Abstract
Ever since the discovery of high-Tc superconductivity in layered cuprates, the roles that individual layers play have been debated, due to difficulty in layer-by-layer characterization. While there is similar challenge in many Fe-based layered superconductors, the newly-discovered Ca10(Pt4As8)(Fe2As2)5 provides opportunities to explore superconductivity layer by layer, because it contains both superconducting building blocks (Fe2As2 layers) and intermediate Pt4As8 layers. Cleaving a single crystal under ultra-high vacuum results in multiple terminations: an ordered Pt4As8 layer, two reconstructed Ca layers on the top of a Pt4As8 layer, and disordered Ca layer on the top of Fe2As2 layer. The electronic properties of individual layers are studied using scanning tunneling microscopy/spectroscopy (STM/S), which reveals different spectra for each surface. Remarkably superconducting coherence peaks are seen only on the ordered Ca/Pt4As8 layer. Our results indicate that an ordered structure with proper charge balance is required in order to preserve superconductivity.
Similar content being viewed by others
Introduction
Since the discovery of Fe-based superconductors in 2008, tremendous effort has been expended to understand the origin of their physical properties, that exhibit strong coupling between structure, magnetism, and superconductivity. Similar to high-temperature (Tc) cuprate superconductors, Fe pnictide superconductors form a layered structure with Fe2As2 building blocks. Depending on the spacers that separate these building blocks, these superconductors are often categorized as “111” (e.g. LiFeAs)1, “1111” (e.g. LaFeAsO and CaFeAsF)2,3, and “122” (e.g. BaFe2As2)4 families etc. Surprisingly, these compounds exhibit only small anisotropy in their physical properties, in spite of their layered structure5,6,7,8,9. This suggests strong Fe2As2 interlayer coupling. Cleaving a single crystal of such a system usually results in the Fe2As2 layer covered by atoms from the spacer, thus difficult to study the role a spacer plays using surface sensitive techniques. Recently, a new Fe-based superconducting system Ca10PtnAs8(Fe2As2)5 with n = 3 (Ca10-3-8) and n = 4 (Ca10-4-8) has been reported10,11,12. As shown in Fig. 1a there is additional PtnAs8 layer sandwiched in between two Ca layers within adjacent Fe2As2 building blocks: Ca10-3-8 is triclinic, while three different structures—tetragonal, triclinic, and monoclinic—have been reported for the Ca10-4-8 compound10,11,12,13. According to first-principles calculations11,14,15 and an angle-resolved photoemission spectroscopy study16, these PtnAs8 layers are conducting, in contrast to insulating spacers in 111, 1111, 122 families, and more complex compounds such as Sr3Sc2O5Fe2As217 and Sr4V2O6Fe2As218. Thus, this new Ca10PtnAs8(Fe2As2)5 system offers an excellent platform to explore the role of interlayer spacers in interlayer superconductivity.
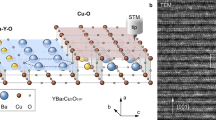
Structure of the Ca10-4-8.
(a) Schematic of the Ca10-4-8 bulk structure with the relative spacings between the different planes. Preferred cleavage plane is marked with a blue arrow. (b) HAADF-STEM image taken along [210] direction shows detailed structure of Ca10Pt4As8(Fe2−xPtxAs2)5: one of Pt4As8 layers and the corresponding plane in (a) are marked with a red arrow. Intensity profile measured from the yellow rectangle provides the configuration of atomic planes stacked along c direction: alternating Pt doped Fe2As2 and Pt4As8 layers with Ca ions in between them. Double stack of the structure in Fig. 1a is shown in the image. (c) In-plane resistivity (ρab) shows a clear superconducting transition with onset Tc = 34 K and zero resistivity Tc = 31 K. Out-of-plane resistivity (ρc) has slightly broad superconducting transition with a peak near 38 K. Bulk-truncated Pt4As8 layer (d) and Fe2−xPtxAs2 layer (e). The unit cell of each surface is marked with green (Pt1- ) and red (1 × 1) squares.
) and red (1 × 1) squares.
In this paper, we report experimental investigations of superconducting Ca10Pt4As8(Fe2−xPtxAs2)5 single crystals layer-by-layer using scanning tunneling microscopy and spectroscopy (STM/S). The layer-by-layer probing of these layered superconductors is extremely important as illustrated in the recent study of Bi2Sr2CaCu2O8+δ, where it was found that “the well-known pseudogap feature observed by STM is inherently property of the BiO planes and thus irrelevant directly to Cooper pairing”19. By creating a fresh surface through single-crystal cleavage under ultra-high vacuum, one can study both structural (STM topography) and electronic properties (scanning tunneling spectroscopy (STS)) of the exposed surfaces. By comparing STS spectra taken on different layers, we address how the surface structure and Ca concentration affect superconductivity on the surfaces of Ca10-4-8.
Results
For the Ca10Pt4As8(Fe2−xPtxAs2)5 single crystals we used for this study, an atomically-defined layered structure is clearly imaged by high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) taken along [210] direction (Fig. 1b). Similar to previous report12, our crystals form a tetragonal structure with a lattice parameter a = 8.733 Å, and interlayer distances indicated in Fig. 1a. The intensity of HAADF-STEM images strongly depends on the averaged atomic number (Z) in the projected atomic columns. As shown in Fig. 1b, the higher intensity of Fe (Z = 26) column than that of As (Z = 33) column indicates that there exists some Pt in Fe2−xPtxAs2 layer, i.e. x ≠ 0. Noticeable Pt doping in Fe2−xPtxAs2 layers was reported in previous studies10,12, which is responsible for various reported Tc values of the material. For our study, Ca10Pt4As8(Fe2−xPtxAs2)5 single crystals exhibit a superconducting transition at Tc = 34 K, as shown in both the in-plane and out-of-plane resistivity in Fig. 1c.
