., Villarroya-Lidon, S. & O’Regan, B. J. Mater. Chem. 22, 23387–23394 (2012).
Unno, M. et al. Silanol dyes for solar cells: higher efficiency and significant durability. Appl. Organometal. Chem. 24, 247–250 (2010).
Kakiage, K. et al. High performance of Si-O-Ti bonds for anchoring sensitizing dyes on TiO2 electrodes in dye-sensitized solar cells evidenced by using alkoxysilylazobenzenes. Chem. Lett. 39, 260–262 (2010).
Kakiage, K. et al. Adsorption and sensitizing properties of azobenzenes having different numbers of silyl-anchor groups in dye-sensitized solar cells. Key Eng. Mater. 497, 61–66 (2012).
Usami, A. et al. Temperature dependence of open-circuit voltage in dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 93, 840–842 (2009).
Chen, P. et al. High open-circuit voltage solid-state dye-sensitized solar cells with organic dye. Nano Lett. 9, 2487–2492 (2009).
Yum, J.-H. et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 3, 631 (2012).
Ito, S. et al. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. Photovolt: Res. Appl. 15, 603–612 (2007).
Gaussian 09, Revision D.01, Frisch, M. J. et al. Gaussian, Inc., Wallingford CT (2009).
Acknowledgements
This work was partly supported by the “Element Innovation” Project by Ministry of Education, Culture, Sports, Science & Technology in Japan and by JSPS KAKENHI Grant Number 15H03848.
Author information
Authors and Affiliations
Contributions
K.K., H.O. and Y.A. carried out most of the experimental work and drafted most of the manuscript. T.Y. and K.O. analyzed the data. S.I. carried out the preparation of Mg-doped TiO2. J.F. analyzed the data and revised the manuscript. M.H. conceived the idea, analyzed the data, and revised the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kakiage, K., Osada, H., Aoyama, Y. et al. Achievement of over 1.4 V photovoltage in a dye-sensitized solar cell by the application of a silyl-anchor coumarin dye. Sci Rep 6, 35888 (2016). https://doi.org/10.1038/srep35888
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35888
This article is cited by
-
Role of rare-earth oxides, conjugated with \({\mathrm{TiO}}_{2}\), in the enhancement of power conversion efficiency of dye sensitized solar cells (DSSCs)
Environmental Science and Pollution Research (2023)
-
Hybrid metal complex with TiO2/SiO2 composite-doped polymer for the enhancement of photo energy conversion in silicon solar panels
Journal of Materials Science: Materials in Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.