Abstract
The resistance of fall armyworm (FAW), Spodoptera frugiperda, has been characterized to some Cry and Vip3A proteins of Bacillus thuringiensis (Bt) expressed in transgenic maize in Brazil. Here we evaluated the effective dominance of resistance based on the survival of neonates from selected Bt-resistant, heterozygous, and susceptible (Sus) strains of FAW on different Bt maize and cotton varieties. High survival of strains resistant to the Cry1F (HX-R), Cry1A.105/Cry2Ab (VT-R) and Cry1A.105/Cry2Ab/Cry1F (PW-R) proteins was detected on Herculex, YieldGard VT PRO and PowerCore maize. Our Vip3A-resistant strain (Vip-R) exhibited high survival on Herculex, Agrisure Viptera and Agrisure Viptera 3 maize. However, the heterozygous from HX-R × Sus, VT-R × Sus, PW-R × Sus and Vip-R × Sus had complete mortality on YieldGard VT PRO, PowerCore, Agrisure Viptera, and Agrisure Viptera 3, whereas the HX-R × Sus and Vip-R × Sus strains survived on Herculex maize. On Bt cotton, the HX-R, VT-R and PW-R strains exhibited high survival on Bollgard II. All resistant strains survived on WideStrike, but only PW-R and Vip-R × Sus survived on TwinLink. Our study provides useful data to aid in the understanding of the effectiveness of the refuge strategy for Insect Resistance Management of Bt plants.
Similar content being viewed by others
Introduction
Fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith), is the primary pest of maize (Zea mays L.) and cotton (Gossypium hirsutum L.) in South American countries1,2. In Brazil, some biological characteristics of FAW—such as polyphagy3, a high reproductive capacity and adult dispersion4, and multiple generations per year5,6— favor high infestation rates of this pest on maize and cotton throughout the year.
The use of transgenic maize and cotton varieties expressing insecticidal proteins from Bacillus thuringiensis Berliner (Bt) is the main control strategy for FAW in Brazil7,8,9,10. During the 2014–2015 crop season, Bt maize and cotton were cultivated on approximately 80 and 40%, respectively, of the total area used for these crops11. Among the several available commercial Bt maize and cotton varieties, most express Bt proteins from the Cry1 group, with some proteins showing cross-resistance, such as Cry1F, Cry1A.105, Cry1Ac, and Cry1Ab12,13,14,15,16. In the current Brazilian crop production system, overlap of the cultivation of some Bt plants (i.e., maize, cotton and soybean) occurs, and cross-crop resistance among Bt plants can contribute to the evolution of resistance in field populations of FAW16,17.
The evolution of resistance in populations of S. frugiperda is the primary threat to the sustainability of Bt maize and cotton varieties. In Brazil, FAW has evolved field-relevant resistance to the Cry1F protein expressed in Herculex maize6 and to the Cry1Ab protein in MON810 maize18. This species has also developed resistance to Cry1F maize in Puerto Rico19 and in some areas of the southeastern United States13. Laboratory studies have also indicated the resistance of FAW to YieldGard VT PRO maize, which expresses Cry1A.105/Cry2Ab212,16; PowerCore maize, which expresses Cry1A.105/Cry2Ab2/Cry1F20; and Agrisure Viptera and Agrisure Viptera 3 maize, which express Vip3Aa20 and Vip3Aa20/Cry1Ab21, respectively. The laboratory selection of these resistant strains from field populations of FAW is indicative of the presence of resistance alleles in the field, which reveals a potential risk of the evolution of resistance if resistance management strategies are neglected.
The resistance management strategies called ‘high-dose/refuge’ and ‘gene pyramiding’ have been proposed to prevent or delay the evolution of resistance in FAW to Bt plants in Brazil. The first strategy combines refuge areas, where susceptible insects are generated for mating with the rare resistant insects that survive in the Bt areas, with the high-dose expression of Bt protein(s), which causes the resistance to be “functionally” recessive22,23,24. The second strategy uses plants that express two or more Bt proteins with high insecticidal activity against the same target pest25,26. Theoretically, only the individuals who are homozygous for resistance to all proteins survive on Bt plants under this strategy27. Mathematical models suggest that the use of the gene pyramiding strategy more efficiently delays the evolution of resistance compared with the use of plants that express only one insecticidal protein28,29. In this context, to support resistance management programs, we evaluated the functional dominance of FAW resistance to Bt maize, based on the survival of resistant, heterozygous, and susceptible strains on the Bt maize and cotton varieties used commercially in Brazil.