Fresh surfaces were created by cleaving single crystals under ultrahigh vacuum (~10−10 torr) at ~90–100 K. Since Ca atoms are weakly bounded to adjacent layers but closer to the Fe2−xPtxAs2 layer than the Pt4As8 layer (see Fig. 1a), naively two surface terminations should be expected with roughly the same probability: (1) a full Ca layer on the top of Fe2−xPtxAs2 and (2) a bare Pt4As8 layer. Bulk-truncated Pt4As8 layer and Fe2−xPtxAs2 layer with their relative surface unit cells are shown in Fig. 1d,e, respectively. The Fe2−xPtxAs2 surface has a (1 × 1) structure with  . Since the x value is yet to be determined, showing Fig. 1e is the structure of Fe2As2 without indicating the location of Pt. (marked with a red “square” (□) represents a (1 × 1) structure in Fig. 1e). The Pt4As8 surface has a different (1 × 1) structure with
. Since the x value is yet to be determined, showing Fig. 1e is the structure of Fe2As2 without indicating the location of Pt. (marked with a red “square” (□) represents a (1 × 1) structure in Fig. 1e). The Pt4As8 surface has a different (1 × 1) structure with  (marked with a green “square” (□)), which has the same size as the primitive unit cell for bulk-truncation (Fig. 1a). This green “square” crystal unit cell for Pt4As8 is commensurate with the surface (1 × 1) Fe2−xPtxAs2 lattice. To facilitate consistent notation every structure will be denoted relative to the (1 × 1) Fe2−xPtxAs2 surface unit cell: the bulk-truncated (1 × 1) Pt4As8 structure (Fig. 1d) will be denoted as the Pt1-
(marked with a green “square” (□)), which has the same size as the primitive unit cell for bulk-truncation (Fig. 1a). This green “square” crystal unit cell for Pt4As8 is commensurate with the surface (1 × 1) Fe2−xPtxAs2 lattice. To facilitate consistent notation every structure will be denoted relative to the (1 × 1) Fe2−xPtxAs2 surface unit cell: the bulk-truncated (1 × 1) Pt4As8 structure (Fig. 1d) will be denoted as the Pt1- structure. All observed structures are summarized in Table 1, which will be presented and discussed later.
structure. All observed structures are summarized in Table 1, which will be presented and discussed later.
Figure 2a shows the STM image of cleaved surfaces, displaying large flat terraces. Figure 2b shows the line profile recorded along the marked location in Fig. 2a, where the step height is ~10 Å, which corresponds to a reported unit cell height11,12. In addition to these large steps, there are regions that show much smaller steps (~1 Å). These are marked with arrows in Fig. 2a,b. The magnified image of such an area is shown in Fig. 2c. Notably, it consists of two terraces (A and B) with a height difference of only ~1 Å (see Fig. 2d). The observed surfaces can be identified using structural information displayed in Fig. 1a. Figure 3a shows the topography of region A marked in Fig. 2c. Note it has a “square” unit cell with a lattice size ~8.7 Å with some local electronic inhomogeneity (Supplementary Fig. S1). This is identical to the lattice size of the bulk-truncated (1 × 1) Pt4As8 structure (Fig. 1d, “Pt1- ”). Thus, we conclude that the region A is the Pt4As8 layer without reconstruction nor the presence of Ca. As shown in Fig. 1a,d, there are four Pt sites in the crystal unit cell: Pt1 is above the plane formed by Pt2 and Pt3, and Pt4 is below the plane. STM should observe primarily the Pt1 atoms, forming the “square” unit cell with the edge length of ~8.7 Å, as illustrated in Fig. 1d (green square) and labeled Pt1-
”). Thus, we conclude that the region A is the Pt4As8 layer without reconstruction nor the presence of Ca. As shown in Fig. 1a,d, there are four Pt sites in the crystal unit cell: Pt1 is above the plane formed by Pt2 and Pt3, and Pt4 is below the plane. STM should observe primarily the Pt1 atoms, forming the “square” unit cell with the edge length of ~8.7 Å, as illustrated in Fig. 1d (green square) and labeled Pt1- .
.
Pt1- surface and Ca2-
surface and Ca2- surface.
surface.
(a,b) STM image (taken at 4.3 K) of the stepped Pt4As8 surfaces (Vsample = 0.7 V, I = 2.3 pA) and a line profile showing steps with a reported unit cell height11,12. Ca covered areas are marked with arrows. One of such Ca covered area is shown in (c): STM image (taken at 4.3 K) showing both Pt1- surface (the region A) and Ca2-
surface (the region A) and Ca2- surface (the region B) (Vsample = 0.2 V, I = 2 pA). (d) Line profile shows Pt1-
surface (the region B) (Vsample = 0.2 V, I = 2 pA). (d) Line profile shows Pt1- layer with a small step height (~1 Å) to the Ca2-
layer with a small step height (~1 Å) to the Ca2- surface.
surface.
Observed surfaces and their relative structures.
STM image of (a) Pt1- surface (Vsample = 0.7 V, I = 2.4 pA), (b) Ca2-
surface (Vsample = 0.7 V, I = 2.4 pA), (b) Ca2- surface (Vsample = 0.2 V, I = 2 pA), and (c) disordered Ca covered Fe2−xPtxAs2 layer (Vsample = 0.2 V, I = 2.4 pA). All images are taken at 4.3 K. (insets) corresponding Fourier transform patterns. (d) Schematic shows relative relations of each surface structure. The green solid square represents
surface (Vsample = 0.2 V, I = 2 pA), and (c) disordered Ca covered Fe2−xPtxAs2 layer (Vsample = 0.2 V, I = 2.4 pA). All images are taken at 4.3 K. (insets) corresponding Fourier transform patterns. (d) Schematic shows relative relations of each surface structure. The green solid square represents  unit cell of the Pt1-
unit cell of the Pt1- surface (as also shown in Fig. 1d), the purple solid square represents (1 × 1) structure of Ca layer with a full-monolayer (ML) coverage, and the red dashed square is
surface (as also shown in Fig. 1d), the purple solid square represents (1 × 1) structure of Ca layer with a full-monolayer (ML) coverage, and the red dashed square is  unit cell of the Ca2-
unit cell of the Ca2- surface. Pt1 (Pt4) is located at
surface. Pt1 (Pt4) is located at  above (below) the Pt in-plane (Pt2, Pt3) at
above (below) the Pt in-plane (Pt2, Pt3) at  . Ca2 atoms forming
. Ca2 atoms forming  unit cell are located at
unit cell are located at  , which is ~0.15 Å lower than other Ca atoms (Ca1 at
, which is ~0.15 Å lower than other Ca atoms (Ca1 at  ).
