Abstract
A scaffold-hopping strategy toward Agomelatine based on in silico screening and knowledge analysis was employed to design novel antidepressant agents. A series of 3, 4-dihydroisoquinoline compounds were selected for chemical synthesis and biological assessment. Three compounds (6a-1, 6a-2, 6a-9) demonstrated protective effects on corticosterone-induced lesion of PC12 cells. Compound 6a-1 also displayed low inhibitory effects on the growth of HEK293 and L02 normal cells and it was further evaluated for its potential antidepressant effects in vivo. The forced swim test (FST) results revealed that compound 6a-1 remarkably reduced the immobility time of rats and the open field test (OFT) results indicated a better general locomotor activity of the rats treated with compound 6a-1 than those with Agomelatine or Fluoxetine. Mechanism studies implied that compound 6a-1 can significantly reduce PC12 cell apoptosis by up-regulation of GSH and down-regulation of ROS in corticosterone-induced lesion of PC12 cells. Meanwhile, the down-regulation of calcium ion concentration and up-regulation of BDNF level in PC12 cells may account for the neuroprotective effects. Furthermore, compound 6a-1 can increase cell survival and cell proliferation, promote cell maturation in the rat hippocampus after chronic treatment. The acute toxicity data in vivo indicated compound 6a-1 exhibited less hepatotoxicity than Agomelatine.
Similar content being viewed by others
Introduction
Depression characterized by sadness, loss of interest or pleasure, low self-esteem, poor concentration and always associated with disturbed sleep and appetite, affects approximately 350 million people all over the world1,2. According to the World Health Organization’s Global Burden of Disease project, major depressive disorder will become the leading cause of disability and a major contributor to the overall disease burden worldwide. Patients with major depression have an increased onset risk of aging-related somatic diseases such as heart disease, diabetes, obesity and cancer3,4. At its worst, depression can lead to suicide. Over 800 000 people die due to suicide every year and more than 70 percent suicides suffer from major depression1,2. Currently, most medications for treatment of depression target serotonergic and/or noradrenergic transmitter systems or inhibit monoamine oxidase to reduce the degradation of serotonin and noradrenaline. Despite that a large number of antidepressant drugs commercially available, there are still many issues leading to risks of depression therapy. It was reported that a number of patients who took antidepressant drugs experienced serious side effects and drug-drug interactions, with fewer than half of patients responding well to currently available treatments5. Besides, the long-lasting therapy period gives rise to poor patient compliance6.
The monoamine hypothesis of depression has dominated thinking about mood disorders since 50 years ago owing to the fact that both monoamine oxidase inhibitors and tricyclic antidepressants increased brain levels of monoamines. However, rapid drug-induced elevations of monoamine levels and symptom improvement require weeks of antidepressant treatment7. Neuroscientists have made great efforts to investigate the neurobiological and structural changes correlated with the clinical course over the last decade. Neuronal plasticity, neurogenesis in the adult brain, and the ability of antidepressants to regulate the expression of genes related to plasticity and resilience, have attracted great amount of attention in the past years8,9,10,11,12,13.
Several studies14,15,16,17 showed that hippocampal volume decreased in patients with stress-related major depression, which might be due to glial and neuronal atrophy or loss related in part to increases in corticosteroids and excitatory amino acids; such relationships have been demonstrated in animal models18,19,20, while still under investigation in humans21,22. Meanwhile, Agomelatine, a recently marketed antidepressant drug, was reported to induce neurogenesis and cell proliferation in the ventral part of dentate gyrus, resulting to the rapid and early increase in maturation at a critical period of neuronal development, which likely influences the functional integration of new born cells into hippocampal circuitry. The mentioned above formed the basis for the neuroplasticity hypothesis of major depression. Fluoxetine and many other antidepressants in clinic also shared above neurogenetic effects23,24,25,26,27,28. In addition, many studies indicated that antidepressant drugs are able to prevent neuronal damage and cell loss that may occur in the brain of patients with mood disorders29,30,31,32. Although the links between hippocampal neurogenesis and psychiatric disorders are far to be elucidated, a better understanding of the regulation of neurogenesis by antidepressants and how they influence distinct phases of progenitor cell development may yield insights into the physiological mechanisms that underlie antidepressant behavioral efficacy.
As stated before, Agomelatine, Launched in European Union in 2009, was reported to induce neurogenesis and cell proliferation in the ventral part of dentate gyrus of patients, and brought great expectation in the clinic treatment of major depression. However, it was soon reported to have considerable hepatotoxicity, which should be the major reason why it was discontinued development for the US market in October 201133. A number of observations imply that it is urgently desirable to find new chemical entities (NCE) as potential antidepressant candidates with enhanced benefit-risk balance.
In the field of modern medicinal chemistry, scaffold hopping strategy, a lead optimization method, has been widely used to discover novel drug candidates that bind to the same receptor or possess similar pharmacological effects. A change in the central chemical template of the lead compound can also lead to a granted patent and even enhanced ADME/T properties. There are now a lot of computational approaches to scaffold hopping. For example, the popular Maestro modelling software provides us ligand-based, structure-based and isosteric matching core hopping methods. However, it is still challenging to get alternative structures with synthetic tractability and at the same time conserve essential pharmacophore features. Furthermore, complicated similarity descriptors are hard to manage by the experimental pharmaceutial chemists and of little use if the scaffold hopping campaign starts from a single active compound only.
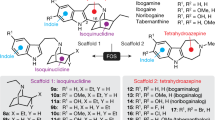
Inspired by aforementioned reasons and as a part of the ongoing work in our research groups aimed at the search of novel antidepressants6,34 with neuroprotective mechanism, we started a scaffold hopping campaign of Agomelatine in silico. Combination of the scoring function of fitting values and expertise, 3, 4-dihydroisoquinoline skeleton was selected as novel scaffold for chemical synthesis and the structure-activity relationship on C-1 position of this scaffold was extensively explored.
As known, PC12, a cell lineage derived from a pheochromocytoma of rat adrenal medulla, has been widely used to investigate the mechanisms involved in neurotoxicity, neuroprotection and neurorestoration35,36. Glucocorticoids at high concentration lead to PC12 neuronal damage under depressive disorder, and this feature makes PC12 cells very useful as a model system for in vitro screening37. As such, in this paper, we used corticosterone-induced lesion of PC12 cells as a rapid in vitro screening model for preliminary assessment of neuroprotective activity of the synthesize novel 3,4-dihydroisoquinoline compounds.
Thus, we disclose our studies on the design, synthesis and biological evaluation of a novel series of 3, 4-dihydroisoquinoline compounds as antidepressants. In the in vitro screening, compound 6a-1 was identified to possess highly neuroprotective effect and low cytotoxicities. Further in vivo FST and OFT experiments implies that compound 6a-1 is a potential potent antidepressant. To make clear the underlying mechanism of 6a-1 for the observed neuroprotective on PC12 cells, hoechst 33258 staining was performed to check out the apoptosis of corticosterone-induced PC12 cells. On the other hand, real-time PCR and ELISA method were also employed to analyze the trophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), Vascular endothelial growth factor (VEGF), Insulin-like growth factor 1 (IGF-1) in vitro or in vivo. Furthermore, the potential of compound 6a-1 to increase neuron cells survival, proliferation and maturation in the rat hippocampus was also investigated after chronic treatment.
Scaffold Hopping
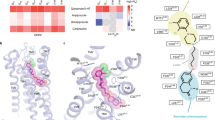
As shown in Fig. 1, the 3D chemical structure of Agomelatine was generated by adding hydrogen and minimization with CHARMm field and Common Feature Pharmacophore protocol in Discovery studio 2.55 was employed to produce four pharmacophores (Figure S1), which were applied to screen Chinese Nature Products Database (CNPD) with Ligand Profiler protocol. 8254 compounds were successfully profiled and the top 500 hits with highest fitting values of each pharmacophore were combined and the duplicated hits were removed. 1061 compounds were remained for further analysis. Brain-blood barrier penetration is of first importance for antidepressants and favored by low molecular weight, lack of ionization at physiological pH, and lipophilicity. To meet the requirement of lack of ionization at physiological pH, 13 alkaloid compounds (as shown below) were visually inspected and selected from the 1061 molecules libarary. In order for facilely synthetic access and getting analogues with low molecular weight of nature products, the common structural moiety of the selected alkaloids, dihydroisoquinoline (this article) or tetrahydroisoquinoline (to be explored) core structure, can be extracted as novel scaffold for chemical synthesis and structure-activity relationship exploration.
Chemistry
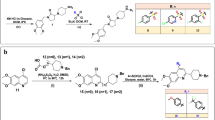
The target compounds 6a and 6b were obtained via multi-step synthesis according to Fig. 2. The first two steps are very straightforward. The N-protected β-alanine 2 was readily prepared by refluxing the mixture of isobenzofuran-1,3-dione 1, β-alanine and acetic acid for 4 hours according to literature38,39 with appropriate revision. Then intermediate 2 was condensed with 2-(4-methoxyphenyl)ethanamine hydrochloride at room temperature to give intermediate 3 in high yield40.
General synthetic route of 3, 4-dihydroisoquinoline compounds 6a and 6b.
Reagents and conditions: (i) β-alanine, AcOH, reflux, 4 h; (ii) 2-(4-methoxyphenyl)ethan–1-amine hydrochloride, EDCI, Pyridine, r.t., 24 h; (iii) P2O5, POCl3, 130 °C, 4 h; (iv) HCl(aq.), reflux, 8 h; (v) Acyl chloride or anhydride/EDCI, r. t., 10 h; carboxylic acid/EDCI/pyridine, r.t., 10 h; (vi) amine, CDI, 0 °C-r.t., 10 h; (vii) sulfonyl chloride, EDCI, r.t., 10 h.
According to a reported method, intermediate 4 can be conveniently obtained by strategy of Bischler-Napieralski reaction41. However, the usual reaction and workup conditions for Bischler-Napieralski reaction did not work in our case. The key issue is of incomplete conversion and mixed unknown impurities. After several attempts, we finally found that heating the intermediate 3 with 6.0 eq. POCl3 and 3.0 eq. P2O5 at 130 °C for 4 hours could reach a complete and neat conversion to the target compound. The reaction mixture was cooled down to room temperature naturally, and the excessive POCl3 was decanted carefully. Then the semisolid residue was gradually buffered to pH 10-11 by diluted NaOH solution with stirring at 60–70 °C. The aqueous solution was extracted with ethyl acetate/dichloromethane (4/1, v/v). To note, the working efficiency is extremely low if this workup was performed at room temperature because the asphalt-like residue was hard to agitate, although generally low temperature was believed to be beneficial for the product stability.
Accordingly, compound 5 was obtained by acid hydrolysis of the key intermediate 4 in a yield of 86.7%. The final compounds 6a were easily made by acylation of compound 5 with the corresponding acyl chloride, or acid anhydride, or carboxylic acid, or sulfonyl chloride at appropriate conditions. Meanwhile, compounds 6b were afforded by condensation compound 5 with corresponding amine with carbonyl diimidazole (CDI) under mild condition42. Through above procedures, 56 novel 3, 4-dihydroisoquinoline compounds were synthesized and isolated in moderate to high yields.
Result and Discussion
Protective effects on corticosterone-injured PC12 cells
As reported before37, corticosterone-injured PC12 cells is a fruitful in vitro model for preliminary screening of antidepressant drugs. All the target compounds were tested for their protective activity on this in vitro model at drug concentration of 1.25, 2.5 and 5 μM. The data were shown in Table 1 (and Supplementary material Table S1). Ten of them (6a-1, 6a-2, 6a-9, 6a-21, 6a-27, 6b-3, 6b-4, 6b-12, 6b-17 and 6b-18) demonstrated potential protective effects (above 10%) on corticosterone-induced lesion of PC12 cells at concentration of 1.25 μM. Three amide compounds (6a-1, 6a-2 and 6a-9) showed pronounced efficacy with protection rates of 25.4%, 32.7% and 20.3% respectively and compound 6a-1 showed a good concentration-dependant manner. As for structure-activity relationship, two prominently active compound 6a-1 and 6a-2 are substituted with methyl and ethyl group at the same position, which suggests that steric hindrance of the side chain may have an influence on the activity. Last, another active compound 6a-9 was introduced with a furan ring into its side chain, where the electron-rich furan ring may contribute to decrease the ROS level in the stimulated PC12 cells.
Cytotoxicities
In order for assessment of their potential cytotoxicities, the inhibitory effects of the final 3, 4-dihydroisoquinoline compounds were assessed in vitro on L02 cells and HEK293 cells. As shown in present data(see Supplementary material Table S2), most of the target compounds displayed low toxicities on the tested cells. Four compounds (6a-1, 6a-16, 6b-6 and 6b-13) showed inhibitory rates lower than 20% on both cells at concentration of 100 μM. The neuroprotection active compound 6a-9 exerted higher inhibition on growth of HEK293 and L02 cells. Other compounds displayed comparable effects to the positive control, Agomelatine. The most promising compounds 6a-1 exerted higher safety profile on HEK293 (10.3%) and L02 (13.7%) cells, superior to Agomelatine (47.5% and 41.8%).
BBB penetration ability calculation and assay
Lipophilicity is generally regarded as a most important physicochemical property largely related to ultimate success in drug discovery and development. It was also identified as an important determinant of central nervous system (CNS) exposure, including both the rate and extent of drug distribution into the brain. Accordingly, a CLogP value of around 2.0 were suggested to be the most optimal for CNS drugs. Described as a surrogate measure of hydrogen-bonding capacity and molecular polarity, the tPSA is a commonly used metric during the optimization of a drug’s ability to permeate cell membranes43. Therefore, CLogP and tPSA were calculated for early assessment of their CNS drug-likeness. The values of ClogP and tPSA for the synthesized compounds were calculated. Accordingly, we find out that the CLogP values of all target compounds fall into the CLogP range (0.16–6.59) of the marketed CNS drugs. As for tPSA, three compounds (6b-15, 6b-16 and 6b-19) go beyond the tPSA range (4.63–108) of the marketed CNS drugs. It suggested that the nitro group in these three compounds is not favored by BBB penetration(see Supplementary material Table S3).
Further, a PAMPA-BBB experiment was performed to measure the CNS permeation ability according to a report method with minor revision44. The results were listed in Table 2. Pe values of eleven drugs were also assayed for validation the method. The Pe value of compound 6a-1 is very close to that of Agomelatine, which make us believe compound 6a-1 may have antidepressant effects in vivo.
Forced swim test
The forced swim test has been widely used as a predictive model of depressive behavior in pre-clinical test45. The FST data of SD rats were shown in Fig. 3a. In the group treated with compound 6a-1, the mean immobility time of the swimming SD rats was reduced by 62.5% compared to the model group. The same parameter for Fluoxetine is 50.7%, for Agomelatine is 8.4%. In our animal models, Agomelatine did not significantly reduce the immobility time of the swimming SD rats. Compound 6a-1 displayed pronounced antidepressant-like activity compared to the model group treated with vehicle. In this test, its effects is similar to that of Fluoxetine.
In vivo antidepressive effects evaluation.
Forced swim test and Open field locomotor activity test. (a) Effects of compound 6a-1 and positive controls in forced swim test; (b) Antidepressant-like effects of compound 6a-1 and positive controls in the open field test. SD rats were treated at the same time of each day of days 2–15 (ig.32 mg/kg/day). Data represent the mean ± S.D. of 10 rats per group. Values are significant at *P < 0.05 when compared with model group treated with vehicle.