Results
Survival of FAW strains on Bt maize varieties
A significant interaction was present between Bt maize varieties and FAW strains (df = 40, deviance = 1905.00, P < 0.0001). The effect of Bt maize (df = 5, deviance = 3536.80, P < 0.0001) and FAW strains (df = 8, deviance = 1103.70, P < 0.0001) was also significant.
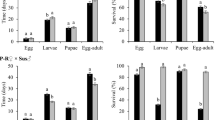
Figure 1 shows the effect of the Bt maize variety on the survival of the FAW strains. The HX-R strain had the highest larval survival rate on Herculex — greater than 96%. In this Bt maize variety, resistant larvae from the VT-R, PW-R and Vip-R strains had significantly lower survival than did the HX-R strain but were not different from each other. Heterozygous larvae from HX-R × Sus (~2% survival) and Vip-R × Sus (~8% survival) exhibited significantly lower survival than the HX-R, VT-R, PW-R and Vip-R strains on Herculex. In contrast, the Sus, VT-R × Sus and PW-R × Sus strains did not survive on this maize variety (Fig. 1). The VT-R and PW-R strains exhibited significantly higher survival than other strains on YieldGard VT PRO and PowerCore — greater than 75% survival—whereas the HX-R strain had a survival rate less than 11%. In contrast, Sus, Vip-R, and all of the heterozygous strains did not survive on YieldGard VT PRO or PowerCore (Fig. 1). Only resistant larvae from the Vip-R strain survived on Agrisure Viptera and Agrisure Viptera 3 at a rate greater than 83% (Fig. 1). On non-Bt maize, all of the FAW strains had survival rates greater than 82% (Fig. 1).
Survival of Bt-resistant, heterozygous, and susceptible strains of FAW on the leaves of Bt and non-Bt maize at 7 days.
Bars (±SE) with the same letter are not significantly different when confidence intervals overlap (95% CI). An asterisk (*) indicates that confidence intervals were not estimated because no variability existed.
The larval survival results of the Bt-resistant, heterozygous, and susceptible FAW strains on the leaves of Bt and non-Bt maize after 7 days are presented in Supplementary Table S1. The HX-R strain had a high survival rate (>92%) on Herculex and non-Bt maize but significantly lower survival on YieldGard VT PRO and PowerCore. In contrast, the HX-R strain did not survive on Agrisure Viptera and Agrisure Viptera 3. The VT-R strain exhibited larval survival greater than 99% on YieldGard VT PRO, which did not differ from its survival on non-Bt maize. In contrast, this strain had significantly lower survival (less than 75%) on Herculex and PowerCore. The VT-R strain also did not survive on Agrisure Viptera or Agrisure Viptera 3. In contrast, this strain had greater than 98% survival on non-Bt maize. The PW-R strain had similar survival rates on Herculex, YieldGard VT PRO and PowerCore, ranging from 78 to 83%. In contrast, no live larvae of the PW-R strain were found on Agrisure Viptera or Agrisure Viptera 3, but on non-Bt maize, this strain had greater than 96% survival. The Vip-R strain had greater than 83% survival on Agrisure Viptera and Agrisure Viptera 3, which was not different than on Herculex or non-Bt maize. In contrast, the Vip-R strain did not survive on YieldGard VT PRO or PowerCore. With the exception of the heterozygous larvae from HX-R × Sus and Vip-R × Sus, which survived on Herculex, all of the heterozygous larvae suffered complete mortality on all of the Bt maize tested. The Sus strain survived only on non-Bt maize, with 82% survival.
Survival of FAW strains on Bt cotton varieties
A significant interaction occurred between Bt cotton varieties and FAW strains (df = 24, deviance = 602.86, P < 0.0001). The larval survival also differed significantly among the Bt cotton varieties (df = 3, deviance = 2255.18, P < 0.0001) and the FAW strains (df = 8, deviance = 427.92, P < 0.0001).
