Abstract
Thermodynamic calculation has been applied to predict the inclusion formation in molten SS400 steel. When the Cerium addition in liquid iron is 70 ppm and the initial Oxygen and Sulphur are both 110 ppm, the formation of oxides containing Cerium would experience the transformation from Ce2O3 to CeO2 and also the formation of sulfides containing Cerium would experience the transformation from CeS to Ce2S3 and then to Ce3S4. Below 2000 K the most thermodynamic stable matter is CeO2 and the less thermodynamic stable inclusion is CeS. Only when the amount of [O] is extremely low and the amount of [S] and [Ce] is relatively high, Ce2S3 has the possibility to form.
Similar content being viewed by others
Introduction
Rare earth (RE) metals have many applications1,2,3,4,5 and their addition to molten iron has attracted increasing research attention6. Such addition affects inclusion structures7 and can be used to purify steel8. The conjugation between oxygen and RE metals9 and between sulfur and RE metals10 is very strong. A lot of research11,12,13 has been done on the equilibrium relation between O, S, and RE metals. It has been found that extremely low oxygen and sulfur concentrations in steel can be achieved via the addition of an RE metal14. A lot of research15,16,17,18 has also been done on steel deoxidization and desulfurization via titanium and magnesium. RE metals can be used to deoxidize and desulfurize steel to control inclusion size and chemical composition. Few studies have performed thermodynamic calculations on the use of cerium to modify inclusions.
This paper focuses on the thermodynamic calculations of the cerium-oxygen-sulfur system in molten SS400 steel. The formation conditions of CeS, Ce2S3, Ce3S4, CeO2, and Ce2O3 in molten steel are examined using Wagner’s relation and Lupis’s relation based on the Gibbs free energy change. The transformation mechanism is analyzed by determining the thermodynamic conditions of Ce-desulfurized and Ce-deoxidized steel. The segregation of Ce2O3 in molten iron is also analyzed. In addition, a model for predicting the formation of various inclusions is established for SS400 steel with cerium addition.
Calculations
The thermodynamic calculations of the Ce-O-S system are based on Wagner’s relation19 and Lupis’ relation20. These calculations were implemented in C++. The segregation of Ce2O3 in molten SS400 steel, whose chemical composition is shown in Table 1, was calculated in Matlab 2015a.
The Ce-O-S system is the thermodynamic relation between the dissolved Oxygen, Sulphur and Cerium in liquid iron to explore the formations of inclusions containing Cerium. The first stage for thermodynamic calculation is to derive the thermodynamic equations for the inclusion formations by Wagner’s relation19 and Lupis’ relation20. Then the second stage is to use C++ programming software to derive the unknown chemical composition values for every equation.
Results and Discussion
For the addition of cerium into molten SS400 steel, the reactions of [O], [S], and [Ce] are of interest because Ce has strong affinity with S and O. As reported previously21, when w(RE)/(w[O] + w[S]) = 3.9, the function of cerium is optimal. To determine the separation sequence for various oxides and sulfides of cerium, the amount of cerium in the calculations was set as 1 mol to compare the Gibbs free energy of formation for various inclusions, which can be derived as:


where J denotes the reaction quotient (unitless), ∆G is the Gibbs free energy change of reaction (J/mol), ∆Gθ denotes the Gibbs free energy change of reaction for unmixed reactants and products at standard conditions (J/mol), R is the gas constant (J·mol−1·K−1), T is temperature (K), and K is the equilibrium constant (unitless).
The Gibbs free energy of oxides, sulfides and oxysulfides of cerium are shown in Table 2 14,21,22,23,24,25,26.
Below 2000 K, the most thermodynamically stable inclusion was CeO2, as shown in Fig. 1. Therefore, CeO2 likely formed in the molten iron when the temperature reached the simulated steelmaking temperature of 1873 K. In Fig. 1, it could be read that the least thermodynamic stable inclusion is CeS and the thermodynamic stale sequence of the possible inclusion formed in liquid steel is CeO2 > Ce2O3 > Ce2O2S > Ce2S3 > Ce3S4 > CeS. However, the most thermodynamically stable matter does not guarantee the formation of CeO2, because the formation of oxides containing cerium are controlled not only by the equilibrium constant but also by the concentrations of cerium and oxygen in the molten iron. That is to say, the formation of CeO2 at 1873 K is also determined by the solubility product of CeO2 and the concentration of cerium and oxygen, even though the Gibbs Free Energy of CeO2 is the lowest at 1873 K.
The activities and activity coefficient of Ce, O and S can be written as Eqs (9) and (10) from Wagner’s relation7 and Lupis’ relation20 as follow,


where fi is the Henrian activity coefficient of component i relative to the dilute solution and  is the first-order interaction parameter of i on j in molten iron; w[i] and w[ j] are the mass percentages of elements i and j, respectively (Table 3); αi is the activity of element i.
is the first-order interaction parameter of i on j in molten iron; w[i] and w[ j] are the mass percentages of elements i and j, respectively (Table 3); αi is the activity of element i.
 of cerium, oxygen, and sulfur at 1873 K30.