Open field locomotor activity
The open field test (OFT) evaluates the general locomotor and exploratory behavior of rats. Sometimes the nonspecific motor activities may lead to false-positive results in the forced swim test. Hence, we employed open field apparatus to further test its spontaneous locomotor activity and the data was shown in Fig. 3b. After treated with compound 6a-1, SD rats travelled longer distance than the ones did not treated drugs, which improved the travelled distance by 131.2%. When rats were treated with Fluoxetine or Agomelatine, the percentage of improved travelled distance was 69.8% and 91.2%, respectively. The above data implied that compound 6a-1 can treat the depressant behavior in SD rats model.
Measurement of intracellular GSH and ROS level
Accumulation of oxidative stress has been noted in the brain of stress-induced animal models and oxidative stress is considered to be a mechanism of major depression46. High corticosterone level positively correlates with the oxidative stress in stress-induced animal models and stress-triggered depression patients. It helps antidepressant mechanism elucidation to investigate the anti-oxidative effects of our compound. The GSH level and ROS level of vehicle-treated PC12 cells were designated as 100%. In corticosterone injured PC12 cells, the GSH level was reduced to 60.9%, compared with vehicle control (Fig. 4a). However, compound 6a-1 can antagonize the GSH down-regulation induced by corticosterone effectively, where the GSH level was 87.7%. The data for Fluoxetine and Agomelatine were 96.3% and 93.4%, respectively. On the other hand, the ROS level in corticosterone injured PC12 cells was increased to 170.4% (Fig. 4b). Compound 6a-1 can antagonize the ROS up-regulation induced by corticosterone effectively, where the ROS level was 140.3%. The ROS level of Fluoxetine and Agomelatine were 120.7% and 125.0%. The remarkable decrease of ROS level and up-regulation of GSH level induced by compound 6a-1 may alleviate the oxidative stress in nerve cells, which may improve the treatment of depressed subjects.
Mechanism studies in vitro of compound 6a-1.
Effect of compound 6a-1 regulate the total GSH level or intracellular ROS level or BDNF mRNA levels of corticosterone injured PC12 cells. PC12 cells were treated with normal saline (control); 200 μM of corticosterone (vehicle); 200 μM of corticosterone and 5 μM of drugs, respectively; Values are expressed as mean ± S.D. (n = 6) *P < 0.05.
Detection of mRNA level of BDNF and NGF
To examine whether brain-derived neurotrophic factor Trophic factor (BDNF) and nerve growth factor (NGF) were implicated in the neuoprotective effects of compound 6a-1 on corticosterone injured PC12 cells. The BDNF mRNA level of corticosterone injured PC12 cells decreased by 87.8% compared with control (Fig. 4c). Compound 6a-1 was shown to up-regulate the BDNF level by 42.8% compared with corticosterone-injured group, while the Agomelatine and fluoxetine only increased mRNA levels of BDNF by 37.2% and 32.1% compared with corticosterone-injured group. These data suggested BDNF play a part in protective effect of compound 6a-1 on corticosterone-injured PC12 cells. On the other hand, the NGF mRNA levels almost remained untouched among different groups (See see Supplementary material Figure S4).
Hoechst 33258 staining
It is well established that endogenous oxidative stress can induce neuron cell apoptosis47. To detect the antagonism effect of compound 6a-1 on corticosterone-induced PC12 cells apoptosis, we took advantage of Hoechst 33258 staining (see Supplementary material Figure S5). Cells exhibiting abnormal nuclei (crenation, condensation, and fractionation) were regarded as apoptotic cells. It is no difficulty finding out that apoptosis of corticosterone injured PC12 cells were the most severe. Compound 6a-1effectively reversed apoptosis of PC12 cells induced by corticosterone.
Intracellular calcium ion concentration analysis
Although the calcium intake is helpful for the depression patients, intracellular Ca2+ overload can trigger either necrotic or apoptotic cell death. And prevention of intracellular Ca2+ overload will be of importance for neuroprotection48. Thus we employed Thermo Scientific Array Scan Infinity to test fluorescence intensity of free intracellular calcium ion (see Supplementary material Figure S6). As the results showed, in corticosterone-injured PC12 cells, free calcium ion concentration was obviously overloaded compared with normal control. Fluoxetine and Agomelatine exhibited weaker fluorescence intensity than corticosterone-injured PC12 cells, suggested their suppression on calcium ion overload. To our delight, compound 6a-1 demonstrated much weaker fluorescence intensity than Fluoxetine and Agomelatine, implied compound 6a-1 may possess better neuroprotective activity.
Cell survival, proliferation and maturation in rat hippocampus
It has been clarified that cell proliferation and neurogenesis are generally reduced in animal models of depression and increased by chronic antidepressant treatments49. Bromodeoxyuridine (5-bromo-2′-deoxyuridine, BrdU) is a synthetic nucleoside that is an analog of thymidine and commonly used in the detection of cell survival and proliferation in living tissues. To evaluated effects of compound 6a-1 on cell survival and proliferation after 21 days treatment, BrdU was injected either at beginning (survival) or the end (proliferation) of drug treatments. Rats were treated once daily with compound 6a-1 or Agomelatine (i.p. 40 mg/kg). Hippocampal BrdU-labeled cells were quantified and the results showed the number of BrdU-labeled neuron cells were remarkably increased after compound 6a-1 treatment in both survival (Fig. 5a, Supplementary material Figure S7c) and proliferation (Fig. 5b, Supplementary material Figure S7d) groups, much significantly than that of Agomelatine. The data indicated that compound 6a-1 increases neuron cell survival and proliferation in vivo.
Mechanism studies in vivo of compound 6a-1.
Analysis of survival, cell proliferation, maturation and BNDF level in the rat hippocampus. (a) Compound 6a-1 increases cell survival in vivo; (b) Compound 6a-1 increases cell proliferation in vivo; (c,d) Compound 6a-1 increases cell maturation in vitro. Results are means ± SD of the number of BrdU-labeled cells for n = 6rats per group. (e) Compound 6a-1 increases BDNF levelin vivo. Results are means ± SD pg/mg total protein for six rats per group.
On the other hand, the degree of maturation of newly formed cells labeled with BrdU in vivo was determined at 21 days of development by a combination of polysialic acid form of neural cell adhesion molecule (PSA-NCAM) and a neuronal nuclear antigen (NeuN) labeling. Treatments with either compound 6a-1 or Agomelatine for 21 days reduced the expression of PSA-NCAM compared with vehicle-treated group. The compound 6a-1 group showed an approximately three-fold decrease in the number of PSA-NCAM-labeled cells than that of Agomelatine group (Fig. 5c, Supplementary material Figure S7a). In addition, compared with vehicle-treated group, only compound 6a-1 induced a significant (P < 0.001) increase in the number of NeuN cells (Fig. 5d, Supplementary material Figure S7b). The results showed a highly significant increase in number of mature neurons after chronic treatment with compound 6a-1.
Detection of BDNF, VEGF and IGF-1 Level In Vivo
As accumulating evidence supports that antidepressants stimulate growth factors such as BDNF, IGF-1 and/or VEGF expression generally enhance adult neurogenesis and may exert behavioral antidepressant-like effects50,51. Levels of VEGF, IGF-1 and BDNF protein were measured in the hippocampus after 21-day treatment (i.p. 40 mg/kg). Hippocampal extracts were analyzed by ELISA assays. Compared with Agomelatine treatment, compound 6a-1 induced similar increase in hippocampal BDNF level (Fig. 5e), while neither compound 6a-1 nor Agomelatine exerted any influence on the level of VEGF, IGF-1 when compared with vehicle-treated group (see Supplementary material Figure S8).
In vivo toxicity evaluation
Compound 6a-1 was administered to adult C57 mice in an intragastric manner at dose of 140 mg/kg/day. No visible clinical signs of toxicity, such as irritability, twisting, righting reflex, tremors, convulsions, breathing, weight loss, or death, were observed during 7-day administration. Ten mice of each group were sacrificed on the 7th day, their heart, liver, spleen, lung and kidney were harvested and H&E histological staining was performed. Figure 6A,F represents an optical micrograph of heart tissue of mice treated with compound 6a-1 and Agomelatine, respectively. Cardiac myocytes were clear and arranged in good order, with no necrosis, hemorrhage or inflammatory exudates were observed in Agomelatine group. As for compound 6a-1 group, cardiac myocytes were fragmented and arranged promiscuously after treated with compound 6a-1. The hepatic cords were distinctly clear in the group of compound 6a-1(Fig. 6B), while in Agomelatine group (Fig. 6G) they were severely damaged. As shown in Fig. 6C,H, the tissue structure of the spleen in two groups were unchanged, spleen sinus did not show any pathological changes. The lung and kidney treated with compound 6a-1 (Fig. 6D,E) also did not show any significant difference compared with normal. The above results showed that liver toxicity, the major concern of Agomelatine, was not observed in compound 6a-1.
Inhibitory effects of compound 6a-1 on H9C2 cells growth and hERG K+ channel
Although compound 6a-1 significantly decreased the hepatotoxicity, the unexpected cardiotoxicity raised a critical issue and drew our concerns. It is desirable to clarify the underlying mechanism for further structural optimization. Then we firstly evaluated the in vitro cytotoxicity of compound 6a-1 on H9C2 cells, a permanent cell line derived from rat cardiac tissue. The IC50 value of compound 6a-1 was 455.0 μM, higher than Agomelatine (374.7 μM, Table S4). This result implicated the compound 6a-1 has little effects on H9C2 cells growth.
As we know, the in vitro hERG potassium channel activity in mammalian cell lines can be tested to predict QT prolongation risk, which is frequently associated with potentially lethal arrhythmias42. Thus the effects of compound 6a-1 on hERG channel was further investigated to clarify its QT prolongation risk. As a result, we tested the hERG K+ channel inhibition of compound 6a-1 (Table S4) and the IC50 value was over 40 μM, indicated that compound 6a-1 has minor effects on hERG K+ channel.
From above in vitro studies, a reasonable interpretation of the observed in vivo cardiotoxicity could not be reached and more studies are still needed. Interestingly, Neferine, one of the four natural products we used to extract the chemical scaffold in this paper, was reported to possess potential cardiotoxicity by disruption of calcium homeostasis52. In our case, compound 6a-1 downregulated the calcium overload in corticorsterone-injured PC12 cells. It will help to decipher the intrinsic relationship between these two observations and suggest a probable direction in future studies.
Test on FLIPR assays
Given that 5-HT2C receptor was involved in the effect of Agomelatine on cell proliferation, maturation and survival, we tested compound 6a-1 on 5-HT2C antagonism effects with FLIPR assays. As shown in Table S5, the IC50 value of compound 6a-1 was higher than 100 μM, indicated the major biological target of compound 6a-1 is different from Agomelatine. This observation tells us that there may be a possibility that novel bioactive compounds discovered from scaffold hopping strategy may take biological effects with changed biological target(s).
Conclusions
Based on virtual screening of CNPD library with pharmacophores generated from the marketed drug Agomelatine, 3, 4-dihydroisoquinoline scaffold was selected as new scaffold of novel Agomelatine analogues for further chemical synthesis and structure-activity relationship investigation. Accordingly, fifty-six novel 3, 4-dihydroisoquinoline compounds were synthesized by altering the C-1 substituted groups of the skeleton. The compounds 6a-1 and 6a-2 with small steric hindrance were found to possess highly neuroprotective effects on corticosterone-injured PC12 cells. Further results from in vitro cytotoxicities tests of compound 6a-1 on HEK293 and L02 cells, and BBB permeation ability make it worthy of in vivo animal studies. The FST and OFT of SD rats implied compound 6a-1 possesses obvious antidepressant effects on animal models. Mechanism studies implicated that compound 6a-1 can significantly reduce PC12 cell apoptosis by up-regulation of GSH and down-regulation of ROS in corticosterone-induced lesion of PC12 cells. Meanwhile, the down-regulation of calcium ion concentration and up-regulation of BDNF level in PC12 cells may account for the neuroprotective effects. Analysis of cell survival, cell proliferation and maturation in the rat hippocampus show compound 6a-1 can increase cell survival and cell proliferation, promote cell maturation after a chronic treatment. Besides, the acute toxicity data in vivo indicated compound 6a-1 exhibited less hepatotoxicity than Agomelatine. Although more studies are needed to elucidate the exact action molecular target(s) and mechanism of the observed cardiotoxicity in vivo, compound 6a-1 provides us insights for further structural optimization and discovery of novel antidepressant agents with neuroplasticity mechanism. Our study also demonstrated a successful scaffold hopping approach in the process of drug lead discovery by a combination of in silico screening and knowledge-based analysis.
Experiment Section
Corticosterone-induced PC12 cells lesion and protective effects of the 3, 4-dihydroisoquinoline compounds
PC12 cells were purchased from American Type Culture Collection and were maintained in DMEM medium supplemented with penicillin (100 unit/ml), streptomycin (100 μg/ml), 5% fetal bovine serum (FBS), and 10% horse serum at 37 °C in humidified atmosphere with 5% CO2. The detailed procedures were performed referring to the reported literatures6. Briefly, PC12 cells of logarithmic growth phase were collected, re-suspended and then seeded at a density of 1 × 105 cells per well in 96-well plates and cultured in the DMEM medium with 5% horse serum, 10% FBSfor 24 h.
After that, the upper medium in the 96-well plates was absorbed and then the PC12 cells were treated with 100 μl of 200 μM corticosterone for 1 h and then respectively co-incubated with Fluoxetine, Agomelatine or other compounds for another 24 hour.
After incubation with compounds, 20 μl of 5 mg/ml MTT solution was added to each well and cultured at 37 °C for 2 h–4 h. Then, the culture medium was removed, and 150 μl dimethyl sulfoxide(DMSO) was added to each well for 15 min at room temperature. The absorbance of each well was measured at 570 nm using a microplate reader. The protective rates (PR) were calculated according to equation (1).

Cytotoxicity evaluation
Human normal hepatic L02 cells, human embryonic kidney cell line 293 cells (HEK293) and Rattus myoblast cell line H9C2 cells were respectively seeded in 96-well plates at a density of 4 × 103 cells per well and cultured in the 1640 or DMEM medium, with the supplement of 10% fetal bovine serum, penicillin (100 unit/ml), streptomycin (100 μg/ml) in a humidified incubator with 5% CO2 for 24 h. After adherence, L02, HEK293 and H9C2 cells were exposed to different compounds for another 48 h. The cytotoxicities of the compounds were evaluated by MTT method.
BBB permeation ability measurement
Parallel artificial membrane permeation assay (PAMPA) was performed according to the literature44 with minor modification. The PVDF (Poly(vinylidene fluoride)) membrane of the donor plate was coated with PBL (Polar Brain Lipid, Avanti, USA) in dodecane (4 μL of 20 mg/mL PBL in dodecane) and the acceptor well was filled with 350 μL of PBS/EtOH (70/30, v/v, pH 7.4) buffer (VD). The tested compound was dissolved in DMSO and then diluted with PBS/EtOH (70/30, v/v, pH 7.4) buffer to reach the concentration of 100 μg/mL in the donor well.