Figure 2 shows data for the Bt cotton varieties as a fixed effect. On Bollgard II, the HX-R, VT-R, and PW-R strains had similar larval survival, ranging from 41 to 46%. In contrast, less than 4% survival was observed for larvae of the Vip-R, HX-R × Sus and VT-R × Sus strains. On this Bt cotton, the Sus, PW-R × Sus and Vip-R × Sus strains did not survive (Fig. 2). On WideStrike, the HX-R, VT-R and PW-R strains had survival rates greater than 70% and did not differ from each other. In contrast, significantly lower survival was observed for the Vip-R and Vip-R × Sus strains — less than 43%. Larvae from the Sus, HX-R × Sus, VT-R × Sus and PW-R × Sus strains did not survive on WideStrike (Fig. 2). On TwinLink, only larvae of the PW-R (28% survival) and Vip-R × Sus strains (1% survival) survived (Fig. 2). Larval survival was greater than 63% for all strains on non-Bt cotton (Fig. 2).
Survival of Bt-resistant, heterozygous and susceptible strains of FAW on leaves of Bt and non-Bt cotton at 7 days.
Bars (±SE) with the same letter were not significantly different when the confidence intervals overlap (95% CI). An asterisk (*) indicates that confidence intervals were not estimated because no variability existed.
High survival of the FAW strains was found on all Bt cotton varieties tested (Supplementary Table S2). Larvae from the HX-R strain had a high survival on WideStrike and non-Bt cotton — greater than 65%. This strain also exhibited approximately 40% survival on Bollgard II, but it did not survive on TwinLink. The VT-R strain exhibited greater than 83% survival on WideStrike and non-Bt cotton; however, its survival was less than 42% on Bollgard II, and it did not survive on TwinLink. The PW-R strain had a similar survival on WideStrike (78%) and non-Bt cotton (88%), but a higher survival rate on Bollgard II (45%) and TwinLink (28%). The Vip-R strain larvae showed 43% and 1.6% survival on WideStrike and Bollgard II, respectively; however, a higher survival rate of approximately 64% was detected on non-Bt cotton. Heterozygous Vip-R × Sus and VT-R × Sus larvae survived on WideStrike and Bollgard II, but less than 2% survival was observed for larvae from the Vip-R × Sus strain exposed to Bollgard II. Neonates from the Sus strain did not survive on any Bt cotton variety tested, but their survival was greater than 86% on non-Bt cotton.
Discussion
The Bt-resistant FAW strains (HX-R, VT-R, PW-R, and Vip-R) had high survival on the studied Bt maize varieties to which they were known to be resistant. In contrast, the Bt-susceptible FAW strain (Sus) showed complete mortality on all Bt maize and cotton varieties tested. These results demonstrate that Bt resistance is largely genetic, as found in previous studies6,20,21,30. In addition, the HX-R strain also survived on YieldGard VT PRO and PowerCore maize, and the VT-R and PW-R strains survived on Herculex maize. In contrast, the Vip-R strain only survived on Agrisure Viptera and Agrisure Viptera 3 maize. The survival of the HX-R strain on YieldGard VT PRO and PowerCore maize and of the VT-R and PW-R strains on Herculex maize is due to cross-resistance between the Cry1F and Cry1A.105 proteins. This hypothesis is supported by previous studies that showed a high resistance ratio of Cry1F-resistant strains when exposed to Cry1A.10512,13,14,16. This cross-crop resistance occurs due to the similar amino acid sequences of Cry1F and Cry1A.105 proteins, with 72% similarity26. Additionally, the Cry1 proteins also share the same binding site in the midgut of the FAW larvae15. The YieldGard VT PRO and PowerCore maize varieties also express Cry2Ab2, which has ~35 and 34% similarity with Cry1F and Cry1A.105, respectively, but does not share the same binding site with the Cry1 proteins15,26. However, expression of Cry2Ab2 did not cause complete mortality of the FAW strains resistant to Cry1F and/or Cry1A.105 due to the low toxicity of this Bt protein to FAW12,16. We also detected that the Vip-R strain showed complete mortality on YieldGard VT PRO and PowerCore, which indicated no cross-resistance between the Cry1 and Vip3A proteins. This lack of cross-resistance was also previously reported in FAW and tobacco budworm, Chloridea virescens12,13,14,31,32,33,34. In contrast, the Vip-R strain had a high survival rate on Herculex, but the HX-R strain did not survive on the Agrisure Viptera and Agrisure Viptera 3 maize varieties, indicating the presence of multiple resistances in the Vip-R strain to the Vip3Aa20 and Cry1F proteins. In other words, during the F2 screening tests to obtain a Vip3Aa20-resistant strain, the resistance allele to the Cry1F protein was also selected. This probably occurred due to a high frequency of the resistance allele to the Cry1F protein in Brazilian populations of FAW, as reported by Farias et al.35,36.