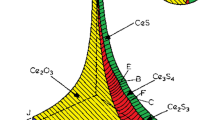
of cerium, oxygen, and sulfur at 1873 K30.By using data22,23 from Tables 2 and 3, the following curves for Ce-S and Ce-O in Fig. 2 can be calculated. The key to derive every line in Fig. 2 is the relation of equilibrium constant, Gibbs free energy and the amount of the chemical compositions for every possible inclusion according to Wagner’s relation19 and Lupis’ relation20. When the equilibrium constant is linked to the amount of the chemical compositions for every possible inclusion, equations for Fig. 2 can be obtained. When the weight percentage of cerium, oxygen and sulphur are known in the molten iron at 1873 K, the main inclusion formed would be found in Fig. 2. As shown in Fig. 2, if the cerium addition in liquid iron is 70 ppm and the initial oxygen and sulphur are both 110 ppm, the formation of oxides containing cerium would experience the transformation from Ce2O3 to CeO2 and also the formation of sulfides containing cerium would experience the transformation from CeS to Ce2S3 and then to Ce3S4. From Fig. 2, when the temperature of molten iron reached 1873 K, Ce3S4 is the main product, as the amount of cerium in molten iron is high and the amount of sulphur is relatively lower compared to the formation of CeS and Ce2S3.
In order to investigate the formation conditions of Ce2O3, Ce2S3 and Ce2O2S, the doubly saturated curve with Ce2O3/Ce2O2S and Ce2S3/Ce2O2S are calculated, using the thermodynamic data derived in Tables 2 and Equation 1, 2.
In molten iron, it is assumed that  and
and 
 . Based on the reaction Ce2O2S + [O] = Ce2O3 + [S], it is found that [%S] = 10[%O] when Ce2O2S and Ce2O3 coexist. When Ce2O2S and Ce2S3 coexist in molten iron, it is derived that [%S] = 100[%O], based on the thermodynamic calculation of the reaction Ce2S3 + 2[O] = Ce2O2S + 2[S]. Figure 3 was derived from the above calculations. In Fig. 3, it can be concluded that Ce2O3 and Ce2O2S can exist in molten iron in a wide amount range of [Ce], [O] and [S]. More importantly, only when the amount of [O] is extremely low and the amount of [S] and [Ce] is relatively high, Ce2S3 has the possibility to form.
. Based on the reaction Ce2O2S + [O] = Ce2O3 + [S], it is found that [%S] = 10[%O] when Ce2O2S and Ce2O3 coexist. When Ce2O2S and Ce2S3 coexist in molten iron, it is derived that [%S] = 100[%O], based on the thermodynamic calculation of the reaction Ce2S3 + 2[O] = Ce2O2S + 2[S]. Figure 3 was derived from the above calculations. In Fig. 3, it can be concluded that Ce2O3 and Ce2O2S can exist in molten iron in a wide amount range of [Ce], [O] and [S]. More importantly, only when the amount of [O] is extremely low and the amount of [S] and [Ce] is relatively high, Ce2S3 has the possibility to form.
Cerium is a perfect deoxidizer and desulfurizer for steel purification. Compared with other elements, for example Aluminum, Titanium, Magnesium and Calcium27,28, which can also deoxidize and desulfurize, cerium can formed a complex compound Ce2O2S which contains Oxygen and Sulphur together. The formation possibility of Ce2O2S has been verified by Hu’s research29 when they studied the effect of Ce addition on the C-Mn steel microstructure. It is reproted by Wang26 that Ce2O3 is easier to form in molten iron when the iron molten temperature is 1873 K. However, the thermodynamic conditions were changed when the temperature decreases from 1873 K to solidus temperature. On the other hand, when the temperature of molten iron decreases to that at which solid steel starts to form, the cerium and oxygen in the molten iron begin to segregate. Their amounts are respectively:


where W(Ce) and W(O) are the percentage amounts of cerium and oxygen of molten iron during the molten iron solidification, respectively;  and
and  are the initial percentage amounts of cerium and oxygen in the liquid phase, respectively; kCe (=0.005) and kO (=0.022) are the solvent partition coefficients at equilibrium for cerium and oxygen, respectively; fs is the solid fraction.
are the initial percentage amounts of cerium and oxygen in the liquid phase, respectively; kCe (=0.005) and kO (=0.022) are the solvent partition coefficients at equilibrium for cerium and oxygen, respectively; fs is the solid fraction.