).
Since the step height between regions A (Pt4As8) and B is ~1 Å, the region B can only be a Ca covered area on the top of Pt4As8. According to Fig. 1a, there are five Ca atoms on one Pt1- unit cell: four (Ca1) are located at z/c = 0.2418 and one (Ca2) at z/c = 0.2294 (where z/c = 0 is the plane formed by Pt2 and Pt3)12. As illustrated in Fig. 3d, the weakly bounded Ca layer with a full-monolayer (ML) coverage should have a (1 × 1) structure with the lattice parameter of 3.9 Å (where 1 ML is defined as the amount of Ca atoms: four Ca1 and one Ca2 per primitive unit cell), the same structure as the bulk-truncated (1 × 1) Fe2−xPtxAs2 (Fig. 1e). However, the region B (see Fig. 3b) shows a different structure than the one with a full ML coverage. The Fourier transform pattern in the inset of Fig. 3b shows the same spots as observed in Pt1-
unit cell: four (Ca1) are located at z/c = 0.2418 and one (Ca2) at z/c = 0.2294 (where z/c = 0 is the plane formed by Pt2 and Pt3)12. As illustrated in Fig. 3d, the weakly bounded Ca layer with a full-monolayer (ML) coverage should have a (1 × 1) structure with the lattice parameter of 3.9 Å (where 1 ML is defined as the amount of Ca atoms: four Ca1 and one Ca2 per primitive unit cell), the same structure as the bulk-truncated (1 × 1) Fe2−xPtxAs2 (Fig. 1e). However, the region B (see Fig. 3b) shows a different structure than the one with a full ML coverage. The Fourier transform pattern in the inset of Fig. 3b shows the same spots as observed in Pt1- (see the inset of Fig. 3a): Ca2 atoms form a “square” unit cell with the lattice size of ~8.7 Å, which is commensurate with the Pt1-
(see the inset of Fig. 3a): Ca2 atoms form a “square” unit cell with the lattice size of ~8.7 Å, which is commensurate with the Pt1- underneath. While we expect to observe a fully Ca covered Fe2−xPtxAs2 layer and a bare Pt4As8 layer, the existence of Ca2-
underneath. While we expect to observe a fully Ca covered Fe2−xPtxAs2 layer and a bare Pt4As8 layer, the existence of Ca2- can be traced back to the Ca distribution along the c axis. Ca2 is ~0.15 Å closer to the Pt4As8 surface compared to Ca1, and is located on the top of Pt4 (Figs 1a and 3d). When the crystal is cleaved, Ca2 atoms remain on the Pt4As8 surface while Ca1 atoms are peeled off. The resultant Ca2-
can be traced back to the Ca distribution along the c axis. Ca2 is ~0.15 Å closer to the Pt4As8 surface compared to Ca1, and is located on the top of Pt4 (Figs 1a and 3d). When the crystal is cleaved, Ca2 atoms remain on the Pt4As8 surface while Ca1 atoms are peeled off. The resultant Ca2- unit cell is marked in Fig. 3d with the red dashed square. By counting atoms shown in Fig. 3b, the Ca coverage is estimated to be ~0.17 ML, which is close to 0.2 ML, the amount of Ca2 to form a perfect
unit cell is marked in Fig. 3d with the red dashed square. By counting atoms shown in Fig. 3b, the Ca coverage is estimated to be ~0.17 ML, which is close to 0.2 ML, the amount of Ca2 to form a perfect  structure (Ca1:Ca2 = 4:1). However, the additional weak and broad spots marked with arrows in the Fourier transform patterns (see the inset of Fig. 3b) indicate that there is additional short-range order formed by Ca atoms.
structure (Ca1:Ca2 = 4:1). However, the additional weak and broad spots marked with arrows in the Fourier transform patterns (see the inset of Fig. 3b) indicate that there is additional short-range order formed by Ca atoms.
In addition to the bare Pt1- (region A) and Ca2-
(region A) and Ca2- (regions B) surfaces, we observe another surface. As shown in Fig. 3c, this surface does not form any ordered structure. As will be discussed later, this is identified as a Ca covered Fe2−xPtxAs2 layer (“Ca-disordered”).
(regions B) surfaces, we observe another surface. As shown in Fig. 3c, this surface does not form any ordered structure. As will be discussed later, this is identified as a Ca covered Fe2−xPtxAs2 layer (“Ca-disordered”).