We also observed that all Bt-resistant FAW strains had a relatively high survival rate on the Bollgard II and WideStrike cotton varieties, whereas the PW-R strain showed some survival on TwinLink cotton. The survival of FAW on Bollgard II and WideStrike is due to the low toxicity and low expression of Cry1Ac against FAW37,38,39,40. In addition, the survival of FAW on Bollgard II can also be explained by the low toxicity of Cry2Ab2 against this species12,16; however, on WideStrike, the resistance of FAW was due to field-evolved resistance to Cry1F6,33. In contrast, the HX-R, VT-R, and Vip-R strains did not survive on TwinLink, which expresses Cry1Ab and Cry2Ae. In this Bt cotton variety, the Cry1Ab protein did not cause high larval mortality of FAW9,32,33,41,42. The tolerance of this species to Cry1Ab can be explained by its low affinity for the midgut tissue and more rapid degradation in the larval midgut43,44. Therefore, the mortality of FAW on TwinLink is basically caused by the expression of the Cry2Ae protein, which has a different binding site from the Cry1 proteins and relatively high toxicity against FAW45. In contrast, the survival of the PW-R strain on TwinLink is due to the low toxicity of Cry1Ab and cross-resistance between the Cry2Ab and Cry2Ae proteins, as reported by Gouffon et al.45.
Our results also showed that all of the heterozygous FAW strains had complete mortality on the YieldGard VT PRO, PowerCore, Agrisure Viptera, and Agrisure Viptera 3 maize varieties. These results demonstrate that the in-plant expression of the Bt proteins meets the definition of high-dose, which causes the resistance to be ‘functionally’ recessive. The mortality of the heterozygous strains supports the adoption of refuge areas for resistance management of FAW in Brazil. In contrast, larvae from the reciprocal crosses HX-R × Sus and Vip-R × Sus survived on Herculex maize. The survival of the HX-R × Sus strain on Herculex maize was expected because this Bt maize variety does not meet the definition of high-dose against FAW6,46. In contrast, the unexpected survival of the Vip-R × Sus strain on Herculex maize is due to the multiple resistances of the Vip-R strain, as discussed above.
The heterozygous HX-R × Sus and VT-R × Sus larvae survived on the Bollgard II cotton, whereas Vip-R × Sus had some survival on WideStrike and TwinLink. The survival of these heterozygous strains on the Bt cotton varieties presents a consistent risk for the evolution of resistance because these insects are mainly responsible for carrying the resistance alleles23,47. The survival of the FAW heterozygotes on Bt cotton varieties can increase the frequency of resistance alleles to Bt proteins expressed on Bt maize varieties and vice-versa. The survival of the FAW heterozygotes on Herculex maize and the low adoption of refuge areas were the primary contributors to the field-evolved resistance to the Cry1F protein in Brazil6. In other words, resistance to the Cry1 maize varieties can compromise the efficacy of WideStrike cotton, as demonstrated in our study and as was also shown by Yang et al.33.