The concentration of DMSO did not exceed 0.5% (v/v) in the donor solution. 200 μL of the donor solution was added to the donor wells (VA) and the donor filter plate was carefully put on the acceptor plate so that the coated membrane contacted both donor solution and acceptor buffer. Test compound diffused from the donor well through the lipid membrane (area = 0.28 cm2) to the acceptor well. The concentration of the drug in both donor and the acceptor wells was assessed after 18 hours of incubation at room temperature in triplicate using Varioskan Flash Multimode Reader (Thermo Scientific) at the maximum absorption wavelength of tested compound. Concentration of the compound was calculated from the standard curve and expressed as the permeability (Pe) according to Equation (2):

Animals
All animal methods in this study were carried out in accordance with guidelines and regulations of the Ethical Committee of Sichuan University for the use of Laboratory Animals and approved by the Institutional Animal Care and Treatment Committee of Sichuan University and all animals in this study were treated humanely throughout the experimental period. Sprague Dawley (SD) rats weighing 200–230 g were placed individually in cages maintained at 25 °C and were fasted overnight but free access to water before experiments. 6~8 week-old male Wistar rats were group-housed under standard conditions (12-h light/dark cycle, 22 ± 2 °C, food and water ad libitum). BALB/c mice (8 to 12 weeks old; 20–30 g) used in this study were kept in temperature controlled (24 ± 1 °C) rooms with food and water given ad libitum. The detailed procedures were described in the following different experiments.
Forced swim test
The detailed procedures were performed according to our previous studies6,34. Briefly, each SD rat was placed in a vertical Plexiglas cylinder (height 50 cm; diameter 20 cm) containing 18 cm height of water at 25 ± 2 °C and was forced to swim individually for 15 min on1st day. From 2nd day to 15th day, the rats were intragastrically administered compound 6a-1 or control drugs at dosage of 32 mg/kg/day. On the 2nd day and 15th day, 30 minutes after drug treatment, each rat was placed again into water and forced to swim for 6 min. The rat behavior was recorded by a video camera placed directly facing cylinders. The duration of immobility during the last 4 minutes was analyzed by Xeye Animal behavior analysis system (Xeye Aba V3.2). The rat was considered as immobile when its moving speed was less than 20 mm/s. The dosage of is 32 mg/kg every day. The water in the cylinder was changed each trial. After each exposure, animals were partially dried with a towel and returned to their home cages. All tests were performed in a quiet room.
Open field locomotor activity
The detailed procedures were performed according to our previous studies6,34. In brief, each SD rat was placed at the center of the open field (50 × 50 cm2 chamber, 50-cm-high walls, with a 25 cm2 area in the middle of floor defined as the central square) for 6 min in a quiet room. The animals were gently placed in the center of the platform and were allowed to explore the surroundings. A camera was installed above the center of the field. Immediately after a rat was placed at the center in the open field, the movements and position of the animals were recorded and registered automatically by computerized system. Next test was performed after cleaning the chamber. The traveled tracks of rats were recorded for 6 minutes and the videos of the last 4 minutes were analyzed also by Xeye Animal behavior analysis system.
Measurement of intracellular ROS and GSH level
The detailed procedures for ROS level detection were performed according to the reported literatures53,54. In brief, PC12 cells were washed with D-Hanks after drug treatment. Then cells were incubated with 2′, 7′-dichloro-fluorescein diacetate (DCF-DA, 20 μM) for 30 min at 37 °C in darkness. The ROS induced fluorescence intensity was measured by microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
The GSH level was measured by reference of literatures’ methods55,56 with appropriate revision. Briefly, A total number of 1 × 106 cells treated with compound 6a-1 or positive controls were collected and centrifuged at 2000 rpm for 5 min and the cell pellets were lysed using ultrasonic irradiation in 200 μL of ice-cold RIPA lysis buffer with protease inhibitor cocktail. After being incubated on ice for 10 min, the lysate was centrifuged at 10 000 rpm for 10 min. 100 μL of the supernatant was mixed with 200 μL of trichloroacetic acid (25%) and 200 μL of saline. The mixture was centrifuged at 3000 × g for 10 min at 4 °C, and then 200 μL of the resulting supernatant was mixed with 1 mL of phosphate buffer (100 mM, pH 8.0) and 50 μL of 5, 5-dithiobis-2-nitrobenzoic acid (DTNB). The solution was maintained at room temperature for 5 min and its absorbance measured at 412 nm.
Real-time PCR detection mRNA level of BDNF and NGF
The detailed procedures were performed according to the reported literatures57,58. Briefly, total RNA from hippocampus of drug treated C57 mice was isolated using TRIzol® reagent (Invitrogen, USA) according to the manufacturer’s instructions. Quantification of mRNAs was performed on Bio-rad CFX96TM Real-Time PCR Detection System (Bio-rad, USA). The sequences of gene-specific primers were as follows: BDNF forward primer, CCCATCACAATCTCACGGTA, BDNF reverse primer, ACAGGACGGAAACAGAACGA; NGF forward primer, CCTTCAACAG -GACTCACAGGA, NGF reverse primer, TCTCCAACCCACACACTGAC.
Hoechst 33258 staining
To detect apoptotic cells, drug treated cells were stained with the DNA dye Hoechst 33258. Cells with the indicated treatment were fixed with methanol for 10 min at 4 °C before incubation with Hoechst 33258 for 10 min at room temperature. After washes with PBS, the apoptotic cells were mounted onto slides and observed under the fluorescence microscope BX61 (Olympus, Japan). Images were captured using DP71 CCD digital camera (Olympus).
Intracellular Calcium ion concentration analysis
The detailed procedures were performed according to the reported literatures59. In brief, calcium ion concentration was measured by Fura-2 AM (Thermo Scientific). PC12 cells were treated with compound 6a-1 for 48 hours. Then cells were detached with 0.25% trypsin and centrifuged at 1000 r/min for 5 minutes. The supernatant was removed and the cells were stained with Fura-2 AM according to the manufacturer’s instructions. Fluorescence intensity of labeled cells was photographed for 25 random sights each group using Thermo Scientific Array Scan Infinity.
Analysis of cell survival, proliferation and maturation in the rat hippocampus
The detailed procedures were performed according to the reported literatures49.
Wistar rats were used herein. Agomelatine were purchased from Dalian Meilun Biotech Company. Compound 6a-1 (40 mg/kg) or Agomelatine (40 mg/kg) was injected as a suspension in 1% Hydroxyethyl cellulose at 40 mg/kg i.p. once a day for 21 days. For cell proliferation study, BrdU (200 mg/kg i.p.) was administered 2 h before perfusion. For cell maturation and survival studies, animals received five injections of BrdU (75 mg/kg, 2 h intervals) the first day of treatment and were killed 21 days later. Each mouse was randomly selected 8 visual fields for statistical analysis.
BDNF levels measurement in vivo
The detailed procedures were performed according to the reported literatures49.
SD rats were killed by decapitation and hippocampi were dissected out and stored at −80 °C until use. Tissue samples were homogenized at 4 °C in Promega lysis buffer for ELISA. Samples were then sonicated and lysates were cleared by centrifugation at 4 °C, 15 000 g. Protein concentrations were determined according to the Bradford assay using bovine serum albumin as standard. Quantifications of VEGF and IGF-1 proteins were measured with Quantikine M mouse VEGF and IGF-1enzyme immunoassay kits and BDNF protein with a mouse ELISA kit.
Acute toxicity evaluation
In brief, all C57 mice were carefully observed after administration of compound 6a-1 or Agomelatine at doses of 140 mg/Kg/day for their general conditions. Ten mice of each group were sacrificed on the 7th day, their heart, liver, spleen, lung and kidney were harvested and H&E histological staining was performed. Then the stained tissues were observed by two pathologists in a blinded manner.
Inhibition evaluation on hERG K+ channel
The detailed procedures were performed according to the reported literatures60. In brief, A Chinese hamster ovary (CHO) cell line stably expressing hERG potassium channels were cultured into appropriate cell density. Then cells were automatically prepared for application to chips. Whole-cell recordings were performed using automated QPatch (Sophion) and the data were analyzed using Assay Software provided by Graphpad Prism 5.0.
FLIPR assays for 5-HT2c antagonism
Seed cells at the density of 10 K cell/well, 37 °C, 5% CO2 incubate for 16–24 hours, then loading cells with 30 ul of Calcium 5 and 37 °C, 5% CO2 incubate for 1 hour. After transfer compound to compound plate with 30 ul Assay Buffer by Echo, add 15 ul/well of compound, incubate for 10 mins. Then 22.5 ul/well of inducer were added and measure calcium flux signal with FLIPR.
Statistical analysis
Data are showed as mean ± S.D. Statistical analyses were performed with one-way ANOVA followed by Dunnett’s test for multiple comparison procedures. The statistics were performed by GraphPad Prism 5.0 software. The level of statistical significance was set at P < 0.05. (*P < 0.05, **P < 0.01,***P < 0.001).
Chemistry
All solvents and reagents were analytical reagents and used directly without further purification. All melting points were determined on a SGW X-4 MicroMelting Point apparatus without corrected. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance (Varian Unity Inova) 400 MHz spectrometer, with TMS took as internal reference chemical shift in δ, ppm. High-resolution mass (HRMS) spectrometry was carried out on a Waters Q-TOF Premier mass spectrometer. All compounds used for biological assays were at least of 98% purity based on HPLC analytical results monitored with full wavelengths.
3-(1,3-Dioxoisoindolin-2-yl) propanoic acid (2)
To a flask was added 1, 3-isobenzofurandione(300.0 g, 2.03 mol), β-alanine (180.0 g, 2.03 mol) and acetic acid (2 L) and then refluxed at 120 °C for 4 h. After the completion of reaction, the solvent was evaporated under reduced pressure and the solid residue was washed with water to pH 7 and dried to afford the title compound(421.2 g, yield: 95%), mp: 150–151 °C. The NMR and MS spectral data were in accordance with the literature.
3-(1,3-Dioxoisoindolin-2-yl)-N-(4-methoxyphenethyl) propanamide(3)
Compound 2 (384.0 g,1.75 mol), 2-(4-methoxyphenyl) ethanamine hydrochloride(394.1 g, 2.10 mol) and EDCI (671.7 g, 3.50 mol) were dissolved in 4 L pyridine. After stirring at room temperature for 4 h, the resulting mixture was concentrated under reduced pressure, then buffered to pH 2 with hydrochloric acid and extracted with DCM (500 mL × 3). The combined organic layer were concentrated under reduced pressure to obtained the crude product, which was recrystallized with ethyl acetate. The title compound was obtained as pale white powder (483.6 g, yield: 78.3%). mp: 150–151 °C. 1H-NMR (CDCl3): δ 7.84 (dd, J1 = 5.6 Hz, J2 = 3.2 Hz, 2H), 7.19 (dd, J1 = 5.2 Hz, J2 = 2.8 Hz, 2H), 7.08 (d, J = 4.2 Hz, 2H), 6.81 (d, J = 8.4 Hz, 2H), 5.06 (br, 1H), 3.99 (t, J = 7.2 Hz, 2H), 3.78 (s, 3H), 3.48 (dd, J1 = 12.8 Hz, J2 = 6.8 Hz, 2H), 2.73 (t, J = 7.2 Hz, 2H), 2.57 (t, J = 7.2 Hz, 2H); MS (ITMS) m/z calcd. for C20H20N2O4 [M+H+]: 352.14, found: 352.14.
2-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) isoindoline-1,3-dione(4)
Compound 3 (70 g, 0.20 mol) were dissolved in xylene (400 mL), and then P2O5 (36 g, 0.25 mol) and POCl3 (110 mL) were added in order. The mixture was heated to 125 °C and string. After 4 h, the xylene was removed and the mixture was buffered to pH 10 with hydrochloric acid, extracted with ethyl acetate and DCM (EA/DCM: 3/1, 800 mL × 5). The organic layer was combined and evaporated and the crude products was separated by flash column (elute DCM/MeOH: 100/1) to obtained the title compound as yellow solid (33.5 g, yield 50.8%), mp: 161–163 °C; 1H-NMR (DMSO-d6): δ 7.81 (dd, J1 = 5.6 Hz, J2 = 3.2 Hz, 2H), 7.69 (dd, J1 = 5.6 Hz, J2 = 2.8 Hz, 2H), 7.09–7.06 (m, 2H), 6.84 (dd, J1 = 8.4 Hz, J2 = 2.4 Hz, 1H), 4.07 (dd, J = 7.6 Hz, 2H), 3.81 (s, 3H), 3.64 (t, J = 7.6 Hz, 2H), 3.08 (t, J = 7.2 Hz, 2H), 2.61 (t, J = 7.2 Hz, 2H); 13C NMR (DMSO-d6): δ 167.72, 163.61, 158.12, 134.27, 131.61, 129.13, 128.97, 128.29, 122.87, 116.11, 110.08, 55.17, 46.57, 35.44, 33.28, 24.25; HRMS (TOF) m/z calcd. for C20H18N2O3 [M+H+] 335.1396, found: 335.1339.
2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethanamine hydrochloric acid (5)
To a flask with compound 4 (33.5 g, 0.1 mol) was added concentrated hydrochloric acid (500 mL) and heated to reflux for 8 h. After the completion of reaction, the solvent was evaporated. By adding acetone, a white solid was separated. After filtration, compound 5 was obtained as pale white solid (20.9 g, yield 86.7%). 1H NMR (DMSO-d6): δ 7.58 (s, 1H), 7.44 (d, J = 8.4 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 3.89 (s, 3H), 3.80 (t, J = 8.0 Hz, 2H), 3.61 (t, J = 7.2 Hz, 2H), 3.23–3.22 (m, 2H), 3.03 (t, J = 7.6 Hz, 2H).
General procedure for the preparation of compound 6a
A-with different substituted acyl chlorides or sulfonyl chlorides or anhydrides
2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethanamine hydrochloride (5) (300 mg, 1.43 mmol) and K2CO3 (494 mg, 3.58 mmol) was dissolved in CH2Cl2 (10 mL) and DMF (2 mL), then appropriate substituted acyl chloride or sulfonyl chloride or anhydride (3.12 mmol) was added dropwise under ice bath. After stirring at room temperature for 10 h, the reaction mixture was diluted with CH2Cl2 (20 mL), then washed with H2O (20 mL). The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure and the residue was purified by chromatography or preparative TLC (eluent DCM/MeOH:80/1, v/v) to afford the desired crude product. The crude product was then dissolved in acetone, titrated with oxalic acid and the white solid were separated. After filtration, the oxalate form was obtained.
B- with different carboxylic acids
2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethanamine hydrochloride (5) (300 mg, 1.43 mmol) and EDCI (548 mg, 2.86 mmol) was dissolved in 10 mL pyridine, then carboxylic acid (1.72 mmol) was added. After stirring at room temperature for 10 h, the solvent was removed by evaporation and the residue was diluted with CH2Cl2 (20 mL), then washed with H2O (20 mL). The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure and the residue was purified by chromatography or preparative TLC (eluent DCM/MeOH: 80/1, v/v) to afford the desired crude product. The crude product was then dissolved in acetone, titrated with oxalic acid and the white solid were separated. After filtration, the oxalate form was obtained.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) acetamide oxalate (6a-1)
Following general procedure to obtain pure product as white solid 167 mg (yield: 39.9%). mp: 115–117 °C; 1H-NMR (D2O): 7.34 (d, 1H, J = 2.4 Hz, Ar-H), 7.31 (d, 1H, J = 8.4 Hz, Ar-H), 7.25 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 3.78 (s, 3H, -OCH3), 3.73 (t, 2H, J = 8.0 Hz, -CH2-), 3.47 (t, 2H, J = 5.2 Hz, -CH2-), 3.17 (t, 2H, J = 4.8 Hz, -CH2-), 2.93 (t, 2H, J = 8.0 Hz, -CH2-), 1.72 (s, 3H, -CH3); 13C-NMR (D2O): δ 178.3, 174.3, 164.8, 158.4, 130.7, 129.9, 125.6, 123.3, 114.8, 55.8, 41.6, 37.5, 33.9, 23.6, 21.3; HRMS (TOF) m/z calcd. for C14H18N2O2 [M+H+]: 247.1450, found: 247.1447.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) propionamide oxalate (6a-2)
Following general procedure to obtain pure product as white solid 154 mg (yield: 30.7%). mp: 103–105 °C; 1H-NMR (DMSO-d6): δ 8.35 (br, s, 1H, -NH-), 7.60 (s, 1H, Ar-H), 7.41 (d, 1H, J = 8.4 Hz, Ar-H), 7.34 (d, 1H, J = 8.0 Hz, Ar-H), 3.87 (s, 3H, -OCH3), 3.73 (t, 2H, J = 7.6 Hz, -CH2-), 3.41 (t, 2H, J = 5.2 Hz, -CH2-), 3.31 (t, 2H, J = 4.8 Hz, -CH2-), 2.98 (t, 2H, J = 8.4 Hz, -CH2-), 1.99 (d, 2H, J = 8.4 Hz, -CH2-), 0.88 (t, 3H, J = 7.2 Hz, -CH3); 13C-NMR (DMSO-d6): δ 176.5, 173.6, 161.5, 158.5, 130.1, 129.6, 126.3, 122.1, 114.6, 55.8, 40.9, 37.3, 33.1 28.3, 23.4, 9.6; HRMS (TOF) m/z calcd. for C15H20N2O2 [M+H+]: 261.1603, found: 261.1580.