Returning to the Ca2- surface, we now illustrate that it is metastable. In the scanned area of ~9 μm2 from 3 different as-cleaved samples, Pt1-
surface, we now illustrate that it is metastable. In the scanned area of ~9 μm2 from 3 different as-cleaved samples, Pt1- surface (86%) and Ca2-
surface (86%) and Ca2- layer (14%) on top of Pt1-
layer (14%) on top of Pt1- surface are observed. In some Ca2-
surface are observed. In some Ca2- regions, Fourier transform patterns show stronger additional spots (marked with arrows in the inset of Fig. 3b) with weaker
regions, Fourier transform patterns show stronger additional spots (marked with arrows in the inset of Fig. 3b) with weaker  spots, indicating the Ca2-
spots, indicating the Ca2- surface is not a ground state. Figure 4a shows the Ca layer after the sample is warmed from 4.3 K to room temperature (~290 K) for an hour, revealing a new structure with a
surface is not a ground state. Figure 4a shows the Ca layer after the sample is warmed from 4.3 K to room temperature (~290 K) for an hour, revealing a new structure with a  unit cell (“Ca2-
unit cell (“Ca2- ”) displayed in Fig. 4a,b. Ca2-
”) displayed in Fig. 4a,b. Ca2- is the (
is the ( )R45° reconstruction of the original Ca2-
)R45° reconstruction of the original Ca2- unit cell, suggesting the Ca surface undergoes a structure change during the warming process. Since the Ca coverage for the perfect Ca2-
unit cell, suggesting the Ca surface undergoes a structure change during the warming process. Since the Ca coverage for the perfect Ca2- structure is 0.2 ML, there are excess Ca atoms (0.1 ML) after forming Ca2-
structure is 0.2 ML, there are excess Ca atoms (0.1 ML) after forming Ca2- structure. These excessive Ca atoms form clusters on the surface as shown in Fig. 4a. With an additional hour-long annealing at room temperature, all surface Ca atoms are clustered (“Ca-clustered”), revealing the Pt1-
structure. These excessive Ca atoms form clusters on the surface as shown in Fig. 4a. With an additional hour-long annealing at room temperature, all surface Ca atoms are clustered (“Ca-clustered”), revealing the Pt1- surface underneath (Fig. 4c and the region C in Fig. 4d): the square lattice of Pt1-
surface underneath (Fig. 4c and the region C in Fig. 4d): the square lattice of Pt1- surface can be seen in between clusters (one of such area is marked with a yellow circle in Fig. 4c). Thus, the Ca-clustered surface is stable and the Ca2-
surface can be seen in between clusters (one of such area is marked with a yellow circle in Fig. 4c). Thus, the Ca-clustered surface is stable and the Ca2- and Ca2-
and Ca2- surfaces are metastable.
surfaces are metastable.
Annealing effect on Ca2- and disordered Ca surfaces.
and disordered Ca surfaces.
(a,b) STM image (taken at 4.3 K) of Ca2- surface after warmed from 4.3 K to room temperature (~290 K) and schematic of the structure (Vsample = 100 mV, I = 2 pA). (inset) Fourier transform pattern. STM image of Ca2-
surface after warmed from 4.3 K to room temperature (~290 K) and schematic of the structure (Vsample = 100 mV, I = 2 pA). (inset) Fourier transform pattern. STM image of Ca2- surface taken at 80 K is shown in Supplementary Fig. S3: there is no significant difference between images taken at 4.3 K and 80 K. (c) Additional hour long annealing at room temperature causes surface Ca atoms become clustered, revealing underneath Pt1-
surface taken at 80 K is shown in Supplementary Fig. S3: there is no significant difference between images taken at 4.3 K and 80 K. (c) Additional hour long annealing at room temperature causes surface Ca atoms become clustered, revealing underneath Pt1- surface (taken at 80 K, Vsample = 1 V, and I = 100 pA). One of such revealed Pt1-
surface (taken at 80 K, Vsample = 1 V, and I = 100 pA). One of such revealed Pt1- surfaces is marked with a yellow circle. (d) STM image showing two different layers (taken at 80 K, Vsample = 1 V, and I = 100 pA): Pt1-
surfaces is marked with a yellow circle. (d) STM image showing two different layers (taken at 80 K, Vsample = 1 V, and I = 100 pA): Pt1- with Ca clusters (the region C) and disordered Ca surface on Fe2−xPtxAs2 layer (the region D). (e) Distance between Pt4As8 layer and Ca covered Fe2−xPtxAs2 layer is shown in the line profile.
with Ca clusters (the region C) and disordered Ca surface on Fe2−xPtxAs2 layer (the region D). (e) Distance between Pt4As8 layer and Ca covered Fe2−xPtxAs2 layer is shown in the line profile.
As for the Ca-disordered Fe2−xPtxAs2 surface (Fig. 3c), it remains disordered after annealing (Fig. 4d). According to the line profile shown in Fig. 4e, the step height between regions C and D is ~3 Å. Since the region C is the Pt4As8 layer, the region D must be the Ca layer on the top of Fe2−xPtxAs2 layer. We also observed the same disordered surface located about 3.5 Å lower than the Ca2- surface (Supplementary Fig. S2), after initial warming up from 4.3 K to 290 K. The morphological feature is also the same as that shown in Fig. 3c, which is obtained from the as-cleaved Ca/Fe2−xPtxAs2 surface. For comparison, the surface of CaFe2As2 has a (1 × 2) structure formed by 0.5 ML Ca on the top of Fe2As2 layer20. For Ca10Pt4As8(Fe2−xPtxAs2)5, the absence of ordered Ca/Fe2−xPtxAs2 surface is undoubtedly due to the asymmetric interlayer spacing: the Ca layer is closer to the Fe2−xPtxAs2 layer than to the Pt4As8 layer. When the sample is cleaved, most of Ca atoms stay on the top of Fe2−xPtxAs2 layer, which are disordered as shown in Fig. 3c (as-cleaved) and 4d (after annealing). In contrast to the Ca/Pt4As8 surface, the disordered Ca/Fe2−xPtxAs2 surface is insensitive to annealing. Only the surface corrugation changes from ~1 Å (as-cleaved) to ~2 Å (after annealing). The large amount of disordered Ca atoms prevents from seeing the bare Fe2−xPtxAs2 layer.
surface (Supplementary Fig. S2), after initial warming up from 4.3 K to 290 K. The morphological feature is also the same as that shown in Fig. 3c, which is obtained from the as-cleaved Ca/Fe2−xPtxAs2 surface. For comparison, the surface of CaFe2As2 has a (1 × 2) structure formed by 0.5 ML Ca on the top of Fe2As2 layer20. For Ca10Pt4As8(Fe2−xPtxAs2)5, the absence of ordered Ca/Fe2−xPtxAs2 surface is undoubtedly due to the asymmetric interlayer spacing: the Ca layer is closer to the Fe2−xPtxAs2 layer than to the Pt4As8 layer. When the sample is cleaved, most of Ca atoms stay on the top of Fe2−xPtxAs2 layer, which are disordered as shown in Fig. 3c (as-cleaved) and 4d (after annealing). In contrast to the Ca/Pt4As8 surface, the disordered Ca/Fe2−xPtxAs2 surface is insensitive to annealing. Only the surface corrugation changes from ~1 Å (as-cleaved) to ~2 Å (after annealing). The large amount of disordered Ca atoms prevents from seeing the bare Fe2−xPtxAs2 layer.