In the current Brazilian crop production system, Bt maize and cotton varieties are grown simultaneously in adjacent areas. The same FAW populations infest both crops in these areas17. Several other aspects also increase the selection pressure for resistance to Bt crops in the FAW population, such as (i) multiple crop seasons per year, (ii) a high rate of adoption of Bt crops, (iii) low compliance with refuge areas, (iv) cross-resistance among the Cry1 proteins, (v) several generations of FAW per year, and (vi) the high reproductive capacity of FAW. In this scenario, FAW developed resistance to Cry1F and Cry1Ab6,18, and if the same conditions prevailed, resistance to other Bt proteins or Bt crops would also quickly develop. In this study, we demonstrated that the functional dominance of FAW resistance to Bt proteins is recessive due to the high mortality of the heterozygous insects on Bt maize and cotton varieties. Therefore, to support the high-dose refuge strategy, increasing the adoption of refuge areas by farmers is a key point that requires the stakeholders’ urgent attention in an attempt to delay or prevent the evolution of resistance in the FAW population and to prolong the lifetime of the use of Bt crops for controlling this species in Brazil.
Methods
Fall armyworm strains
Herculex-resistant strain (HX-R)
The strain resistant to Herculex maize, which expresses the Cry1F protein, was selected from a field population collected in Barreiras, Bahia, Brazil, as described in detail by Farias et al.6. This strain was subsequently used in eight repeated back crossings, each followed by sib-mating and selection among susceptible and resistant insects on diet-overlay bioassays with the Cry1F protein to obtain a nearly isogenic resistant strain30. The resistance ratio to the Cry1F protein was greater than 5000-fold, and the inheritance of resistance was characterized as autosomal6.
YieldGard VT PRO-resistant (VT-R) and PowerCore-resistant (PW-R) strains
The strain resistant to the YieldGard VT PRO (Cry1A.105 and Cry2Ab2) and the PowerCore (Cry1A.105, Cry2Ab2 and Cry1F) maize varieties was selected in the laboratory from positive F2 screening tests of field populations as described by Bernardi et al.16,20. After selection, the resistant strains were reared for nine generations on the respective maize event. The resistance ratios of the VT-R and PW-R strains to the Cry1F, Cry1A.105 and Cry2Ab2 proteins were greater than 2700-, 3300- and 10-fold, respectively. The inheritance of the resistance was characterized as autosomal recessive and polygenic16,20.
Agrisure Viptera-resistant strain (Vip-R)
The Vip-R strain resistant to the Agrisure Viptera (Vip3Aa20) and Agrisure Viptera 3 (Cry1Ab/Vip3Aa20) maize varieties was selected via an F2 screening of a field population from Correntina, Bahia, Brazil21,48. After selection, the Vip-R strain was reared for seven generations on Agrisure Viptera maize leaves. The resistance ratio to Vip3Aa20 was greater than 3200-fold, and the inheritance of resistance was characterized as autosomal recessive and monogenic21.
Susceptible strain
The susceptible strain was obtained from EMBRAPA Milho e Sorgo, Sete Lagoas, Minas Gerais, Brazil, has been maintained in the laboratory since 1995 and was free of selection pressure by insecticides and Bt proteins.
Heterozygous strains
The heterozygous strains were obtained from a reciprocal cross of resistant♀ × Sus♂, with at least 40 pairs. In the bioassays, only the F1 progeny of these crosses were used because inheritance of resistance is not sex-linked in any of the resistant strains, and the heterozygous insects showed similar mortality and growth inhibition responses to the Sus strain in diet-overlay bioassays6,16,20,21.
Bt plants
Five Bt maize and three Bt cotton varieties that are commercially used in Brazil were tested (Table 1). Non-Bt maize and cotton plants were use as control treatments. Maize and cotton plants were cultivated under field conditions at a density of 10 plants per linear meter with 0.5 m between rows. Before the bioassays, all plants were tested for the expression of insecticidal proteins using detection kits (Envirologix, QuickStix™).
Leaf-bioassays
Bioassays were performed with plants from the V4 to V8 (maize) and squaring (cotton) phenological stages. Maize and cotton bioassays were performed by taking leaves from the corn whorls or the upper third of the plants, respectively. In the laboratory, the leaves were cut into ~4-cm2 pieces and placed in a gelled mixture of 2% agar-water in 32-well plastic plates (Advento do Brasil, São Paulo, Brazil). Then, each well was infested with one neonate (<24 h old). The plates were sealed with their plastic covers and placed in a room at 27 ± 1 °C and 60 ± 10% RH, with a photoperiod of 14 h. A completely randomized experimental design was used, with 128 larvae/strain/Bt plant (4 replicates of 32 neonates). The leaves were replaced every three days. The larval survival was assessed at 7 days.