2,2,2-Trifluoro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) acetamideoxalate (6a-3)
Following general procedure to obtain pure product as white solid 89 mg (yield: 18.3%). mp: 41–43 °C; 1H-NMR (DMSO-d6): δ 8.32 (br, s, 1H, -NH-), 7.56 (s, 1H, Ar-H), 7.36 (d, 1H, J = 8.0 Hz, Ar-H), 7.17 (d, 1H, J = 8.0 Hz, Ar-H), 3.87 (s, 3H, -OCH3), 3.73 (t, 2H, J = 8.4 Hz, -CH2-), 3.41 (t, 2H, J = 5.2 Hz, -CH2-), 3.23 (t, 2H, J = 4.8 Hz, -CH2-), 2.79 (t, 2H, J = 8.0 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 178.3, 173.2, 164.6, 158.5, 157.6, 130.2, 129.3, 128.7, 119.4, 116.8, 113.9, 55.7, 46.4, 37.4, 33.2; HRMS (TOF) m/z calcd. for C14H15F3N2O2 [M+H+]: 301.1164, found: 301.1245.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) cyclopropanecarboxamideoxalate (6a-4)
Following general procedure to obtain pure product as white solid 130 mg (yield: 35.9%). mp: >220 °C; 1H-NMR (DMSO-d6): δ 8.71 (br, s, 1H, -NH-), 7.72 (s, 1H, Ar-H), 7.53 (d, 1H, J = 8.0 Hz, Ar-H), 7.21 (d, 1H, J = 8.4 Hz, Ar-H), 3.83 (s, 3H, -OCH3), 3.73 (t, 2H, J = 7.6 Hz, -CH2-), 3.43 (t, 2H, J = 5.6 Hz, -CH2-), 3.28 (t, 2H, J = 4.8 Hz, -CH2-), 2.93 (t, 2H, J = 8.4 Hz, -CH2-), 1.32 (m, 1H, -CH-), 1.16 (m, 2H, -CH2-), 0.97 (m, 2H, -CH2-); 13C-NMR (DMSO-d6): δ 177.1, 173.8, 164.4, 158.5, 130.2, 129.4, 127.7, 121.5, 114.5, 55.6, 42.3, 37.4, 33.2, 25.5, 16.7, 8.7; HRMS (TOF) m/z calcd. for C16H20N2O2 [M+H+]: 273.1603, found: 273.1578.
4-Chloro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamideoxalate (6a-5)
Following general procedure to obtain pure product as gray solid 160 mg (yield: 46.7%). mp: 90–92 °C; 1H-NMR (DMSO-d6): δ 8.95 (br, s, 1H, -NH-), 7.77 (d, 2H, J = 8.4 Hz, Ar-H), 7.57–7.52 (m, 3H, Ar-H), 7.38 (d, 1H, J = 8.0 Hz, Ar-H), 7.26 (d, 1H, J = 7.6 Hz, Ar-H), 3.82 (s, 3H, -OCH3), 3.72 (t, 2H, J = 8.4 Hz, -CH2-), 3.62 (t, 2H, J = 5.6 Hz, -CH2-), 3.30 (t, 2H, J = 4.8 Hz, -CH2-), 2.91 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.7, 165.7, 162.8, 158.4, 136.1, 129.9, 129.4, 129.0, 128.3, 126.7, 121.0, 114.0, 55.6, 42.1, 37.9, 33.6, 23.6; HRMS (TOF) m/z calcd. for C19H19ClN2O2 [M+H+]: 343.1213, found: 343.1110.
4-Methoxy-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamideoxalate (6a-6)
Following general procedure to obtain pure product as pale yellow oil 192 mg (yield: 44.8%). 1H-NMR (DMSO-d6): δ 8.73 (br, s, 1H, -NH-), 7.74 (d, 2H, J = 8.4 Hz, Ar-H), 7.55 (d, 1H, J = 8.4 Hz, Ar-H), 7.36 (d, 1H, J = 7.6 Hz, Ar-H), 7.24 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 6.96 (d, 2H, J = 8.0 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.79 (s, 3H, -OCH3), 3.70 (t, 2H, J = 8.4 Hz, -CH2-), 3.60 (t, 2H, J = 5.2 Hz, -CH2-), 3.28 (t, 2H, J = 4.8 Hz, -CH2-), 2.89 (t, 2H, J = 8.0 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.3, 166.2, 163.2, 161.6, 158.4, 129.9, 129.3, 128.9, 126.9, 126.1, 120.9, 113.9, 113.4, 55.6, 42.2, 37.8, 33.8, 30.7, 23.6; HRMS (TOF) m/z calcd. for C20H22N2O3 [M+H+]: 339.1709, found: 339.1609.
2-Chloro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamide oxalate (6a-7)
Following general procedure to obtain pure product as white solid 274 mg (yield: 44.2%). mp: >220 °C; 1H-NMR (DMSO-d6): δ 9.07 (br, s, 1H, -NH-), 7.73 (t, 2H, J = 8.4 Hz, Ar-H), 7.51–7.45 (m, 3H, Ar-H), 7.35 (s, 1H, Ar-H), 7.19 (d, 1H, J = 8 Hz, Ar-H), 3.83 (s, 3H, -OCH3), 3.75 (t, 2H, J = 7.6 Hz, -CH2-), 3.59 (t, 2H, J = 5.6 Hz, -CH2-), 3.13 (t, 2H, J = 4.8 Hz, -CH2-), 2.97 (t, 2H, J = 8.0 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.8, 165.5, 162.3, 158.3, 135.4, 132.8, 131.3, 129.2, 128.3, 126.7, 120.9, 115.5, 55.9, 44.7, 37.9, 33.9, 24.6; HRMS (TOF) m/z calcd. for C19H19ClN2O2 [M+H+]: 343.1213, found: 343.2206.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) -4-(trifluoromethyl) benzamideoxalate (6a-8)
Following general procedure to obtain pure product as yellow solid 78 mg (yield: 16.7%). mp: >220 °C; 1H-NMR (DMSO- d6): δ 8.93 (br, s, 1H, -NH-), 7.73 (d, 2H, J = 8.4 Hz, Ar-H), 7.63 (s, 1H, Ar-H), 7.36 (d, 2H, J = 8.0 Hz, Ar-H), 7.28 (d, 1H, J = 7.6 Hz, Ar-H), 7.21 (dd, 1H, J1 = 8.4 Hz, J2 = 2.8 Hz, Ar-H), 3.83 (s, 3H, -OCH3), 3.73 (t, 2H, J = 8.0 Hz, -CH2-), 3.58 (t, 2H, J = 5.6 Hz, -CH2-), 3.31 (t, 2H, J = 4.8 Hz, -CH2-), 2.93 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 176.6, 166.7, 163.5, 158.5, 137.9, 133.6, 129.8, 129.7, 128.4, 127.1, 124.9, 123.3, 120.8, 114.3, 55.9, 45.1, 37.5, 34.3, 25.5. HRMS (TOF) m/z calcd. for C20H19F3N2O2 [M+H+]: 377.1477, found: 377.1724.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) furan-2-carboxamideoxalate (6a-9)
Following general procedure to obtain pure product as pale gray solid 497 mg (yield: 90.3%). mp: >220 °C; 1H-NMR (DMSO-d6): δ 8.85 (t, 1H, J = 5.6 Hz, -NH-), 7.82 (s, 1H, Ar-H), 7.62 (d, 1H, J = 2.0 Hz, Ar-H), 7.40 (d, 1H, J = 8.4 Hz, Ar-H), 7.31 (d, 1H, J = 8.4 Hz, Ar-H), 7.10 (d, 1H, J = 3.2 Hz, Ar-H), 6.60 (s, 1H, Ar-H), 3.83 (s, 3H, -OCH3), 3.75 (t, 2H, J = 7.6 Hz, -CH2-), 3.59 (t, 2H, J = 5.6 Hz, -CH2-), 3.37 (t, 2H, J = 6.0 Hz, -CH2-), 2.98 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 176.3, 161.4, 158.5, 158.1, 154.5, 147.4, 145.2, 130.1, 129.5, 126.3, 114.5, 113.7, 111.8, 55.7, 37.3, 33.3, 33.4; HRMS (TOF) m/z calcd. for C17H18N2O3 [M+H+]: 299.1396, found: 299.2260.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl)benzamide oxalate (6a-10)
Following general procedure to obtain pure product as pale yellow grease 362 mg (yield: 82.1%). 1H-NMR (DMSO-d6): δ 8.98 (br, s, 1H, -NH-), 7.95 (d, 1H, J = 8.4 Hz, Ar-H), 7.76 (d, 1H, J = 8.0 Hz, Ar-H), 7.61 (t, 1H, J = 7.6 Hz, Ar-H), 7.49 (t, 2H, J = 8.4 Hz, Ar-H), 7.41 (dd, 2H, J1 = 8.4 Hz, J2 = 2.8 Hz, Ar-H), 7.28 (d, 1H, J = 5.2 Hz, Ar-H), 3.83 (s, 3H, -OCH3), 3.73 (t, 2H, J = 8.0 Hz, -CH2-), 3.67 (t, 2H, J = 5.6 Hz, -CH2-), 3.31 (t, 2H, J = 4.8 Hz, -CH2-), 2.97 (t, 2H, J = 8 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 175.2, 166.7, 163.5, 158.5, 137.9, 133.6, 129.8, 128.4, 127.1, 124.9, 123.3, 120.8, 114.3, 55.9, 45.1, 37.5, 34.3, 25.5. HRMS (TOF) m/z calcd. for C19H20N2O2 [M+H+]: 309.1603, found: 309.1607.
4-(2-Bromoethyl) -N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamide oxalate (6a-11)
Following general procedure to obtain pure product as white powder 172 mg (yield: 23.8%). 1H-NMR (DMSO- d6): δ 7.89 (s, 4H, Ar-H), 7.40 (s, 1H, Ar-H), 7.34 (d, 1H, J = 8.4 Hz, Ar-H), 7.18 (d, 1H, J = 12.8 Hz, Ar-H), 3.94 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.65 (t, 2H, J = 7.6 Hz, -CH2-), 3.28 (t, 2H, J = 5.6 Hz, -CH2-), 2.79 (t, 2H, J = 4.8 Hz,-CH2-), 2.55 (t, 2H, J = 8.0 Hz, -CH2-), 1.30 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.3, 167.7, 161.9, 158.4, 142.9, 134.4, 131.5, 129.2, 129.1, 119.4, 112.3, 55.5, 43.3, 39.1, 33.5, 32.1, 31.6, 23.8; HRMS (TOF) m/z calcd. for C21H23BrN2O2 [M+H+]: 415.1021, found: 415.1307.
4-Fluoro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamide oxalate (6a-12)
Following general procedure to obtain pure product as white powder 152 mg (yield: 25.5%). mp: 149–151 °C; 1H-NMR (DMSO-d6): δ 8.92 (br, s, 1H, -NH-), 7.80 (d, 2H, J = 8.4 Hz, Ar-H), 7.53–7.50 (m, 3H, Ar-H), 7.34 (d, 1H, J = 8.0 Hz, Ar-H), 7.22–7.20 (m, 1H, Ar-H), 3.81 (s, 3H, -OCH3), 3.69 (t, 2H, J = 7.2 Hz, -CH2-), 3.61 (d, 2H, J = 5.6 Hz, -CH2-), 3.24 (t, 2H, J = 4.8 Hz, -CH2-), 2.85 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 172.7, 165.5, 162.9, 158.4, 136.1, 132.8, 129.8, 129.2, 129.1, 128.3, 127.2, 120.1, 113.4, 55.6, 42.9, 37.8, 33.8, 23.7; HRMS (TOF) m/z calcd. for C19H19FN2O2 [M+H+]: 327.1509, found: 327.1393.
4-Isopropyl-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamide oxalate (6a-13)
Following general procedure to obtain pure product as white powder 167 mg (yield: 39.9%). mp: 160–162oC; 1H-NMR (DMSO-d6): δ 9.04 (br, s, 1H, -NH-), 7.73 (d, 2H, J = 8.0 Hz, Ar-H), 7.66 (d, 1H, J = 2.0 Hz, Ar-H), 7.40 (d, 1H, J = 8.4 Hz, Ar-H), 7.30–7.27 (m, 3H, Ar-H), 3.81 (s, 3H, -OCH3), 3.71 (t, 2H, J = 7.6 Hz, -CH2-), 3.64 (d, 2H, J = 5.6 Hz, -CH2-), 3.44 (t, 2H, J = 4.8 Hz, -CH2-), 2.99 (d, 2H, J = 8.0 Hz, -CH2-), 2.95–2.90 (m, 1H, -CH-), 1.20 (d, 6H, J = 6.8 Hz, -CH3); 13C-NMR (DMSO-d6): δ 176.7, 166.7, 161.2, 158.5, 151.8, 131.4, 130.0, 129.5, 127.3, 126.4, 126.0, 122.1, 114.7, 55.8, 40.9, 38.0, 33.3, 33.1, 23.6, 23.4; HRMS (TOF) m/z calcd. for C22H26N2O2 [M+H+]: 351.2073, found: 351.1982.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl) ethyl)-1-(methylsulfonyl) methanamide oxalate (6a-14)
Following general procedure to obtain pure product as white powder 127 mg (yield: 18.6%). mp: 193–195 °C; 1H-NMR (DMSO-d6): δ 8.01 (br, s, 1H, -NH-), 7.73 (t, 1H, J = 8.0 Hz, Ar-H), 7.59 (d, 2H, J = 5.6 Hz, Ar-H), 7.42 (d, 2H, J = 8.4 Hz, Ar-H), 7.34 (m, 2H, Ar-H), 3.83–3.80 (d, 4H, J = 12.8 Hz, -CH2-), 3.72 (s, 3H, -OCH3), 3.29 (s, 1H, -CH2-), 3.17–3.13 (m, 1H, -CH2-), 2.98 (s, 1H, -CH2-), 2.35 (s, 3H, -CH3), 1.27–1.21 (m, 1H, -CH2-); 13C-NMR (DMSO-d6): δ 174.4, 166.7, 161.2, 158.5, 137.7, 131.6, 130.3, 127.9, 126.4, 119.6, 113.6, 55.8, 40.9, 38.0, 33.3, 33.1, 23.6, 23.4; HRMS (TOF) m/z calcd. for C19H22N2O3S [M+H+]: 359.1429, found: 359.1367.