Cleaving a Ca10Pt4As8(Fe2−xPtxAs2)5 single crystal results in three surfaces and two additional surfaces via annealing as summarized in Table 1. In the Ca2- surface, the step height (~1 Å) observed in Fig. 2d is ~0.7 Å shorter than the corresponding bulk spacing between Ca2 and Pt1 (1.67 Å). This STM result is consistent with the behavior reported at the surface of CaFe2As2 with 0.5 ML Ca coverage20, where Ca atoms are pulled down by ~0.5 Å (~30%) determined by low energy electron diffraction (LEED). In addition, the calculated charge states using the Bader scheme in ref. 15 [
surface, the step height (~1 Å) observed in Fig. 2d is ~0.7 Å shorter than the corresponding bulk spacing between Ca2 and Pt1 (1.67 Å). This STM result is consistent with the behavior reported at the surface of CaFe2As2 with 0.5 ML Ca coverage20, where Ca atoms are pulled down by ~0.5 Å (~30%) determined by low energy electron diffraction (LEED). In addition, the calculated charge states using the Bader scheme in ref. 15 [ ,
,  , and
, and  ] can be utilized to support the large inward relaxation. In bulk, positively charged Ca atoms are positioned in between two negatively charged layers, Fe2−xPtxAs2 and Pt4As8. When the negatively charged Fe2−xPtxAs2 layer is removed by cleaving there would be too many positive charged Ca atoms unless some are removed with the Fe2−xPtxAs2 layer. If the remaining Ca is indeed above Pt4 atom, this specific Ca atom (Ca2) has more room to be pulled down than other four Ca atoms (Ca1) since Pt4 is located at 0.72 Å below the plane formed by Pt2 and Pt3 at z/c = 0. In the Ca-disordered surface, the remaining Ca atoms do not form any ordered structure as shown in Figs 3c and 4d. This may be the reason that the observed spacing (~3 Å) between Pt4As8 layer and Ca covered Fe2−xPtxAs2 layer (Fig. 4e) is close to the spacing in the bulk (3.25 Å).
] can be utilized to support the large inward relaxation. In bulk, positively charged Ca atoms are positioned in between two negatively charged layers, Fe2−xPtxAs2 and Pt4As8. When the negatively charged Fe2−xPtxAs2 layer is removed by cleaving there would be too many positive charged Ca atoms unless some are removed with the Fe2−xPtxAs2 layer. If the remaining Ca is indeed above Pt4 atom, this specific Ca atom (Ca2) has more room to be pulled down than other four Ca atoms (Ca1) since Pt4 is located at 0.72 Å below the plane formed by Pt2 and Pt3 at z/c = 0. In the Ca-disordered surface, the remaining Ca atoms do not form any ordered structure as shown in Figs 3c and 4d. This may be the reason that the observed spacing (~3 Å) between Pt4As8 layer and Ca covered Fe2−xPtxAs2 layer (Fig. 4e) is close to the spacing in the bulk (3.25 Å).
The most significant observation on the cleaved Ca10-4-8 surfaces comes from the STS measurements. From the bulk measurements, Tc of Ca10-4-8 is ~34 K (see Fig. 1c). Thus, STS measurements at a much lower temperature (4.3 K) than Tc are expected to show the opening of superconducting energy gap. This is indeed observed on the ordered Ca surfaces on Pt4As8. Figure 5a,b show spectra taken on the Ca2- and Ca2-
and Ca2- surfaces with corresponding STM images (insets). The annealed surface (Ca2-
surfaces with corresponding STM images (insets). The annealed surface (Ca2- ) shows enhanced superconducting coherence peaks (Fig. 5b) when compared to the spectrum taken on the as-cleaved surface (Ca2-
) shows enhanced superconducting coherence peaks (Fig. 5b) when compared to the spectrum taken on the as-cleaved surface (Ca2- ) (Fig. 5a). Note that the spectra shown in Fig. 5 are raw data (no normalization) so information about absolute conductance cannot be obtained. However, from the coherence peak positions in Fig. 5b, one can estimate the energy gap Δ ~4.4 meV at T = 4.3 K. This results in that
) (Fig. 5a). Note that the spectra shown in Fig. 5 are raw data (no normalization) so information about absolute conductance cannot be obtained. However, from the coherence peak positions in Fig. 5b, one can estimate the energy gap Δ ~4.4 meV at T = 4.3 K. This results in that  . According to the BCS theory,
. According to the BCS theory,  for weakly coupled superconductors. The smaller
for weakly coupled superconductors. The smaller  may result from underestimated Δ due to finite temperature and/or overestimated Tc at the surface. It is worth pointing out that the spectrum (Fig. 5b) exhibits a finite zero bias conductance (ZBC). Such a feature has been observed previously in other Fe-based superconductors20,21,22,23,24,25,26, even when probed at much lower temperatures than their Tc, indicating the ZBC is not due to the thermal broadening effect. It was also shown that spectral features can vary on different reconstructed surfaces in BaFe2−xCoxAs226 suggesting reconstructions may be closely related to the observed ZBC in Fe-based superconductors. The origin of the ZBC, however, is not clear at the moment and requires further investigation.
may result from underestimated Δ due to finite temperature and/or overestimated Tc at the surface. It is worth pointing out that the spectrum (Fig. 5b) exhibits a finite zero bias conductance (ZBC). Such a feature has been observed previously in other Fe-based superconductors20,21,22,23,24,25,26, even when probed at much lower temperatures than their Tc, indicating the ZBC is not due to the thermal broadening effect. It was also shown that spectral features can vary on different reconstructed surfaces in BaFe2−xCoxAs226 suggesting reconstructions may be closely related to the observed ZBC in Fe-based superconductors. The origin of the ZBC, however, is not clear at the moment and requires further investigation.
STM images of four observed surfaces (inset) and spectra corresponding to each surface taken at 4.3 K: (a) Ca2- , (b) Ca2-
, (b) Ca2- , (c) Pt1-
, (c) Pt1- , and (d) Ca-disordered.