Statistical analysis
A binomial generalized linear model was fit to the larval survival data with a linear interaction predictor (Bt plant vs insect genotype). The goodness-of-fit was evaluated using half-normal plots with a simulated envelope49. An analysis of deviance was performed to assess the significance of the interaction between the factors (i.e., the Bt plant and insect genotypes) and whether the effect was non-significant (P = 0.05). All of these analyses were performed using R50, but the means were compared via the confidence intervals (95%) for the linear predictors of the fitted model. The CIs were obtained using the PROC GENMOD (link function = logit) procedure in SAS 9.351. Using the OUTPUT statement, it was possible to obtain the fitted values and their confidence intervals, using the option command output out = pred p = predi, lower = lcl upper = ucl, but the tables and figures contain the original means.
Additional Information
How to cite this article: Horikoshi, R. J. et al. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management. Sci. Rep. 6, 34864; doi: 10.1038/srep34864 (2016).
References
Pogue, M. G. A world revision of the genus Spodoptera Guenee (Lepidoptera: Noctuidae). Mem Am. Entomol. Soc . 43, 1–202 (2002).
Martinelli, S., Barata, R. M., Zucchi, M. I., Silva-Filho, M. C. & Omoto, C. Molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations associated to maize and cotton crops in Brazil. J. Econ. Entomol . 99, 519–526 (2006).
Barros, E. M., Torres, J. B., Ruberson, J. R. & Oliveira, M. D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 137, 237–245 (2010).
Nagoshi, R. N. et al. Haplotype profile comparisons between Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Rico, South America, and the United States and their implications to migratory behavior. J. Econ. Entomol. 108, 135–144 (2015).
Fitt, G. P. et al. Resistance Risks of Bt Cotton and their Management in Brazil. (ed. Hilbeck, A., Andow, D. A. & Fontes, E. M. G. ). Environmental risk assessment of genetically modified organisms, Volume 2, Methodologies for assessing Bt cotton in Brazil Ch. 12 . 300–345 (CAB International, Wallingford, UK, 2006).
Farias, J. R. et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot . 64, 150–158 (2014).
Okumura, R. S. et al. Agronomic efficiency of Bacillus thuringiensis (Bt) maize hybrids in pests control on Lucas do Rio Verde city, State of Mato Grosso, Brazil. Afr. J. Agric. Res . 8, 2232–2239 (2013).
Waquil, J. M. et al. Management of Lepidopteran pests in maize crop using the Bt pyramided event Cry1A. 105 and Cry2Ab2. Pesqui. Agropecu. Bras . 48, 1529–1537 (2013).
Bernardi, O. et al. Resistance risk assessment of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Crambidae) to Vip3Aa20 insecticidal protein expressed in corn. J. Econ. Entomol. 108, 2711–2719 (2015).
Sorgatto, R. J., Bernardi, O. & Omoto, C. Survival and development of Spodoptera frugiperda and Chrysodeixis includens (Lepidoptera: Noctuidae) on Bt cotton and implications for resistance management strategies in Brazil. Environ. Entomol. 44, 186–192 (2015).
Céleres. 3rd Follow-up on agricultural biotechnology adoption for the 2014/15 Crop (2015). Available at: http://www.celeres.com.br/. (accessed 13th October 2015).
Santos-Amaya, O. F. et al. Resistance to dual-gene Bt maize in Spodoptera frugiperda: selection, inheritance, and cross-resistance to other transgenic events. Sci. Rep. 5, 18243 (2015).
Huang, F. et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLoS One 9, e0112958 (2014).
Vélez, A. et al. Inheritance of Cry1F resistance, cross-resistance and frequency of resistant alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae). Bull. Entomol. Res. 103, 700–713 (2013).
Hernandez-Rodriguez, C., Hernandez-Martinez, P., Van Rie, J., Escriche, B. & Ferre, J. Shared midgut binding sites for Cry1A. 105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS One 8, e0068164 (2013).