2,4-Dimethoxy-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamide oxalate (6a-15)
Following general procedure to obtain pure product as white powder 286 mg (yield: 43.6%). mp: 85–87 °C; 1H-NMR (DMSO-d6): δ 8.43 (t, 1H, J = 5.6 Hz, -NH-), 7.72 (d, 1H, J = 8.4 Hz, Ar-H), 7.55 (d, 1H, J = 2.4 Hz, Ar-H), 7.35 (d, 1H, J = 7.6 Hz, Ar-H), 7.23 (d, 1H, J = 2.4 Hz, Ar-H), 6.60–6.56 (m, 2H, Ar-H), 3.86 (s, 3H, -OCH3), 3.80 (s, 3H, -OCH3), 3.79 (s, 3H, -OCH3), 3.72 (t, 2H, J = 7.6 Hz, -CH2-), 3.67 (d, 2H, J = 5.6 Hz, -CH2-), 3.31 (t, 2H, J = 4.8 Hz, -CH2-), 2.91 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.9, 164.8, 163.0, 162.9, 158.7, 158.4, 132.4, 129.8, 129.2, 127.0, 120.9, 114.0, 105.5, 98.2, 55.8, 55.6, 55.4, 42.0, 37.7, 33.6, 30.6, 23.6; HRMS (TOF) m/z calcd. for C21H24N2O4 [M+H+]: 369. 1814, found: 369. 1677.
4-Acetyl-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl) ethyl) benzamideoxalate (6a-16)
Following general procedure to obtain pure product as pale yellow grease 130 mg (yield: 20.6%). 1H-NMR (DMSO-d6): δ 9.24 (br, s, 1H, -NH-), 7.97 (d, 2H, J = 8.4 Hz, Ar-H), 7.89 (d, 2H, J = 8.0 Hz, Ar-H), 7.61 (s, 1H, Ar-H), 7.37 (d, 1H, J = 8.4 Hz, Ar-H), 7.25 (dd, 1H, J1 = 8.0 Hz, J2 = 1.6 Hz, Ar-H), 3.80 (s, 3H, -OCH3), 3.72 (t, 2H, J = 7.2 Hz, -CH2-), 3.65 (d, 2H, J = 5.6 Hz, -CH2-), 3.41 (s, 2H, -CH2-), 2.96 (t, 2H, J = 7.2 Hz, -CH2-), 2.59 (s, 3H, -CH3); 13C-NMR (DMSO-d6): δ 197.7, 175.9, 166.0, 163.0, 161.6, 158.4, 138.6, 137.6, 130.0, 128.0, 126.5, 121.7, 114.4, 55.7, 51.4, 48.54, 41.4, 38.0, 33.1, 26.9, 23.5; HRMS (TOF) m/z calcd. for C21H22N2O3 [M+H+]: 351.1709, found: 351.1729.
2-(1H-Indol-2-yl)-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)acetamide oxalate(6a-17)
Following general procedure to obtain pure product as pale yellow grease 160 mg (yield: 24.0%). 1H-NMR (DMSO-d6): δ 8.44 (d, 1H, J = 5.6 Hz, -NH-), 7.85 (s, 3H, Ar-H), 7.35 (s, 1H, Ar-H), 7.30 (d, 1H, J = 8.4 Hz, Ar-H), 7.13 (d, 1H, J = 8.0 Hz, Ar-H), 7.05–6.97 (m, 2H, Ar-H), 3.88 (t, 2H, J = 8.4 Hz, -CH2-), 3.79 (s, 3H, -OCH3), 3.69 (t, J = 5.6 Hz, -CH2-), 3.61 (t, 2H, J = 4.8 Hz, -CH2-), 3.23 (t, 2H, J = 7.2 Hz, -CH2-), 2.74 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.9, 166.0, 161.6, 157.6, 136.6, 129.8, 129.3, 128.8, 126.5, 121.7, 119.9, 114.4, 111.5, 55.7, 48.9, 41.4, 36.9, 33.1, 24.8; HRMS (TOF) m/z calcd. for C21H22N2O3 [M+H+]: 362.1869, found: 362.1826.
4-(tert-Butyl)-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)benzamide oxalate (6a-18)
Following general procedure to obtain pure product as pale yellow solid 144 mg (yield: 22.2%). mp: 63–65 °C; 1H-NMR (DMSO-d6): δ 8.87 (br, s, 1H, -NH-), 7.72 (d, 2H, J = 8.0 Hz, Ar-H), 7.60 (s, 1H, Ar-H), 7.44 (d, 2H, J = 8.0 Hz, Ar-H), 7.38 (d, 1H, J = 8.4 Hz, Ar-H), 7.26 (dd, 1H, J1 = 8.4 Hz, J2 = 1.6 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.72 (t, 2H, J = 7.6 Hz, -CH2-), 3.63 (d, 2H, J = 5.2 Hz, -CH2-), 3.38 (t, 2H, J = 4.8 Hz, -CH2-), 2.93 (t, 2H, J = 7.6 Hz, -CH2-), 1.28 (s, 9H, -CH3); 13C-NMR (DMSO-d6): δ 175.1, 166.6, 163.3, 158.5, 154.0, 131.2, 129.9, 129.4, 127.0, 126.7, 124.9, 121.2, 114.2, 55.6, 41.8, 37.9, 34.5, 33.6, 30.9, 23.6. HRMS (TOF) m/z calcd. for C23H28N2O2 [M+H+]: 365.2229, found: 365.2178.
4-Cyano-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)benzamide oxalate(6a-19)
Following general procedure to obtain pure product as pale yellow solid 170 mg (yield: 28.1%). mp: 180–182 °C; 1H-NMR (DMSO-d6): δ 9.43 (br, s, 1H, -NH-), 7.94 (dd, 4H, J1 = 16.4 Hz, J2 = 8.4 Hz, Ar-H), 7.62 (d, 1H, J = 2.0 Hz, Ar-H), 7.39 (d, 1H, J = 8.4 Hz, Ar-H), 7.28 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.74 (t, 2H, J = 7.6 Hz, -CH2-), 3.66 (d, 2H, J = 5.6 Hz, -CH2-), 3.45 (t, 2H, J = 5.6 Hz, -CH2-), 2.99 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 176.5, 165.3, 161.4, 158.4, 137.7, 132.3, 130.1, 129.6, 128.1, 126.3, 122.1, 118.3, 114.7, 113.6, 55.8, 41.0, 38.1, 32.9, 23.4. HRMS (TOF) m/z calcd. for C20H19N3O2 [M+H+]: 334.1556, found: 334.1392.
2-((2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)carbamoyl)phenylacetate oxalate(6a-20)
Following general procedure to obtain pure product as white solid powder 243 mg (yield: 37.2%). mp: > 220 °C; 1H-NMR (DMSO-d6): δ 9.49 (br, s, 1H, -NH-), 7.88 (t, 1H, J = 8.4 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.64 (s, 1H, Ar-H), 7.43–7.35 (m, 2H, Ar-H), 7.27 (d, 1H, J = 8.0 Hz, Ar-H), 6.91–6.83 (m, 1H, Ar-H), 3.83 (s, 3H, -OCH3), 3.75–3.70 (m, 2H, -CH2-), 3.48 (t, 2H, J = 5.6 Hz, -CH2-), 3.16 (s, 3H, -CH3), 3.00 (t, 2H, J = 4.8 Hz, -CH2-), 1.91 (s, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 172.3, 165.6, 164.0, 161.4, 158.4, 148.4, 132.6, 130.2, 129.4, 128.7, 127.7, 122.4, 118.8, 113.7, 55.6, 44.2, 37.9, 34.2, 24.5, 21.0. HRMS (TOF) m/z calcd. for C21H22N2O4 [M+H+]: 367.2416, found: 367.1658.
2-Chloro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)nicotinamide oxalate(6a-21)
Following general procedure to obtain pure product as pale yellow solid powder 208 mg (yield: 37.2%). mp: 91–93 °C; 1H-NMR (DMSO-d6): δ 9.26 (br, s, 1H, -NH-), 8.54 (d, 1H, J = 4.8 Hz, Ar-H), 7.75 (s, 1H, Ar-H), 7.69 (d, 1H, J = 4.8 Hz, Ar-H), 7.48 (s, 1H, Ar-H), 7.35 (d, 1H, J = 8.4 Hz, Ar-H), 7.22 (d, 1H, J = 8.0 Hz, Ar-H), 3.82 (s, 3H, -OCH3), 3.70 (t, 2H, J = 7.6 Hz, -CH2-), 3.63 (d, 2H, J = 5.6 Hz, -CH2-), 3.26 (t, 2H, J = 5.6 Hz, -CH2-), 2.86 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 172.7, 163.6, 162.9, 158.4, 150.8, 150.6, 144.5, 129.8, 129.2, 127.1, 121.8, 120.7, 120.1, 113.5, 55.6, 42.8, 37.8, 33.4, 30.7, 23.7. HRMS (TOF) m/z calcd. for C18H18ClN3O2 [M+H+]: 344.1166, found: 344.1169.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-methylbenzamide oxalate(6a-22)
Following general procedure to obtain pure product as pale yellow solid powder 267 mg (yield: 45.2%). mp: > 220 °C; 1H-NMR (DMSO-d6): δ 9.04 (t, 1H, J = 5.6 Hz, -NH-), 7.74 (t, 1H, J = 8.4 Hz, Ar-H), 7.65 (d, 1H, J = 5.6 Hz, Ar-H), 7.57 (d, 2H, J = 7.6 Hz, Ar-H), 7.40 (t, 1H, J = 4.8 Hz, Ar-H), 7.29 (t, 2H, J = 8.4 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.72 (t, 2H, J = 7.2 Hz, -CH2-), 3.64 (t, 2H, J = 5.6 Hz, -CH2-), 3.43 (t, 2H, J = 4.8 Hz, -CH2-), 2.99 (t, 2H, J = 8.0 Hz, -CH2-), 2.32 (s, 3H, -CH3); 13C-NMR (DMSO-d6): δ 176.8, 166.9, 161.1, 158.5, 137.4, 133.8, 131.8, 130.0, 129.5, 128.0, 127.7, 126.4, 124.3, 122.2, 114.7, 55.7, 40.98, 38.0, 32.9, 23.4, 20.9. HRMS (TOF) m/z calcd. for C20H22N2O2 [M+H+]: 323.1760, found: 323.1741.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)nicotinamide oxalate(6a-23)
Following general procedure to obtain pure product as pale yellow solid powder 97 mg (yield: 16.9%). mp: 57–59 °C; 1H-NMR (DMSO- d6): δ 9.18 (br, s, 1H, -NH-), 8.91 (s, 1H, Ar-H), 8.68 (d, 1H, J = 4.0 Hz, Ar-H), 8.12 (d, 1H, J = 7.6 Hz, Ar-H), 7.59 (s, 1H, Ar-H), 7.49–7.46 (m, 1H, Ar-H), 7.38 (d, 1H, J = 8.0 Hz, Ar-H), 7.27 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.77–3.71 (m, 2H, -CH2-), 3.65 (d, 2H, J = 5.6 Hz, -CH2-), 3.37 (t, 2H, J = 4.8 Hz, -CH2-), 2.95 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 165.3, 162.0, 158.4, 151.9, 148.3, 134.8, 130.0, 129.5, 129.4, 126.6, 123.3, 121.4, 114.3, 55.7, 41.7, 37.8, 33.3, 30.7, 23.5. HRMS (TOF) m/z calcd. for C18H19N3O2 [M+H+]: 310.1556, found: 310.1578.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)picolinamide oxalate(6a-24)
Following general procedure to obtain pure product as pale yellow solid powder 158 mg (yield: 27.7%). mp: 95–97 °C; 1H-NMR (DMSO-d6): δ 9.14 (t, 1H, J = 6.0 Hz,-NH-), 8.61 (d, 1H, J = 4.4 Hz, Ar-H), 7.95 (dd, 2H, J1 = 12.8 Hz, J2 = 7.2 Hz, Ar-H), 7.59–7.57 (m, 1H, Ar-H), 7.51 (d, 1H, J = 2.0 Hz, Ar-H), 7.31 (d, 1H, J = 8.4 Hz, Ar-H), 7.17 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 3.79 (s, 3H, -OCH3), 3.72–3.65 (m, 4H, -CH2-), 3.29 (t, 2H, J = 6.0 Hz, -CH2-), 2.87 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 173.6, 164.2, 163.4, 158.3, 149.5, 148.3, 137.7, 129.8, 129.1, 127.1, 126.5, 121.8, 120.5, 113.6, 55.5, 42.5, 37.5, 33.6, 23.7. HRMS (TOF) m/z calcd. for C18H19N3O2 [M+H+]: 310.1556, found: 310.1546.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-4-methylbenzamide oxalate(6a-25)
Following general procedure to obtain pure product as pale yellow solid powder 258 mg (yield: 43.7%). mp: 208–210 °C; 1H-NMR (DMSO-d6): δ 8.92 (t, 1H, J = 5.2 Hz, -NH-), 7.69 (d, 2H, J = 8.0 Hz, Ar-H), 7.64 (d, 1H, J = 2.0 Hz, Ar-H), 7.40 (d, 1H, J = 8.4 Hz, Ar-H), 7.29 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 7.23 (d, 2H, J = 8.0 Hz, Ar-H), 3.82 (s, 3H, -OCH3), 3.71 (t, 2H, J = 8.0 Hz, -CH2-), 3.63 (t, 2H, J = 5.6 Hz, -CH2-), 3.39 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 7.6 Hz, -CH2-), 2.34 (s, 3H, -CH3); 13C-NMR (DMSO-d6): δ 175.1, 166.6, 161.8, 158.5, 141.2, 131.04, 130.0, 129.5, 128.7, 127.1, 126.5, 121.8, 114.2, 55.7, 41.3, 37.9, 33.3, 30.9, 23.5, 20.9. HRMS (TOF) m/z calcd. for C20H22N2O2 [M+H+]: 323.1760, found: 323.1693.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)quinoline-2-carboxamide oxalate(6a-26)
Following general procedure to obtain pure product as pale yellow oil 210 mg (yield: 32.6%). 1H-NMR (DMSO-d6): δ 9.21 (t, 1H, J = 4.8 Hz, -NH-), 8.65 (d, 1H, J = 4.4 Hz, Ar-H), 8.07 (d, 2H, J = 8.4 Hz, Ar-H), 7.68 (d, 1H, J = 5.6 Hz, Ar-H), 7.55–7.51 (m, 2H, Ar-H), 7.42 (d, 1H, J = 8.0 Hz, Ar-H), 7.35 (d, 1H, J = 2.0 Hz, Ar-H), 7.33 (d, 1H, J = 2.4 Hz, Ar-H), 3.86 (s, 3H, -OCH3), 3.75 (t, 2H, J = 8.4 Hz, -CH2-), 3.59 (t, 2H, J = 5.6 Hz, -CH2-), 3.45 (t, 2H, J = 4.8 Hz, -CH2-), 2.98 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 173.6, 164.2, 163.4, 158.3, 149.5, 148.3, 137.7, 129.8, 129.1, 127.1, 126.5, 121.8, 120.5, 113.6, 55.5, 42.5, 37.5, 33.6, 23.7. HRMS (TOF) m/z calcd. for C22H21N3O2 [M+H+]: 360.1712, found: 360.1797.