, and (d) Ca-disordered.
Only on the ordered Ca surfaces, superconducting features are observed. Note that spectra are raw data and the tip was stabilized at the sample bias Vsample = 20 mV and the tunneling set current (a) 15 pA, (b) 20 pA, (c) 40 pA, and (d) 25 pA, respectively.
The STS data for the Pt1- surface shows a kink with positive bias but smooth dI/dV with negative bias (Fig. 5c). In addition, the detailed feature in each spectrum varies significantly depending on the site where STS is taken (Fig. S4). Likewise, the STS spectra taken on the Ca-disordered (on the Fe2−xPtxAs2) surface lack any features that could be associated with superconductivity (Fig. 5d). These spectra are strongly site dependent as well.
surface shows a kink with positive bias but smooth dI/dV with negative bias (Fig. 5c). In addition, the detailed feature in each spectrum varies significantly depending on the site where STS is taken (Fig. S4). Likewise, the STS spectra taken on the Ca-disordered (on the Fe2−xPtxAs2) surface lack any features that could be associated with superconductivity (Fig. 5d). These spectra are strongly site dependent as well.
Discussion
What preserves superconductivity on the Ca-ordered Pt4As8 surfaces (Ca2- and Ca2-
and Ca2- ) but diminishes superconductivity on the bare Pt4As8 surface (Pt1-
) but diminishes superconductivity on the bare Pt4As8 surface (Pt1- ) and Ca-disordered Fe2−xPtxAs2 surface? It is well known that the Fe2As2 layer is responsible for superconductivity in bulk Fe-based superconductors, and the Pt4As8 layer is expected to be superconducting as well, given the nature of 3D superconductivity (Fig. 1c). We recall that superconducting coherence peaks have been observed in spectra taken on the surfaces of other Fe-based superconductors20,23,27, in which the surfaces are ordered. For example, the cleaved surface of Ca(Fe0.925Co0.075)2As2 has an ordered stripe phase formed by a half monolayer Ca on the Fe2As2 layer20. Moreover, superconducting features are enhanced in the Ca2-
) and Ca-disordered Fe2−xPtxAs2 surface? It is well known that the Fe2As2 layer is responsible for superconductivity in bulk Fe-based superconductors, and the Pt4As8 layer is expected to be superconducting as well, given the nature of 3D superconductivity (Fig. 1c). We recall that superconducting coherence peaks have been observed in spectra taken on the surfaces of other Fe-based superconductors20,23,27, in which the surfaces are ordered. For example, the cleaved surface of Ca(Fe0.925Co0.075)2As2 has an ordered stripe phase formed by a half monolayer Ca on the Fe2As2 layer20. Moreover, superconducting features are enhanced in the Ca2- (Fig. 5b) with a better ordered surface than the Ca2-
(Fig. 5b) with a better ordered surface than the Ca2- , as seen in the Fourier transform pattern of the Ca2-
, as seen in the Fourier transform pattern of the Ca2- , which is much sharper than the one of Ca2-
, which is much sharper than the one of Ca2- (see insets of Figs 3b and 4a). This suggests that an ordered surface favors superconductivity in this system. Thus, the absence of superconducting coherence peak in our STS spectra obtained from Ca-disordered Fe2−xPtxAs2 is due to the disorder of Ca atoms. However, this cannot explain why there is no coherence peaks seen in the bare Pt4As8 layer, where we observed an ordered structure (Fig. 5c). Here we propose that the lack of superconductivity on a bare Pt4As8 layer is a result of charge imbalance. In comparison with Ca2-
(see insets of Figs 3b and 4a). This suggests that an ordered surface favors superconductivity in this system. Thus, the absence of superconducting coherence peak in our STS spectra obtained from Ca-disordered Fe2−xPtxAs2 is due to the disorder of Ca atoms. However, this cannot explain why there is no coherence peaks seen in the bare Pt4As8 layer, where we observed an ordered structure (Fig. 5c). Here we propose that the lack of superconductivity on a bare Pt4As8 layer is a result of charge imbalance. In comparison with Ca2- and Ca2-
and Ca2- surfaces, the bare Pt4As8 layer has no Ca atoms, which must be responsible for the absence of superconducting coherence peaks. In the bulk, the charge transfer from Ca layer to Pt4As8 layer is much greater than the charge transfer from Ca to Fe2−xPtxAs2 layer15. The lack of Ca makes the Pt1-
surfaces, the bare Pt4As8 layer has no Ca atoms, which must be responsible for the absence of superconducting coherence peaks. In the bulk, the charge transfer from Ca layer to Pt4As8 layer is much greater than the charge transfer from Ca to Fe2−xPtxAs2 layer15. The lack of Ca makes the Pt1- surface not superconducting which is much different than the bulk. Compared to the bare Pt1-
surface not superconducting which is much different than the bulk. Compared to the bare Pt1- surface, the Ca2-
surface, the Ca2- and Ca2-
and Ca2- surfaces have much less severe charge imbalance, thus preserving superconductivity. The effect of charge imbalance is also evidenced at the surface of Sr1−xKxFe2As227, where no sign of superconducting coherence peak is observed on the ordered bare Fe2As2 layer. Moreover, it was shown that superconductivity can be tuned by charge transfer from gated ionic liquid to FeSe28.
surfaces have much less severe charge imbalance, thus preserving superconductivity. The effect of charge imbalance is also evidenced at the surface of Sr1−xKxFe2As227, where no sign of superconducting coherence peak is observed on the ordered bare Fe2As2 layer. Moreover, it was shown that superconductivity can be tuned by charge transfer from gated ionic liquid to FeSe28.