Bernardi, D. et al. Cross-resistance between Cry1 proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLoS One 10, e0140130 (2015).
Martinelli, S. et al. Genetic structure and molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) collected in maize and cotton fields in Brazil. Bull. Entomol. Res. 97, 225–231 (2007).
Omoto, C. et al. Field‐evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 72, 1727–1736 (2016).
Storer, N. P. et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico . J. Econ. Entomol . 103, 1031–1038 (2010).
Bernardi, D. Resistência de Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) a eventos “piramidados” de milho que expressam proteínas inseticidas de Bacillus thuringiensis Berliner. PhD dissertation. Escola Superior de Agricultura “Luiz de Queiroz, Piracicaba, Brazil (2015).
Bernardi, O. et al. Selection and characterization of resistance to the Vip3Aa20 protein from Bacillus thuringiensis in Spodoptera frugiperda. Pest Manag. Sci. 72, 1794–1802 (2016).
Alstad, D. N. & Andow, D. A. Managing the evolution of insect resistance to transgenic plants. Science 268, 1894–1896 (1995).
Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 43, 701–726 (1998).
Tabashnik, B. E. Delaying insect resistance to transgenic crops. Proc. Natl. Acad. Sci. USA 105, 19029–19030 (2008).
Ghimire, M. N., Huang, F., Leonard, R., Head, G. P. & Yang, Y. Susceptibility of Cry1Ab-susceptible and -resistant sugarcane borer to transgenic corn plants containing single or pyramided Bacillus thuringiensis genes. Crop Prot . 30, 74–81 (2011).
Carrière, Y., Crickmore, N. & Tabashnik, B. E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 33, 161–168 (2015).
Brevault, T. et al. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA 110, 5806–5811 (2013).
Roush, R. T. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Phil. Trans. R. Soc. Lond. B 353, 1777–1786 (1998).
Zhao, J. Z. et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 21, 1493–1497 (2003).
Horikoshi, R. J. et al. Near-isogenic Cry1F-Resistant strain of Spodoptera frugiperda (Lepidoptera: Noctuidae) to investigate fitness cost associated with resistance in Brazil. J. Econ. Entomol. 109, 854–859 (2016).
Niu, Y., Meagher, R. L. Jr., Yang, F. & Huang, F. Susceptibility of field populations of the fall armyworm (Lepidoptera: Noctuidae) from Florida and Puerto Rico to purified Cry1F protein and corn leaf tissue containing single and pyramided Bt genes. Fla. Entomol. 96, 701–713 (2013).
Niu, Y., Yang, F., Dangal, V. & Huang, F. Larval survival and plant injury of Cry1 F-susceptible, -resistant, and -heterozygous fall armyworm (Lepidoptera: Noctuidae) on non-Bt and Bt corn containing single or pyramided genes. Crop Prot . 59, 22–28 (2014).
Yang, F. et al. Performance and cross-crop resistance of Cry1F-maize selected Spodoptera frugiperda on transgenic Bt cotton: implications for resistance management. Sci. Rep. 6, 28059 (2016).
Jackson, R. E., Marcus, M. A., Gould, F., Bradley, J. R. Jr. & Van Duyn, J. W. Cross-resistance responses of Cry1Ac-selected Heliothis virescens (Lepidoptera: Noctuidae) to the Bacillus thuringiensis protein Vip3A. J. Econ. Entomol . 100, 180–186 (2007).
Farias, J. R., Horikoshi, R. J., Santos, A. C. & Omoto, C. Geographical and temporal variability in susceptibility to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. J. Econ. Entomol. 107, 2182–2189 (2014).
Farias, J. R. et al. Frequency of Cry1F resistance alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. doi: 10.1002/ps.4274 (2016).
Adamczyk, J. J. Jr. et al. Evaluations of Bollgard®, Bollgard II®, and Widestrike® technologies against beet and fall armyworm larvae (Lepidoptera: Noctuidae). Fla. Entomol. 91, 531–536 (2008).