2-Bromo-5-chloro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)benzamide oxalate(6a-27)
Following general procedure to obtain pure product as colorless oil 152 mg (yield: 20.8%). 1H-NMR (DMSO-d6): δ 8.95 (br, s, 1H, -NH-), 7.67 (dd, 1H, J1 = 8.0 Hz, J2 = 4.8 Hz, Ar-H), 7.60 (s, 1H, Ar-H), 7.41 (d, 1H, J = 8.4 Hz, Ar-H), 7.32 (d, 1H, J = 8.0 Hz, Ar-H), 7.24 (t, 2H, J = 8.4 Hz, Ar-H), 3.86 (s, 3H, -OCH3), 3.77 (t, 2H, J = 7.6 Hz, -CH2-), 3.60 (t, 2H, J = 5.6 Hz, -CH2-), 3.37 (t, 2H, J = 4.8 Hz,-CH2-), 2.94 (t, 2H, J = 6.8 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 166.4, 163.3, 162.1, 159.6, 158.5, 140.0, 134.7, 130.1, 129.5, 126.6, 121.1, 118.3, 116.2, 114.3, 113.6, 55.7, 41.9, 37.7, 33.3, 23.5; HRMS (TOF) m/z calcd. for C19H18BrClN2O2 [M+H+]: 421.0318, found: 421.0287.
2-Bromo-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-5-(trifluoromethyl)benzamide oxalate (6a-28)
Following general procedure to obtain pure product as colorless oil 167 mg (yield: 39.9%). 1H-NMR (DMSO-d6): δ 9.06 (br, s, 1H, -NH-), 7.89 (d, 1H, J = 8.4 Hz, Ar-H), 7.70 (d, 2H, J = 5.6 Hz, Ar-H), 7.41 (d, 2H, J = 8.0 Hz, Ar-H), 7.40–7.32 (m, 3H, Ar-H), 3.85 (s, 3H, -OCH3), 3.78 (t, 2H, J = 8.4 Hz, -CH2-), 3.64 (t, 2H, J = 5.6 Hz, -CH2-), 3.39 (t, 2H, J = 4.8 Hz, -CH2-), 2.94 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.8, 166.4, 163.1, 158.5, 151.5, 139.2, 134.0, 132.4, 130.0, 129.5, 126.7, 123.7, 121.1, 118.1, 115.9, 114.2, 55.7, 42.0, 37.9, 33.2, 23.5; HRMS (TOF) m/z calcd. for C20H18BrF3N2O2 [M+H+]: 455.0582, found: 455.0606.
5-Bromo-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)nicotinamide oxalate(6a-29)
Following general procedure to obtain pure product as white solid 109 mg (yield: 15.9%). mp: 185–187 °C; 1H-NMR (DMSO-d6): δ 9.22 (s, 1H, -NH-), 8.85 (dd, 2H, J1 = 12.8 Hz, J2 = 4.8 Hz, Ar-H), 8.29 (s, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.36 (d, 1H, J = 8.0 Hz, Ar-H), 7.23 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 3.81 (s, 3H, -OCH3), 3.72 (t, 2H, J = 8.4 Hz, -CH2-), 3.64 (d, 2H, J = 5.6 Hz, -CH2-), 3.30 (s, 2H, -CH2-), 2.90 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 163.8, 163.2, 158.4, 152.5, 146.9, 137.2, 131.0, 129.9, 129.3, 126.9, 120.6, 119.9, 113.9, 55.6, 42.3, 37.9, 33.3, 23.6; HRMS (TOF) m/z calcd. for C18H18BrN3O2 [M+H+]: 388.0661, found: 388.0674.
5-Bromo-2-chloro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)nicotinamide oxalate (6a-30)
Following general procedure to obtain pure product as white solid 220 mg (yield: 30.0%). mp: 168–170 °C; 1H-NMR (DMSO-d6): δ 9.02 (s, 1H, -NH-), 8.64 (d, 1H, J = 8.4 Hz, Ar-H), 8.04 (d, 1H, J = 5.6 Hz, Ar-H), 7.46 (s, 1H, Ar-H), 7.35 (d, 1H, J = 8.0 Hz, Ar-H), 7.24 (d, 1H, J = 8.4 Hz, Ar-H), 3.84 (s, 3H, -OCH3), 3.71 (t, 2H, J = 8.0 Hz, -CH2-), 3.59 (d, 2H, J = 5.6 Hz, -CH2-), 3.23 (s, 2H, -CH2-), 2.83 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 163.9, 163.2, 158.4, 150.9, 145.5, 140.2, 133.7, 129.8, 129.2, 127.4, 119.5, 118.7, 113.2, 55.6, 43.3, 37.5, 33.5, 23.8; HRMS (TOF) m/z calcd. for C18H17BrClN3O2 [M+H+]: 422.0271, found: 422.0247.
6-Bromo-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)nicotinamide oxalate (6a-31)
Following general procedure to obtain pure product as white solid 227 mg (yield: 33.2%). 1H-NMR (DMSO-d6): δ 9.28 (s, 1H, -NH-), 8.71 (s, 1H, Ar-H), 8.05 (d, 1H, J = 8.4 Hz, Ar-H), 7.75 (d, 1H, J = 8.0 Hz, Ar-H), 7.56 (s, 1H, Ar-H), 7.37 (d, 1H, J = 7.6 Hz, Ar-H), 7.25 (d, 1H, J = 8.0 Hz, Ar-H), 3.82 (s, 3H, -OCH3), 3.73 (t, 2H, J = 7.6 Hz, -CH2-), 3.64 (d, 2H, J = 4.8 Hz, -CH2-), 3.35 (s, 2H, -CH2-), 2.92 (t, 2H, J = 8.0 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.8, 164.3, 163.1, 158.4, 149.3, 144.0, 138.1, 130.0, 129.4, 129.1, 127.8, 126.6, 121.1, 114.1, 55.7, 41.9, 37.84, 33.2, 23.5; HRMS (TOF) m/z calcd. for C18H18BrN3O2 [M+H+]: 388.0661, found: 388.0643.
N-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-4-methylbenzamide oxalate (6a-32)
Following general procedure to obtain pure product as colorless oil 281 mg (yield: 54.8%). 1H-NMR (DMSO-d6): δ 8.01 (br, s, 1H, -NH-), 7.82 (s, 1H, Ar-H), 7.73 (dd, 1H, J1 = 12.8 Hz, J2 = 4.8 Hz, Ar-H), 7.60 (d, 1H, J = 8.4 Hz, Ar-H), 7.40 (t, 2H, J = 15.6 Hz, Ar-H), 7.34 (t, 2H, J = 8.4 Hz, Ar-H), 3.81 (d, 2H, J = 7.6 Hz, -CH2-), 3.72 (s, 3H, -OCH3), 3.29 (s, 1H, -CH2-), 3.17–3.13 (m, 1H, -CH2-), 2.98 (s, 1H, -CH2-), 2.35 (d, 2H, J = 4.8 Hz, -CH2-), 2.28 (s, 3H, -CH3), 1.27–1.21 (m, 1H, -CH2-); 13C-NMR(DMSO-d6): δ 176.0, 161.8, 160.1, 158.5, 142.9, 137.0, 129.7, 126.4, 125.6, 122.2 114.6, 113.6, 112.5, 55.7, 33.2, 23.4, 20.8, 13.7; HRMS (TOF) m/z calcd. for C19H22N2O3S [M+H+]: 359.1429, found: 359.1367.
4-Fluoro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)benzamide oxalate (6a-33)
Following general procedure to obtain pure product as colorless oil 176 mg (yield: 34.0%). 1H-NMR (DMSO-d6): δ 8.05 (s, 1H, -NH-), 7.84 (s, 2H, Ar-H), 7.42 (t, 2H, J = 8.4 Hz, Ar-H), 7.33 (d, 1H, J = 4.8 Hz, Ar-H), 7.28 (s, 1H, Ar-H), 7.20 (t, 1H, J = 8.0 Hz, Ar-H), 3.82 (s, 3H, -OCH3), 3.68 (t, 2H, J = 7.6 Hz, -CH2-), 3.13 (s, 2H, -CH2-), 2.80 (s, 2H, -CH2-), 1.25 (d, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 166.3, 164.1, 162.6, 158.3, 139.5, 131.7, 130.2, 129.9, 129.1, 118.2, 116.4, 113.7, 55.6, 43.3, 37.6, 33.2, 25.4; HRMS (TOF) m/z calcd. for C18H19FN2O3S [M+H+]: 363.1179, found: 363.1084.
2,6-Difluoro-N-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)benzamide oxalate (6a-34)
Following general procedure to obtain pure product as colorless oil 114 mg (yield: 20.9%). 1H-NMR (DMSO-d6): δ 8.51 (s, 1H, -NH-), 7.85 (s, 3H, Ar-H), 7.45 (s, 1H, Ar-H), 7.35 (t, 1H, J = 15.6 Hz, Ar-H), 7.22 (t, 1H, J = 8.0 Hz, Ar-H), 3.91 (t, 2H, J = 6.0 Hz, -CH2-), 3.80 (s, 3H, -OCH3), 3.66 (t, 2H, J = 12.8 Hz, -CH2-), 3.33 (t, 2H, J = 8.4 Hz, -CH2-), 3.17 (t, 1H, J = 5.6 Hz, -CH2-), 2.83 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 165.5, 163.9, 162.1, 159.6, 139.6, 134.4, 129.9, 129.1, 118.1, 116.3, 114.3, 113.6, 112.5, 55.7, 41.9, 37.7, 33.34 23.5; HRMS (TOF) m/z calcd. for C18H18F2N2O3S [M+H+]: 381.1084, found: 381.1124.
General procedure for the preparation of compound 6b
Into a 50 mL three-neck flask, 2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethanamine hydrochloride (5) 300 mg(1.43 mmol, 1.0 eq), potassium carbonate 279 mg(1.72 mmol, 1.2 eq), DMF 2 mL and DCM 10 mL were added in order. Then the mixture was stirred in ice bath for 10 min. CDI(1.72 mmol, 1.2 eq) was added in three portions. After stirring at room temperature for 10 h, the reaction mixture was diluted with CH2Cl2 (20 mL), then washed with H2O. The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure and the residue was purified by chromatography or preparative TLC (eluent DCM/MeOH: 80/1, v/v) to afford the crude product. The crude product was dissolved in acetone, titrated with oxalic acid and the white solid were separated. After filtration, their oxalate form was obtained.
1-Cyclopropyl-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-1)
Following general procedure to obtain pure product as white solid 78 mg (yield: 18.9%). 1H-NMR (DMSO-d6): δ 8.93 (s, 1H, -NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.51 (s, 1H, Ar-H), 7.35 (d, 1H, J = 5.6 Hz, Ar-H), 7.17 (d, 1H, J = 4.8 Hz, Ar-H), 4.09 (t, 2H, J = 2.4 Hz, -CH2-), 3.84 (s, 3H, -OCH3), 3. 73 (t, 2H, J = 5.6 Hz, -CH2-), 3.31–3.24 (m, 1H, -CH-), 2.95 (t, 2H, J = 7.6 Hz, -CH2-), 2.70–1.60 (m, 2H, -CH2-), 0.86 (s, 1H,-CH2-), 0.64 (s, 1H,-CH2-), 0.52 (s, 1H,-CH2-), 0.27 (s, 1H,-CH2-); 13C-NMR (DMSO-d6): δ164.0, 161.2, 159.9, 158.7, 129.6, 128.8, 119.3, 113.0, 99.9, 55.7, 46.2, 37.6, 35.7, 28.8, 25.5, 6.9; HRMS (TOF) m/z calcd. for C16H21N3O2 [M+H+]: 288.1712, found: 288.1695.
1-(3-Chlorophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-2)
Following general procedure to obtain pure product as brown solid 253 mg (yield: 39.5%). mp: 185–187 °C; 1H-NMR (DMSO-d6): δ 8.88 (s, 1H, -NH-), 8.57 (d, 1H, J = 4.4 Hz, -NH-), 7.58 (s, 3H, Ar-H), 7.49 (d, 1H, J = 1.6 Hz, Ar-H), 7.34 (d, 1H, J = 8.4 Hz, Ar-H), 7.16–7.11 (m, 2H, Ar-H), 4.08 (t, 2H, J = 6.8 Hz, -CH2-), 4.03–3.98 (m, 1H, -CH2-), 3.83 (s, 3H, -OCH3), 3.80–3.70 (m, 2H, -CH2-), 2.96 (t, 2H, J = 8.0 Hz, -CH2-), 2.08 (s, 1H, -CH2-); 13C-NMR (DMSO-d6): δ164.4, 163.6, 158.5, 155.8, 152.5, 134.4, 130.2, 129.4, 128.7, 127.5, 119.5, 118.9, 117.4, 116.5, 111.0, 99.8, 55.5, 40.4, 30.7, 25.6; HRMS (TOF) m/z calcd. for C19H20ClN3O2 [M+H+]: 358.1322, found: 358.1368.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(4-(trifluoromethyl)phenyl)urea oxalate (6b-3)
Following general procedure to obtain pure product as pale yellow oil 238 mg (yield: 334.6%). 1H-NMR (DMSO-d6): δ 9.11 (s, 1H, -NH-), 8.59 (d, 1H, J = 4.4 Hz, -NH-), 7.66 (s, 3H, Ar-H), 7.51 (s, 1H, Ar-H), 7.34 (d, 1H, J = 8.4 Hz, Ar-H), 7.17 (d, 2H, J = 5.6 Hz, Ar-H), 7.06 (t, 1H, J = 8.0 Hz, Ar-H), 4.09 (t, 2H, J = 5.6 Hz, -CH2-), 3.84 (s, 3H, -OCH3), 3.80–3.77 (m, 2H, -CH2-), 3.72 (s, 2H, -CH2-), 2.97 (d, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ163.7, 161.6, 159.9, 158.6, 133.9, 130.2, 129.4, 128.8, 127.4, 119.2, 119.1, 111.2, 99.9, 99.5, 55.6, 40.5, 37.6, 25.5; HRMS (TOF) m/z calcd. for C20H20F3N3O2 [M+H+]: 392.1586, found: 392.1497.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(2-(trifluoromethyl)phenyl)urea oxalate (6b-4)
Following general procedure to obtain pure product as pale yellow oil 189 mg (yield: 33.8%). 1H-NMR (DMSO-d6): δ 9.11 (s, 1H, -NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.51 (s, 1H, Ar-H), 7.37 (t, 2H, J = 7.6 Hz, Ar-H), 7.23 (d, 1H, J = 8.4 Hz, Ar-H), 7.05 (t, 1H, J = 8.0 Hz, Ar-H), 6.88 (dd, 2H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 4.08 (t, 2H, J = 5.6 Hz, -CH2-), 3.84 (s, 3H, -OCH3), 3.72 (d, 2H, J = 4.8 Hz, -CH2-), 2.98–2.89 (m, 2H,-CH2-), 2.72–2.59 (m, 2H, -CH2-); 13C-NMR (DMSO-d6): δ164.0, 161.2, 159.9, 158.7, 134.6, 130.3, 129.6, 128.8, 119.3, 113.0, 99.9, 99.5, 55.7, 46.2, 35.7, 28.8, 25.5; HRMS (TOF) m/z calcd. for C16H21N3O2 [M+H+]: 288.1712, found: 288.1695.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(3,4,5-trichlorophenyl)urea oxalate(6b-5)
Following general procedure to obtain pure product as white solid 143 mg (yield: 23.3%). mp: >220 °C; 1H-NMR (DMSO-d6): δ 8.86 (s, 1H, -NH-), 8.57 (d, 1H, J = 4.8 Hz, -NH-), 7.82 (s, 2H, Ar-H), 7.49 (d, 1H, J = 4.8 Hz, Ar-H), 7.34 (d, 1H, J = 8.0 Hz, Ar-H), 7.16 (d, 1H, J = 5.6 Hz, Ar-H), 4.09 (t, 2H, J = 4.8 Hz, -CH2-), 4.02–3.96 (m, 1H, -CH2-), 3.83 (s, 3H, -OCH3), 3.75 (t, 2H, J = 8.4 Hz, -CH2-), 2.96 (t, 2H, J = 8.0 Hz, -CH2-), 2.08 (s, 1H, -CH2-); 13C-NMR (DMSO-d6): δ164.4, 161.2, 158.3, 155.4, 134.4, 130.2, 129.4, 128.7, 127.5, 127.0, 119.7, 116.7, 55.5, 46.2, 36.7, 31.6, 25.6; HRMS (TOF) m/z calcd. for C19H18Cl3N3O2 [M+H+]: 426.0543, found: 426.0579.