In view of the previous studies of Fe-based superconductors including 11, 111, 122 systems, cleaving creates two identical surfaces, due to structure symmetry. A material like Ca10Pt4As8(Fe2−xPtxAs2)5 is fundamentally different offering great opportunities to study the structural and physical properties layer by layer, and provide important implication on the fabrication of new Fe-based superconductors. We have demonstrated that the electronic properties of the surface depend dramatically upon the stoichiometry and the arrangement of surface atoms. Cleaving and processing of the single crystal sample produce five distinct surface phases: a bare Pt4As8 surface (Pt1- ), an ordered 0.2 ML Ca array on Pt4As8 surface (Ca2-
), an ordered 0.2 ML Ca array on Pt4As8 surface (Ca2- ), an ordered 0.1 ML Ca on Pt4As8 (Ca2-
), an ordered 0.1 ML Ca on Pt4As8 (Ca2- ), a Ca-clustered Pt4As8 layer, and a Ca-disordered layer on Fe2−xPtxAs2.
), a Ca-clustered Pt4As8 layer, and a Ca-disordered layer on Fe2−xPtxAs2.
The fact that STS reveals no sign for superconductivity on the bare Pt4As8 surface and the disordered Ca on Fe2−xPtxAs2 indicates that both structure order and charge balance are crucial for superconductivity. We believe the unequal distance of Ca atoms with respect to both Fe2−xPtxAs2 layer and Pt4As8 layer is the reason for the existence of these strange surface structures. When the surface contains ingredients sufficient to maintain charge balance, properties seen in bulk can be preserved on the surface. This is supported by our observation of superconducting coherent peaks in STS on the Ca2- and Ca2-
and Ca2- surfaces of Ca10Pt4As8(Fe2−xPtxAs2)5.
surfaces of Ca10Pt4As8(Fe2−xPtxAs2)5.
While three-dimensional superconductivity in layered superconductors is considered through tunneling between layers, our STM/STS study of Ca10Pt4As8(Fe2−xPtxAs2)5 reveals that proximity effect would not maintain the superconductivity on the surface if the surface experiences different chemical environment. As can be seen in Fig. 4e, both bare Pt4As8 layer and the disordered Ca layer are just about 3–4 Å from the Fe2−xPtxAs2 layer. The absence of superconductivity in these layers indicates that the top most Fe2−xPtxAs2 layer is not superconducting, even though the adjacent Fe2−xPtxAs2 layer is only ~8 Å below (Fig. 1a).
In summary, we have investigated the detailed surface structures and electronic properties of Ca10Pt4As8(Fe2−xPtxAs2)5 using STM/STS. We observe five different surfaces as summarized in Table 1. Remarkably, STS measurements reveal that only spectra taken on the Ca surfaces with ordered structures and appropriate amount show superconducting coherence peaks with finite zero bias conductance. Neither bare Pt4As8 surface nor disordered Ca/Fe2−xPtxAs2 exhibit superconductivity. Our results indicate that superconductivity can only be preserved on the surface when it has similar chemical arrangement as the bulk counterpart: either missing or disordered Ca would kill superconductivity in the surface planes, due to charge imbalance. Furthermore, our results confirm that the intermediate Pt4As8 layer is superconducting in Ca10Pt4As8(Fe2−xPtxAs2)5, direct evidence for bulk superconductivity.
Methods
Single crystal growth
High quality single crystals of Ca10-4-8 were grown using the self-flux method. The stoichiometric amounts of high purity calcium shot (99.999% Alfa Aesar), Pt powder (99.95% Alfa Aesar), iron powder (99.95% Alfa Aesar), and arsenic powder (99.999% Alfa Aesar) are mixed in the ratio 10:4:10:18. The mixture is placed in an alumina crucible and sealed in a quartz tube under vacuum. The whole assembly is heated in a box furnace to 700 °C at a rate of 150 °C/h and is held at this temperature for 5 h. It is further heated to 1100 °C at a rate of 80 °C/h where it is held for 50 h, and then cooled to 1050 °C at a rate of 1.25 °C/h. It is further cooled to 500 °C at a rate of 5.5 °C/h and finally cooled down to room temperature by turning off the power. Shiny black plate-like single crystals are obtained without requiring any additional process. These crystals have a typical size of 6 × 6 × 0.2 mm3.
STM/S measurements
Ca10-4-8 single crystals are first cleaved at low temperatures (~90–100 K) then transferred to the scanning stage either at 80 K or 4.3 K. 1010 V/A (current amplifier gain) is used at 4.3 K and 109 V/A at 80 K so that different tunneling set currents are used at 4.3 K (2–40 pA) and 80 K (100 pA). No noticeable difference is observed in the surface topology taken at 80 K and 4.3 K. dI/dV spectra are acquired by using a lock-in amplifier with Vmod = 80 μV and fmod = 555 Hz at 4.3 K. WSxM software was among the tools used for image preparation and analysis29.
STEM measurements
STEM imaging is performed using a JEM-ARM200F microscope equipped with probe-corrector operating at 200 kV. Samples for TEM observation along different axis-zone direction are prepared by using Focused Ion Beam (FIB).
Additional Information
How to cite this article: Kim, J. et al. Interrogating the superconductor Ca10(Pt4As8)(Fe2−xPtxAs2)5 Layer-by-layer. Sci. Rep. 6, 35365; doi: 10.1038/srep35365 (2016).
References
Tapp, J. H. et al. LiFeAs: An intrinsic FeAs-based superconductor with Tc = 18 K. Phys. Rev. B 78, 060505 (2008).
Kamihara, Y., Watanabe, T., Hirano, M. & Hosono, H. Iron-based layered superconductor La[O1−xFx]FeAs (x = 0.05–0.12) with Tc = 26 K. J. Am. Chem. Soc. 130, 3296–3297 (2008).
Matsuishi, S. et al. Superconductivity induced by Co-doping in quaternary fluoroarsenide CaFeAsF. J. Am. Chem. Soc. 130, 14428–14429 (2008).
Rotter, M., Tegel, M. & Johrendt, D. Superconductivity at 38 K in the iron arsenide (Ba1−xKx)Fe2As2 . Phys. Rev. Lett. 101, 107006 (2008).
Altarawneh, M. M. et al. Determination of anisotropic Hc2 up to 60 T in Ba0.55K0.45Fe2As2 single crystals. Phys. Rev. B 78, 220505 (2008).