Sivasupramaniam, S. et al. Toxicity and characterization of cotton expressing Bacillus thuringiensis Cry1Ac and Cry2Ab2 proteins for control of lepidopteran pests. J. Econ. Entomol . 101, 546–554 (2008).
Bernardi, O. et al. Low susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically-modified soybean expressing Cry1Ac protein. Crop Prot . 58, 33–40 (2014).
Akin, D., Stewart, S., Layton, M. & Mills, J. Efficacy of cotton expressing pyramided Bacillus thuringiensis insecticidal proteins against lepidopteran pests. Midsouth Entomol . 4, 1–13 (2011).
Adamczyk, J. J. Jr. & Mahaffey, J. S. Efficacy of Vip3A and Cry1Ab transgenic traits in cotton against various lepidopteran pests. Fla. Entomol. 91, 570–575 (2008).
Sousa, F. F. et al. Life-history traits of Spodoptera frugiperda populations exposed to low-dose Bt maize. PloS One 11, e0156608 (2016).
Aranda, E., Sanchez, J., Peferoen, M., Güereca, L. & Bravo, A. Interactions of Bacillus thuringiensis crystal proteins with the midgut epithelial cells of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 68, 203–212 (1996).
Miranda, R., Zamudio, F. Z. & Bravo, A. Processing of Cry1Ab δ-endotoxin from Bacillus thuringiensis by Manduca sexta and Spodoptera frugiperda midgut proteases: role in protoxin activation and toxin inactivation. Insect Biochem. Mol. Biol. 31, 1155–1163 (2001).
Gouffon, C., Van Vliet, A., Van Rie, J., Jansens, S. & Jurat-Fuentes, J. L. Binding sites for Bacillus thuringiensis Cry2Ae toxin on Heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl. Environ. Microbiol. 77, 3182–3188 (2011).
Farias, J. R. et al. Dominance of Cry1F resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) on TC1507 Bt maize in Brazil. Pest Manag. Sci. 72, 974–979 (2015).
Tabashnik, B. E., Van Rensburg, J. B. J. & Carriere, Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. J. Econ. Entomol . 102, 2011–2025 (2009).
Bernardi, O. et al. Frequency of resistance to Vip3Aa20 toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 76, 7–14 (2015).
Demétrio, C. G., Hinde, J. & Moral, R. A. Models for overdispersed data in entomology in Ecological Modelling Applied to Entomology, (ed. Ferreira C. P. & Godoy W. A. ) pp. 219–259 (Springer, 2014).
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
SAS Institute Inc., SAS/STAT: 9.3 User’s Third Edition, SAS Institute Inc, Cary, NC (2010).
Acknowledgements
We thank the National Council for the Improvement of Higher Education (CAPES) for granting a scholarship to the senior author and the Brazilian National Council for Scientific and Technological Development (CNPq) for granting a research fellowship to C.O. (Grant #312086/2013-0).
Author information
Authors and Affiliations
Contributions
R.J.H., D.B., O.B. and C.O. conceived and designed the experiments; R.J.H., D.B., O.B., D.M.O., L.L.M. and F.S.d.A.eA. selected the resistant strains, designed the experiments and collected data; R.J.H., O.B. and J.B.M. performed the statistical analysis; R.J.H., D.B., O.B., J.B.M., D.M.O. and C.O. interpreted the results and discussed and wrote the manuscript; C.O. coordinated the project. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Horikoshi, R., Bernardi, D., Bernardi, O. et al. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management. Sci Rep 6, 34864 (2016). https://doi.org/10.1038/srep34864
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34864
This article is cited by
-
Host plant resistance for fall armyworm management in maize: relevance, status and prospects in Africa and Asia
Theoretical and Applied Genetics (2022)
-
Large-scale assessment of lepidopteran soybean pests and efficacy of Cry1Ac soybean in Brazil
Scientific Reports (2021)
-
Ecological Modelling of Insect Movement in Cropping Systems
Neotropical Entomology (2021)
-
Field Efficacy of Metarhizium rileyi Applications Against Spodoptera frugiperda (Lepidoptera: Noctuidae) in Maize
Neotropical Entomology (2021)
-
Maize Endochitinase Expression in Response to Fall Armyworm Herbivory
Journal of Chemical Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.