The solidus temperature of SS400 is 1777 K. The solubility product of the Ce2O3 formed in molten iron can be expressed as:

The solubility product of the Ce2O3 formed in molten iron at equilibrium can be expressed as:

From Eqs (11) to (14), the solubility products versus solidification ratio (fs) are plotted in Fig. 4. In Fig. 4(a), where the simulated oxygen concentration in liquid steel is 10 ppm and the cerium concentration varies from 0.1% to 0.5%, the solubility products versus solidification ratio (fs) are plotted with the varying cerium concentration (shown in the colorful lines of Fig. 4(a)) and the equilibrium constant of Ce2O3 (KCe2O3) versus solidification ratio fs is curved as the black solid line in Fig. 4(a). It is read in Fig. 4(a) that the colorful lines are all in the above of the black solid line, which means Ce2O3 prefers to segregate in liquid phase with the 10 ppm Oxygen concentration in liquid iron. Moreover, the same conclusion can be drawn from the similar Fig. 4(b–d) with 50 ppm, 100 pmm, 200 ppm oxygen concentration in liquid iron. The inset red diagrams in Fig. 4(a–d) are the detailed solid black curves appeared in Fig. 4(a–d). Figure 4 shows that when the oxygen concentration in molten iron was increased from 10 to 200 ppm and the cerium concentration was in the range of 0.1% to 0.5%, Ce2O3 preferred to segregate in the liquid phase.
Conclusion
By the addition of cerium in molten SS400 steel, when the temperature of molten iron reached 1873 K, at the same time that the Cerium addition in liquid iron is 70 ppm and the initial Oxygen and Sulphur are both 110 ppm, the formation of oxides containing Cerium would experience the transformation from Ce2O3 to CeO2 and also the formation of sulfides containing Cerium would experience the transformation from CeS to Ce2S3 and then to Ce3S4. Below 2000 K the most thermodynamic stable matter CeO2 and the least thermodynamic stable inclusion is CeS. And the thermodynamic stable sequence of the possible inclusions formed in liquid steel is CeO2 > Ce2O3 > Ce2O2S > Ce2S3 > Ce3S4 > CeS. Only when the amount of [O] is extremely low and the amount of [S] and [Ce] is relatively high, Ce2S3 has the possibility to form. With the amount of oxygen in molten iron increasing from 10 ppm to 200 ppm and the amount range of cerium increasing from 0.1% to 0.5%, Ce2O3 prefers to segregate in liquid phase all the time.
Additional Information
How to cite this article: Pan, F. et al. Thermodynamic Calculation among Cerium, Oxygen, and Sulfur in Liquid Iron. Sci. Rep. 6, 35843; doi: 10.1038/srep35843 (2016).
References
Su, Y. & Lai, Y. Performance enhancement of natural pigments on a high light transmission ZrO2 nanoparticle layer in a water‐based dye‐sensitized solar cell. Int. J. Energ. Res. 38, 436–443 (2014).
Kung, P. et al. Down-conversion photoluminescence sensitizing plasmonic silver nanoparticles on ZnO nanorods to generate hydrogen by water splitting photochemistry. Appl. Phys. Lett. 106, 023114 (2015).
Lin, K. & Su, Y. Photoluminescence of Cu: ZnS, Ag: ZnS, and Au: ZnS nanoparticles applied in Bio-LED. Appl. Phys. B: Lasers Opt. 113, 351–359 (2013).
Lai, Y., Su, Y. & Lin, M. Photochemical water splitting performance of Fluorescein, Rhodamine B, and Chlorophyll-Cu supported on ZrO2 nanoparticles layer anode. Dyes Pigm. 103, 76–81 (2014).
Su, Y. et al. Ellipsometric advances for local surface plasmon resonance to determine chitosan adsorption on layer-by-layer gold nanoparticles. Appl. Spectrosc. 61, 1007–1014 (2007).
Fruehan, R. J. The effect of zirconium, cerium, and lanthanum on the solubility of oxygen in liquid iron. Metall. Mater. Trans. B 5, 345−347 (1974).
Wilson, W. G., Kay, D. & Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. J. O. M. 26, 14−23 (1975).
Fischer, W. A. & Bertram, H. The deoxidation, desulphurization and nitrogen removal of iron melts containing oxygen, sulfur or nitrogen using the rare earth metals cerium and lanthanum. Arch. Eisenhuttenwes. 44, 87−95 (1973).
Han, Q. et al. Equilibria between cerium or neodymium and oxygen in molten iron. Metall. Mater. Trans. B 21, 295−302 (1990).
Langenberg, F. C. & Chipman, J. Equilibrium between cerium and sulfur in liquid iron. Trans. Met. Soc. AIME 212, 290−293 (1958).
Pan, F. et al. Effects of rare earth metals on steel microstructures. Materials 9, 417 (2016).
Arunachalam, V. & Ramachandran, S. Rare earths in steel technology. Sci. Technol. Rare Earth Mater. 415–434 (1980).