1-(4-Cyano-2,6-dimethylphenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-6)
Following general procedure to obtain pure product as white solid 217 mg (yield: 40.3%). 1H-NMR (DMSO-d6): δ 8.56 (d, 1H, J = 4.8 Hz, -NH-), 8.19 (s, 1H,-NH-), 7.49 (s, 1H, Ar-H), 7.32 (d, 1H, J = 8.4 Hz, Ar-H), 7.27 (s, 2H, Ar-H), 7.13 (dd, 1H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 4.07 (t, 2H, J = 8.0 Hz, -CH2-), 4.03–3.95 (m, 1H, -CH2-), 3.83 (s, 3H, -OCH3), 3.80–3.71 (m, 2H, -CH2-), 3.17 (s, 1H,-CH2-), 2.96 (t, 2H, J = 7.6 Hz, -CH2-), 2.13 (s, 6H,-CH3); 13C-NMR (DMSO-d6): δ164.5, 161.2, 158.5, 155.8, 138.9, 134.8, 130.6, 130.1, 128.5, 127.5, 118.8, 118.2, 113.5, 99.8, 55.5, 48.5, 37.7, 35.8, 25.6, 16.7; HRMS (TOF) m/z calcd. for C22H24N4O2 [M+H+]: 377.1978, found: 377.1879.
1-(4-Cyano-3-fluorophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-7)
Following general procedure to obtain pure product as white solid 162 mg (yield: 30.9%). mp: 207–209 °C; 1H-NMR (DMSO-d6): δ 9.10 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.67 (s, 2H, Ar-H), 7.51 (d, 1H, J = 5.6 Hz, Ar-H), 7.36 (d, 1H, J = 8.0 Hz, Ar-H), 7.16 (dd, 2H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 4.08 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.73 (t, 2H, J = 4.8 Hz, -CH2-), 2.97 (t, 2H, J = 8.4 Hz, -CH2-), 2.73 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.5, 161.2, 160.8, 158.3, 155.6, 141.0, 134.8, 130.6, 129.9, 121.3, 118.9, 113.5, 110.2, 108.5, 55.6, 48.6, 37.7, 35.8, 25.6; HRMS (TOF) m/z calcd. for C20H19FN4O2 [M+H+]: 367.1570, found: 367.1614.
2-(3-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)ureido)thiazole-5-carboxylate oxalate(6b-8)
Following general procedure to obtain pure product as white solid 72 mg (yield: 12.5%). mp: 125–127 °C; 1H-NMR (DMSO-d6): δ 9.08 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.51 (d, 1H, J = 5.2 Hz, Ar-H), 7.35 (d, 1H, J = 8.4 Hz, Ar-H), 7.15 (t, 2H, J = 8.0 Hz, Ar-H), 4.32 (dd, 2H, J1 = 12.8 Hz, J2 = 2.4 Hz, -CH2-), 4.08 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.80–3.72 (m, 2H, -CH2-), 2.97 (t, 2H, J = 8.0 Hz, -CH2-), 2.91 (t, 1H, J = 4.8 Hz, -CH2-), 2.72–2.59 (m, 1H,-CH2-), 1.27 (s, 3H, -CH3); 13C-NMR (DMSO-d6): δ164.1, 162.7, 161.7, 160.9, 158.6, 155.5, 152.9, 134.1, 129.4, 128.8, 119.2, 111.1, 60.3, 55.6, 40.5, 37.7, 35.3, 25.6, 14.8; HRMS (TOF) m/z calcd. for C19H22N4O4S [M+H+]: 403.1440, found: 402.8830.
1-(4-Chloro-3-(trifluoromethyl)phenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-9)
Following general procedure to obtain pure product as colorless oil 296 mg (yield: 40.2%). 1H-NMR (DMSO-d6): δ 9.08 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.89 (t, 1H, J = 5.6 Hz, Ar-H), 7.76–7.72 (m, 2H, Ar-H), 7.51 (d, 1H, J = 8.0 Hz, Ar-H), 7.33 (d, 1H, J = 8.4 Hz, Ar-H), 7.12 (dd, 1H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 4.08 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.72 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 7.2 Hz, -CH2-), 2.71 (t, 2H, J = 8.0 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.5, 161.2, 158.3, 155.6, 141.0, 134.8, 130.6, 129.9, 128.8, 121.3, 121.4, 118.9, 113.5, 110.2, 108.5, 55.6, 48.6, 37.7, 35.8, 25.8; HRMS (TOF) m/z calcd. for C20H19FN4O2 [M+H+]: 367.1570, found: 367.1614.
1-Isopropyl-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-10)
Following general procedure to obtain pure product as pale yellow solid powder 143 mg (yield: 26.3%). mp: 157–159 °C; 1H-NMR (DMSO- d6): δ 8.86 (s, 1H,-NH-), 8.58 (d, 1H, J = 8.0 Hz, -NH-), 7.50 (s, 1H, Ar-H), 7.32 (t, 1H, J = 8.4 Hz, Ar-H), 7.17–7.11 (m, 1H, Ar-H), 4.08 (t, 2H, J = 7.6 Hz, -CH2-), 4.04–3.97 (m, 1H, -CH2-), 3.84 (s, 3H, -OCH3), 3.81–3.74 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 8.4 Hz, -CH2-), 2.89 (t, 1H, J = 7.6 Hz, -CH2-), 2.09 (m, 1H, -CH-), 1.44 (d, 6H, J = 12.8 Hz, -CH3); 13C-NMR (DMSO-d6): δ164.5, 163.1, 158.6, 155.8, 152.5, 134.4, 129.4, 128.7, 119.6, 118.9, 111.0, 99.7, 55.6, 40.4, 30.7, 25.6; HRMS (TOF) m/z calcd. for C16H23N3O2 [M+H+]: 290.1869, found: 290.9096.
1-(3,5-Dimethylpyridin-4-yl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-11)
Following general procedure to obtain pure product as pale yellow oil 146 mg (yield: 21.1%). 1H-NMR (DMSO-d6): δ 9.09 (s, 1H,-NH-), 8.58 (d, 1H, J = 5.6 Hz, -NH-), 7.51 (s, 1H, Ar-H), 7.33 (d, 1H, J = 8.4 Hz, Ar-H), 7.16 (d, 2H, Ar-H), 7.07–7.00 (m, 1H, Ar-H), 4.08 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.77 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 7.2 Hz, -CH2-), 2.91 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.6, 163.1, 158.3, 155.8, 154.0, 147.5, 134.4, 130.5, 128.8, 119.6, 113.2, 99.7, 55.6, 37.7, 35.8, 30.7, 25.7; HRMS (TOF) m/z calcd. for C18H19Cl2N4O2 [M+H+]: 393.0885, found: 393.0774.
1-(5-Bromopyridin-2-yl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-12)
Following general procedure to obtain pure product as white solid 151 mg (yield: 21.4%). mp: 186–188 °C; 1H-NMR (DMSO-d6): δ 8.79 (s, 1H,-NH-), 8.58 (d, 1H, J = 4.4 Hz, -NH-), 7.51 (s, 1H, Ar-H), 7.33 (dd, 2H, J1 = 15.6 Hz, J2 = 4.8 Hz, Ar-H), 7.16 (dd, 2H, J1 = 8.4 Hz, J2 = 2.0 Hz, Ar-H), 7.12 (d, 1H, J = 8.0 Hz, Ar-H), 4.04 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.78 (t, 2H, J = 7.2 Hz, -CH2-), 2.97 (t, 2H, J = 7.6 Hz, -CH2-), 2.91 (d, 2H, J = 5.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.6, 163.1, 157.5, 155.8, 152.7, 147.5, 130.6, 128.2, 127.8, 119.6, 113.2, 111.3, 104.8, 99.7, 55.6, 46.7, 37.7, 35.8, 25.7; HRMS (TOF) m/z calcd. for C18H19BrN4O2 [M+H+]: 403.0770, found: 403.0736.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(naphthalen-1-yl)urea oxalate(6b-13)
Following general procedure to obtain pure product as brown oil 78 mg (yield: 11.8%). 1H-NMR (DMSO-d6): δ 9.03 (s, 1H,-NH-), 8.59 (d, 1H, J = 8.4 Hz, -NH-), 7.66 (s, 1H, Ar-H), 7.55 (s, 2H, Ar-H), 7.51 (s, 1H, Ar-H), 7.43 (d, 3H, J = 8.0 Hz, Ar-H), 7.34 (d, 2H, J = 7.6 Hz, Ar-H), 7.14 (t, 1H, J = 8.0 Hz, Ar-H), 4.09 (t, 1H, J = 7.2 Hz, -CH2-), 3.86 (s, 3H, -OCH3), 3.84 (s, 1H, -CH2-), 3.78 (t, 2H, J = 8.4 Hz, -CH2-), 3.47 (s, 2H, J = 8.0 Hz, -CH2-), 2.91 (d, 2H, J = 4.8 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.3, 162.7, 157.6, 154.7, 140.6, 134.9, 130.3, 128.2, 127.8, 126.4, 125.0, 124.8, 121.8, 119.6, 113.4, 105.9, 55.6, 46.7, 37.7, 35.8, 25.9; HRMS (TOF) m/z calcd. for C23H23N3O2 [M+H+]: 374.1869, found: 374.2023.
1-(3-Chloro-4-fluorophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-14)
Following general procedure to obtain pure product as pale yellow solid 231 mg (yield: 34.7%). 1H-NMR (DMSO-d6): δ 9.08 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.86 (t, 1H, J = 5.6 Hz, Ar-H), 7.76–7.72 (m, 2H, J = 4.8 Hz, Ar-H), 7.51 (d, 1H, J = 4.8 Hz, Ar-H), 7.42 (d, 1H, J = 8.4 Hz, Ar-H), 7.13 (dd, 1H, J1 = 8.4 Hz, J2 = 2.4 Hz, Ar-H), 4.08 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.72 (t, 2H, J = 5.6 Hz, -CH2-), 2.98 (t, 2H, J = 8.4 Hz, -CH2-), 2.73 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.2, 161.4, 158.0, 155.0, 134.4, 132.2, 130.2, 129.5, 128.8, 124.3, 123.5, 121.4, 118.9, 113.7, 55.6, 46.7, 37.7, 35.6, 25.8; HRMS (TOF) m/z calcd. for C19H19ClFN3O2 [M+H+]: 376.1228, found: 376.2065.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(3-nitrophenyl)urea oxalate(6b-15)
Following general procedure to obtain pure product as yellow oil 126 mg (yield: 19.2%). 1H-NMR (DMSO-d6): δ 8.93 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.61 (s, 2H, Ar-H), 7.50 (s, 1H, Ar-H), 7.35 (d, 1H, J = 5.6 Hz, Ar-H), 7.30–7.22 (m, 1H, Ar-H), 7.17–7.11 (m, 2H, Ar-H), 4.08 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.72 (t, 2H, J = 4.8 Hz, -CH2-), 2.97 (t, 2H, J = 8.0 Hz, -CH2-), 2.71 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.2, 161.2, 157.9, 148.9, 136.5, 130.3, 129.5, 129.1, 128.8, 126.6, 123.5, 119.3, 118.8, 113.4, 113.0, 55.6, 46.7, 37.7, 35.5, 25.8; HRMS (TOF) m/z calcd. for C19H20N4O4 [M+H+]: 369.1263, found: 369.1347.
1-(4-Chloro-2-nitrophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-16)
Following general procedure to obtain pure product as white solid 212 mg (yield: 30.1%). mp: 128–130 °C; 1H-NMR (DMSO-d6): δ 8.93 (s, 1H,-NH-), 8.59 (d, 1H, J = 5.6 Hz, -NH-), 7.50 (d, 1H, J = 4.8 Hz, Ar-H), 7.35 (d, 1H, J = 8.4 Hz, Ar-H), 7.17–7.11 (m, 2H, Ar-H), 7.05 (d, 1H, J = 8.0 Hz, Ar-H), 6.84 (s, 1H, Ar-H), 4.08 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.72 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 8.4 Hz, -CH2-), 2.73 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.2, 161.3, 157.7, 143.7, 135.5, 133.3, 130.2, 129.4, 129.2, 128.8, 126.7, 123.8, 119.7, 117.4, 113.3, 55.6, 46.8, 38.0, 35.2, 25.6; HRMS (TOF) m/z calcd. for C19H19ClN4O4 [M+H+]: 403.1173, found: 402.8860.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(thiazol-2-yl)urea oxalate(6b-17)
Following general procedure to obtain pure product as white solid 109 mg (yield: 18.1%). mp: 160–162 °C; 1H-NMR (DMSO-d6): δ 8.64 (s, 1H,-NH-), 8.58 (d, 1H, J = 7.6 Hz, -NH-), 7.49 (d, 2H, J = 5.2 Hz, Ar-H), 7.34 (dd, 1H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 7.16–7.11 (m, 2H, Ar-H), 4.08 (t, 2H, J = 8.0 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.69 (t, 2H, J = 5.6 Hz, -CH2-), 2.99 (t, 2H, J = 8.4 Hz, -CH2-), 2.71 (t, 2H, J = 7.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.2, 163.1, 161.3, 157.8, 154.3, 132.7, 130.2, 129.3, 128.8, 119.5, 113.7, 112.4, 55.6, 46.8, 37.9, 35.4, 25.7; HRMS (TOF) m/z calcd. for C16H18ClN4O2S [M+H+]: 331.1229, found: 331.1248.
1-(3,4-Dichlorophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-18)
Following general procedure to obtain pure product as yellow solid 163 mg (yield: 23.6%). mp: 134–136 °C; 1H-NMR (DMSO-d6): δ 8.64 (s, 1H,-NH-), 8.58 (d, 1H, J = 8.0 Hz, -NH-), 7.49 (d, 2H, J = 4.8 Hz, Ar-H), 7.49 (d, 1H, J = 8.4 Hz, Ar-H), 7.35 (d, 2H, J = 8.4 Hz, Ar-H), 7.17–7.11 (m, 3H, Ar-H), 4.08 (t, 2H, J = 7.6 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.69 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 7.2 Hz, -CH2-), 2.73 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.4, 163.3, 158.6, 155.8, 152.5, 134.4, 129.4, 128.7, 127.5, 119.6, 118.9, 111.0, 99.8, 55.5, 40.4, 30.7, 25.6; HRMS (TOF) m/z calcd. for C19H19Cl2N3O2 [M+H+]: 392.0933, found: 392.1105.