Yamamoto, A. et al. Small anisotropy, weak thermal fluctuations, and high field superconductivity in Co-doped iron pnictide Ba(Fe1−xCox)2As2 . Appl. Phys. Lett. 94, 062511 (2009).
Yuan, H. Q. et al. Nearly isotropic superconductivity in (Ba,K)Fe2As2 . Nature 457, 565–568 (2009).
Cho, K. et al. Anisotropic upper critical field and possible Fulde-Ferrel-Larkin-Ovchinnikov state in the stoichiometric pnictide superconductor LiFeAs. Phys. Rev. B 83, 060502 (2011).
Jo, Y. J. et al. High-field phase-diagram of Fe arsenide superconductors. Physica C 469, 566–574 (2009).
Kakiya, S. et al. Superconductivity at 38 K in iron-based compound with platinum-arsenide layers Ca10(Pt4As8)(Fe2−xPtxAs2)5 . J. Phys. Soc. Jpn. 80, 093704 (2011).
Löhnert, C. et al. Superconductivity up to 35 K in the iron platinum arsenides (CaFe1−xPtxAs)10Pt4−yAs8 with layered structures. Angew. Chem. Int. Ed. 50, 9195–9199 (2011).
Ni, N., Allred, J. M., Chan, B. C. & Cava R. J. High Tc electron doped Ca10(Pt3As8)(Fe2As2)5 and Ca10(Pt4As8)(Fe2As2)5 superconductors with skutterudite intermediary layers. Proc. Natl. Acad. Sci. USA 108, E1019–E1026 (2011).
Stürzer, T., Derondeau, G. & Johrendt, D. Role of different negatively charged layers in Ca10(FeAs)10(Pt4As8) and superconductivity at 30 K in electron-doped (Ca0.8La0.2)10(FeAs)10(Pt3As8). Phys. Rev. B 86, 060516 (2012).
Nakamura, H. & Machida, M. First-principles study of Ca–Fe–Pt–As-type iron-based superconductors. Physica C 484, 39–42 (2013).
Shein, I. R. & Ivanovskii, A. L. Ab initio study of the nature of the chemical bond and electronic structure of the layered phase Ca10(Pt4As8)(Fe2As2)5 as a parent system in the search for new superconducting iron-containing materials. Theor. Exp. Chem. 47, 292–295 (2011).
Shen, X. P. et al. Electronic structure of Ca10(Pt4As8)(Fe2−xPtxAs2)5 with metallic Pt4As8 layers: An angle-resolved photoemission spectroscopy study. Phys. Rev. B 88, 115124 (2013).
Zhu, X. et al. Sr3Sc2Fe2As2O5 as a possible parent compound for FeAs-based superconductors. Phys. Rev. B 79, 024516 (2009).
Zhu, X. et al. Transition of stoichiometric Sr2VO3FeAs to a superconducting state at 37.2 K. Phys. Rev. B 79, 220512 (2009).
Lv, Y.-F. et al. Mapping the electronic structure of each ingredient oxide layer of high-Tc cuprate superconductor Bi2Sr2CaCu2O8+δ . Phys. Rev. Lett. 115, 237002 (2015).
Li, G. et al. Role of antiferromagnetic ordering in the (1 × 2) surface reconstruction of Ca(Fe1−xCox)2As2 . Phys. Rev. Lett. 112, 077205 (2014).
Teague, M. L. et al. Measurement of a sign-changing two-gap superconducting phase in electron-doped Ba(Fe1−xCox)2As2 single crystals using scanning tunneling spectroscopy. Phys. Rev. Lett. 106, 087004 (2011).
Millo, O. et al. Scanning tunneling spectroscopy of SmFeAsO0.85: Possible evidence for d-wave order-parameter symmetry. Phys. Rev. B 78, 092505 (2008).
Yin, Y. et al. Scanning tunneling spectroscopy and vortex imaging in the iron pnictide superconductor BaFe1.8Co0.2As2 . Phys. Rev. Lett. 102, 097002 (2009).
Massee, F. et al. Nanoscale superconducting-gap variations and lack of phase separation in optimally doped BaFe1.86Co0.14As2 . Phys. Rev. B 79, 220517 (2009).
Massee, F. et al. Pseudogap-less high-Tc superconductivity in BaCoxFe2−xAs2 . EPL (Europhysics Letters) 92, 57012 (2010).
Zhang, H. et al. Sqrt[2] × sqrt[2] structure and charge inhomogeneity at the surface of superconducting BaFe2−xCoxAs2 (x = 0–0.32). Phys. Rev. B 81, 104520 (2010).
Song, C.-L. et al. Dopant clustering, electronic inhomogeneity, and vortex pinning in iron-based superconductors. Phys. Rev. B 87, 214519 (2013).
Lei, B. et al. Evolution of high-temperature superconductivity from a low-Tc phase tuned by carrier concentration in FeSe thin flakes. Phys. Rev. Lett. 116, 077002 (2016).
Horcas, I. et al. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007).
Acknowledgements
This work was supported by NSF-DMR 1504226 (J.K., G.L., A.K., R.J. and E.W.P.), ONR-N00014-14-1-0330 (H.N. and C.K.S.), DOE DE-SC0002136 (Z.W. and J.Z.). The STEM measurement was conducted in Brookhaven National Laboratory which was supported by the U.S. Department of Energy, Office of Basic Energy Science, under Contract No. DE-AC02-98CH10886 (Y.Z.).
Author information
Authors and Affiliations
Contributions
R.J. and E.W.P. proposed and designed the research. A.B.K. and R.J. contributed to single crystal growth. J.K., H.N. and G.L. carried out the STM/STS experiment with the assistance from C.K.S.; Z.W. and Y.Z. carried out the STEM experiment. J.K, J.Z., R.J. and E.W.P. analyzed the data. J.K. wrote the paper with J.Z., R.J. and E.W.P.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kim, J., Nam, H., Li, G. et al. Interrogating the superconductor Ca10(Pt4As8)(Fe2−xPtxAs2)5 Layer-by-layer. Sci Rep 6, 35365 (2016). https://doi.org/10.1038/srep35365
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35365
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.