Wu, Y., Wang, L. & Du, T. Thermodynamics of rare earth elements in liquid iron. J. Less Common Metals 110, 187–193 (1985).
Li, W. C. Thermodynamics of the formation of rare earth inclusions in steel. Iron and Steel 21, 7−12 (1986).
Byun, J., Shim, J., Cho, Y. & Lee, D. Non-metallic inclusion and intragranular nucleation of ferrite in Ti-killed C–Mn steel. Acta Metall. 51, 1593–1606 (2003).
Yang, J., Yamasaki, T. & Kuwabara, M. Behavior of inclusions in deoxidation process of molten steel with in situ produced Mg vapor. ISIJ Int. 47, 699–708 (2007).
Seo, C. et al. Modification and minimization of spinel (Al2O3·xMgO) inclusions formed in Ti-added steel melts. Metall. Mater. Trans. B 41, 790–797 (2010).
Su, Y. et al. Photoelectric characteristics of natural pigments self-assembly fabricated on TiO2/FTO substrate. J. Nanosci. Nanotechnol. 9, 960–964 (2009).
Wagner, C. Thermodynamics of alloys (Massachusetts: Addison-Wesley Press). p. 22 (1952).
Lupis, C. H. Chemical thermodynamics of materials (Amsterdam: North-Holland). p. 31 (1983).
Liu, Y. H., Lin, Q. & Ye, W. Behavior of rare earths in ultralowsulfur microalloyed steel. J. Rare Earths 17, 207−212 (1999).
Wang, L. M. et al. Thermodynamics and application of rare earth elements in steel. J. Chin. RE Soc. 21, 251−254 (2003).
Chen, J. X. Handbook of common datas and graph in steelmaking (Beijing: Metallurgical Industry Press). p. 757−761 (2010).
Katsumata, A. & Todoroki, H. Effect of rare earth metal on inclusion composition in molten stainless steel. Iron Steelmaker 29, 51−57 (2002).
Vahed, A. & Kayd, A. R. Thermodynamics of rare earths in steelmaking. Metall. Mater. Trans. B 7, 375−383 (1976).
Wang, G. C. et al. Experimental and thermodynamic study on formation of inclusions containing cerium in HP295 steel. J. Chin. RE Soc. 31, 161–168 (2013).
Ono, H. et al. Formation conditions of Mg2TiO4 and MgAl2O4 in Ti-Mg-Al complex deoxidation of molten iron. ISIJ Int. 49, 957–964 (2009).
Tomita, Y. Effect of desulphurization and calcium treatments on the inclusion morphology of 0.4 C-Cr-Mo-Ni steel. J. Mater. Sci. 29, 2873–2878 (1994).
Hu, Z. Y. et al. Effect of Ce addition on inclusion and microstructure in C-Mn steel. Metal. Int. 17, 11–17 (2012).
Wang, L. M., Du, T. & Wu, Y. M. Thermodynamic study on formation RES and RE2S3 between rare earth elements and sulphur in iron-based solution. J. Chin. RE Soc. 6, 11 (1987).
Acknowledgements
Our work is sponsored by China Steel Company, National Science Council (MOST104-2622-8-006-001) and Research Center for Energy Technology and Strategy (D105-23008) National Cheng Kung University in Taiwan. Thanks Dr. Ho-Lin Tsai, James Augusto, Shuo-Yen Fang and Guan-Ping Qi for their kind help.
Author information
Authors and Affiliations
Contributions
This paper was proposed by W.-S.H., F.P. and J.Z. contributed to this article equally. This manuscript was written by F.P. The thermodynamic calculations were carried out by F.P. and J.Z. H.-L.C. and Y.-H.S. contributed to data analysis. Y.-H.S. gave us a lot of suggestions to promote our research. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pan, F., Zhang, J., Chen, HL. et al. Thermodynamic Calculation among Cerium, Oxygen, and Sulfur in Liquid Iron. Sci Rep 6, 35843 (2016). https://doi.org/10.1038/srep35843
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35843
This article is cited by
-
As-Cast Grain Refinement of H13 Steel by Cerium Sulfide Formed During Solidification
Metallurgical and Materials Transactions B (2024)
-
Capillary Interaction Between Micron-Sized Ce2O3 Inclusions at the Ar Gas/Liquid Steel Interface
Metallurgical and Materials Transactions B (2022)
-
Role of Cerium on Transformation Kinetics and Mechanical Properties of Low Carbon Steels
Metallurgical and Materials Transactions A (2021)
-
Study of Bauschinger effect of acicular ferrite and polygonal ferrite through ex-situ interrupted bending tests in API X80 linepipe steels
Scientific Reports (2018)
-
Nucleation and Ostwald Growth of Particles in Fe-O-Al-Ca Melt
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.