1-(2-(7-Methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)-3-(2-nitrophenyl)ureaoxalate(6b-19)
Following general procedure to obtain pure product as yellow oil 236 mg (yield: 34.9%). 1H-NMR (DMSO-d6): δ 8.93 (s, 1H,-NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.61 (s, 2H, Ar-H), 7.40 (s, 1H, Ar-H), 7.35 (d, 2H, J = 5.6 Hz, Ar-H), 7.23 (d, 2H, J = 4.8 Hz, Ar-H), 4.09 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.73 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (t, 2H, J = 8.0 Hz, -CH2-), 2.73 (t, 2H, J = 8.4 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 164.2, 162.5, 158.6, 155.4, 143.2, 131.3, 129.7, 129.2, 128.9, 127.2, 119.8, 118.7, 113.4, 55.6, 47.0, 37.2, 34.2, 26.0; HRMS (TOF) m/z calcd. for C19H20N4O4 [M+H+]: 369.1263, found: 369.1198.
1-(4-(Cyanomethyl)phenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate(6b-20)
Following general procedure to obtain pure product as white solid 157 mg (yield: 24.3%). mp: 141–143 °C; 1H-NMR (DMSO-d6): δ 9.11 (s, 1H, -NH-), 8.59 (d, 1H, J = 8.4 Hz, -NH-), 7.63 (s, 2H, Ar-H), 7.48 (s, 1H, Ar-H), 7.31 (d, 1H, J = 8.0 Hz, Ar-H), 7.15 (d, 2H, J = 4.8 Hz, Ar-H), 7.06 (t, 1H, J = 7.2 Hz, Ar-H), 4.32 (m, 2H, -CH2-), 4.09 (t, 2H, J = 8.4 Hz, -CH2-), 3.84 (s, 3H, -OCH3), 3.77–3.72 (m, 2H, -CH2-), 2.97 (d, 2H, J = 5.6 Hz, -CH2-), 2.73 (t, 2H, J = 4.8 Hz, -CH2-); 13C-NMR (DMSO-d6): δ 174.6, 164.1, 161.5, 158.6, 155.5, 152.8, 134.1, 128.8, 119.2, 119.0, 111.1, 99.8, 55.6, 46.3, 37.6, 25.5; HRMS (TOF) m/z calcd. for C21H22N4O2 [M+H+]: 363.1821, found: 363.1228.
1-(4-Bromophenyl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)ureaoxalate(6b-21)
Following general procedure to obtain pure product as yellow oil 162 mg (yield: 23.6%). 1H-NMR (DMSO-d6): δ 9.10 (s, 1H, -NH-), 8.59 (d, 1H, J = 4.8 Hz, -NH-), 7.67 (s, 3H, Ar-H), 7.50 (s, 1H, Ar-H), 7.35 (d, 1H, J = 8.4 Hz, Ar-H), 7.15 (dd, 2H, J1 = 12.8 Hz, J2 = 2.4 Hz, Ar-H), 4.32 (m, 2H, -CH2-), 4.08 (t, 2H, J = 5.6 Hz, -CH2-), 3.84 (s, 3H, -OCH3), 3.73 (t, 2H, J = 5.6 Hz, -CH2-), 2.97 (d, 2H, J = 8.4 Hz, -CH2-), 2.71 (t, 2H, J = 7.2 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.3, 161.6, 158.6, 155.6, 138.6, 130.8, 129.4, 127.5, 122.8, 122.0, 119.5, 113.1, 55.6, 43.3, 39.9, 25.6; HRMS (TOF) m/z calcd. for C19H20BrN3O2 [M+H+]: 402.0817, found: 402.0762.
1-(3-Bromopyridin-2-yl)-3-(2-(7-methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)urea oxalate (6b-22)
Following general procedure to obtain pure product as white solid 267 mg (yield: 37.9%). mp: 131–134 °C; 1H-NMR (DMSO-d6): δ 9.11 (s, 1H, -NH-), 8.58 (d, 1H, J = 4.8 Hz, -NH-), 7.68 (s, 2H, Ar-H), 7.51 (s, 1H, Ar-H), 7.33 (dd, 2H, J1 = 15.6 Hz, J2 = 3.2 Hz, Ar-H), 7.17–7.14 (m, 2H, Ar-H), 4.04 (t, 2H, J = 8.4 Hz, -CH2-), 3.83 (s, 3H, -OCH3), 3.78 (t, 2H, J = 8.0 Hz, -CH2-), 2.99 (t, 2H, J = 7.6 Hz, -CH2-), 2.91 (d, 2H, J = 5.6 Hz, -CH2-); 13C-NMR (DMSO-d6): δ164.1, 163.2, 161.64, 158.6, 155.5, 152.8, 134.1, 129.4, 128.8, 127.5, 123.3, 119.2, 110.1, 99.8, 55.6, 40.5, 37.7, 35.8, 25.6; HRMS (TOF) m/z calcd. for C18H19BrN4O2 [M+H+]: 403.0770, found: 403.0745.
Additional Information
How to cite this article: Yang, Y. et al. Scaffold Hopping Toward Agomelatine: Novel 3, 4-Dihydroisoquinoline Compounds as Potential Antidepressant Agents. Sci. Rep. 6, 34711; doi: 10.1038/srep34711 (2016).
References
Smith, K. Mental Health: A World of Depression. Nature 515, 181 (2014).
Reddy, M. S. Depression - the Global Crisis. Indian J Psychol Med. 34, 201–203 (2012).
Verhoeven, J. E. et al. Major Depressive Disorder and Accelerated Cellular Aging: Results From a Large Psychiatric Cohort Study. Mol Psychiatry 19, 895–901 (2014).
Penninx, B. W., Milaneschi, Y., Lamers, F. & Vogelzangs, N. Understanding the Somatic Consequences of Depression: Biological Mechanisms and the Role of Depression Symptom Profile. BMC MED. 11, 129 (2013).
Berton, O. & Nestler, E. J. New Approaches to Antidepressant Drug Discovery: Beyond Monoamines. Nat. Rev. Neurosci. 7, 137–151 (2006).
Ang, W. et al. Synthesis and biological evaluation of novelnaphthalene compounds as potential antidepressant agents. Eur. J. Med. Chem. 82, 263–273 (2014).
Castren, E. Neuronal Network Plasticity and Recovery From Depression. JAMA Psychiatry. 70, 983–989 (2013).
Duman, R. S., Heninger, G. R. & Nestler, E. J. A Molecular and Cellular Theory of Depression. Arch Gen Psychiatry 54, 597–606 (1997).
Manji, H. K., Drevets, W. C. & Charney, D. S. The cellularneurobiology of depression. NatMed. 7, 541–547 (2001).
Autry, A. E. & Monteggia, L. M. Brain-derivedneurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 64, 238–258 (2012).
Krishnan, V. & Nestler, E. J. The molecularneurobiology of depression. Nature 455, 894–902 (2008).
Samuels, B. A. & Hen, R. Neurogenesis and affective disorders. Eur J Neurosci. 33, 1152–1159 (2011).
Castrén, E. & Rantamäki, T. The role of BDNF andits receptors in depression and antidepressant drugaction: reactivation of developmental plasticity. Dev Neurobiol. 70, 289–297 (2010).
Bremner, J. D. et al. Hippocampal volume reduction in major depression. Am. J. Psychiatry 157, 115–118 (2000).
MacQueen, G. M. et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA 100, 387–392 (2003).
Sheline, Y. I. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry 54, 338–352 (2003).
Cardinali, D. P., Vidal, M. F. & Vigo, D. E. Agomelatine: its role in the management of majordepressive disorder. Clin. Med. Insights Psychiatry 4, 1–23 (2012).
Fuchs, E. & Flügge, G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 23, 295–300 (1998).
Angela, L. L., William, O. O. & Sapolsky, R. M. Stress and depression: Possible linksto neuron death in the hippocampus. Bipolar Disord. 4, 117–128 (2002).
Mcewen, B. S. Effects of adverse experiences for brain structure and function. Biol Psychiatry 48, 721–731 (2000).
Lucassen, P. J. et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid over exposure. Am J Pathol. 158, 453–468 (2001).
Vythilingam, M. et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry 56, 101–112 (2004).
Vimala, P. V., Bhutada, P. S. & Patel, F. R. Therapeutic potential of agomelatinein epilepsy and epileptic complications. Med. Hypotheses. 82, 105–110 (2014).
Gressens, P. et al. Agomelatine, a melatonin receptoragonist with 5-HT (2C) receptor antagonist properties, protects the developing murine white matter againstexcitotoxicity. Eur. J. Pharmacol. 588, 58–63 (2008).
Malberg, J. E. & Schechter, L. E. Increasing hippocampal neurogenesis: a novelmechanism for antidepressant drugs. Curr. Pharm. Des.11, 145–155 (2005).
Duman, R. S. & Monteggia, L. M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 59, 1116–1127 (2006).
Morley-Fletcher, S. et al. Chronic treatment with agomelatineincreasing hippocampal cell proliferation in prenatally stressed rats. Psychopharmacol. (Berl.) 217, 301–313 (2011).
Wang, Y. G. et al. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J. Neurotrauma 28, 259–268 (2011).
Yong, L. T. Neuroprotective effects of antidepressant and mood stabilizingdrugs. J. Psychiatry Neurosci. 27, 8–9 (2012).
Carroll, B. J. Untreated depression and hippocampal volume loss. Am. J. Psychiatry 160, 1516–1518 (2003).
Xu, H. Y., Richardson J. S. & Li, X. M. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 28, 53–62 (2003).
Claire, S. An Investigation into the Possible Neuroprotective Role of Antidepressant Drugs. Rhodes University, Grahamstown, 2002.
Gahr, M. et al. Agomelatine and Hepatotoxicity: Implications of Cumulated Data Derived From Spontaneous Reports of Adverse Drug Reactions. Pharmacopsychiatry 46, 214–220 (2013).
Chang, Y. et al. Synthesis and evaluation of amide side-chain modified Agomelatine analogues as potential antidepressant-like agents. Bioorg Med Chem Lett. 24, 1672–1676 (2014).
Kolla, N. et al. Amitriptyline and fluoxetine protectPC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry Neurosci. 30, 196–201 (2005).
Li, Y. F. et al. The cytoprotective effect of inulin-type hexasaccharide extracted from Morinda officinalis on PC12 cells against the lesioninduced by corticosterone. Life Sci. 75, 1531–1538 (2004).
Zheng, M. et al. Protective Effects of Flavonoid Extract from Apocynum venetum Leaves Against Corticosterone-Induced Neurotoxicity in PC12 Cells. Cell Mol Neurobiol. 31, 421–428 (2011).
Johnson, D. J. et al. The discoveryof a series of N-substituted 3-(4-piperidinyl)-1,3-benzoxazolinones andoxindoles as highly brain penetrant, selective muscarinic M1 agonists. Bioorg. Med. Chem. Lett. 20, 5434–5438 (2010).
Matthias, G. J. B. et al. Defining the mechanism of action and enzymatic selectivity of psammaplin a against its epigenetic targets. J. Med. Chem. 55, 1731–1750 (2012).
Vaid, R. K. et al. A practical synthesis of 2-{4-[4-fluoro-3-(trifluoromethyl)phenyl]-2-(piperidin-4-yl)-1H-imidazol-1-yl}-N,Ndimethylethanamine. Synthesis 44, 2231–2236 (2012).
Okuda, K., Kotake, Y. & Ohta, S. Neuroprotective or neurotoxic activity of 1-methyl-1,2,3,4- tetrahydroisoquinoline and related compounds. Bioorg. Med. Chem. Lett. 13, 2853–2855 (2003).
Petar, A. D. et al. Synthesis and Reactivity of N-Alkyl Carbamoylimidazoles: Development of N-Methyl Carbamoylimidazole as a Methyl Isocyanate Equivalent. J. Org. Chem. 77, 10362–10368 (2012).
Shityakov, S. et al. Analysing molecular polar surface descriptors to predict blood-brain barrier permeation. Int. J. Comput. Biol. Drug Des. 6, 146–156 (2013).
Di, L. et al. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 38(3), 223–232 (2003).
Porsolt, R. D., Pichon, M. L. & Jalfre, M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 (1977).
Michel T. M., Pülschen, D. & Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 18(36), 5890–5899 (2012).
Harrison, J. F. et al. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 33(14), 4660–4671 (2005).
Orrenius, S. & Nicotera, P. The calcium ion and cell death. J Neural. Transm. Suppl. 43, 1–11 (1994).
Soumier, A. et al. Mechanisms Contributing to the Phase-Dependent Regulation of Neurogenesis by the Novel Antidepressant, Agomelatine, in the Adult Rat Hippocampus. Neuropsychopharmacol. 34, 2390–2403 (2009).
Schmidt, H. D. & Duman, R. S. The Role of Neurotrophic Factors in Adult Hippocampal Neurogenesis, Antidepressant Treatments and Animal Models of Depressive-Like Behavior. Behav Pharmacol. 18, 391–418 (2007).
Li, Y. et al. TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment. Neuro. 59, 399–412 (2008).
Yu, Y. et al. Liensinine- and Neferine-Induced Cardiotoxicity in Primary Neonatal Rat Cardiomyocytes and Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Int J Mol Sci. 17 (2016).
Mao, Q. Q. et al. Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca2+ antagonism. Cell. Mol. Neurobiol. 30, 1059–1066 (2010).
Yokozawa, T. et al. Protective effect of the Chinese prescription Kangen-karyu against high glucose-induced oxidative stress in LLC-PK1 cells. J. Ethnopharmacol. 109, 113–120 (2010).
Gulati, K., Chakraborti, A. & Ray, A. Modulation of stress-induced neurobehavioral changes and brain oxidative injury by nitric oxide (NO) mimetics in rats. Behav. Brain. Res. 183, 226–230 (2007).
Rahman, I., Kode, A. & Biswas, S. K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 1, 3159–3165 (2006).
Heid, C. A. et al. Real time quantitative PCR. Genome Res. 6, 986–994 (1996).
Kutchinsky, J. et al. Characterization of potassium channel modulators with QPatch automated patch-clamp technology: system characteristics and performance. Assay Drug Dev. Technol. 1, 685–693 (2003).
Joshi, P. G., Singh, A. & Ravichandra, B. High concentrations of tricyclic antidepressants increase intracellular Ca2+ in cultured neural cells. Neurochem. Res. 24, 391–398 (1999).
Kutchinsky, J., Friis, S., Asmild, M. et al. Characterization of potassium channel modulators with QPatch automated patch-clamp technology: system characteristics and performance. Assay Drug Dev. Technol. 1, 685–693 (2003).
Acknowledgements
This work was supported by New Century Talent Fund by Ministry of Education (NCET-13-0388) of China.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the project together with Y.D., L.Z., Z.L. and W.A., T.Y. performed the chemical synthesis. H.L. and Y.C. performed and evaluated in vitro experiments. Y.Y., H.L. and W.A. performed and evaluated in vivo experiments. H.L. and L.Z. perform the acute in vivo toxicity evaluation. Y.L., Y.Y., H.L. and W.A. wrote the paper with contributions of all co-authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, Y., Ang, W., Long, H. et al. Scaffold Hopping Toward Agomelatine: Novel 3, 4-Dihydroisoquinoline Compounds as Potential Antidepressant Agents. Sci Rep 6, 34711 (2016). https://doi.org/10.1038/srep34711
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34711
This article is cited by
-
5-HTT independent effects of fluoxetine on neuroplasticity
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.