Abstract
Contact heat evoked potentials (CHEPs) represent a neurophysiological approach to assess conduction in the spinothalamic tract. The aim of this study was to establish normative values of CHEPs acquired from cervical dermatomes (C4, C6, C8) and examine the potential confounds of age, sex, and height. 101 (49 male) healthy subjects of three different age groups (18–40, 41–60, and 61–80 years) were recruited. Normal (NB, 35–52 °C) followed by increased (IB, 42–52 °C) baseline stimulation protocols were employed to record CHEPs. Multi-variate linear models were used to investigate the effect of age, sex, and height on the CHEPs parameters (i.e., N2 latency, N2P2 amplitude, rating of perceived intensity). Compared to NB, IB stimulation reduced latency jitter within subjects, yielding larger N2P2 amplitudes, and decreased inter-subject N2 latency variability. Age was associated with reduced N2P2 amplitude and prolonged N2 latency. After controlling for height, male subjects had significantly longer N2 latencies than females during IB stimulation. The study provides normative CHEPs data in a large cohort of healthy subjects from segmentally examined cervical dermatomes. Age and sex were identified as important factors contributing to N2 latency and N2P2 amplitude. The normative data will improve the diagnosis of spinal cord pathologies.
Similar content being viewed by others
Introduction
Sensory deficits are a hallmark of a variety of neurological conditions. As a complementary tool to conventional clinical testing, laser and contact heat evoked potentials (LEPs and CHEPs, respectively) have been adopted as objective measures of small diameter afferents1,2,3,4,5,6,7,8,9,10. These investigations have primarily focused on examining areas of the body commonly associated with sensory impairments, including the face (e.g., trigeminal neuralgia), trunk and back (e.g., post-herpetic neuralgia), and distal parts of the upper and lower extremities (e.g., diabetic neuropathy)11,12,13,14,15. Accordingly, normative values have been well established for these areas3,11,16,17.
The approach of examining a general cutaneous area is suboptimal for the assessment of damage associated with a discrete spinal cord level (e.g., spinal cord injuries). In this case, a more “segmental” approach, involving a level-by-level assessment is needed. Such a segmental approach crucially requires examining dermatomes above, at, and below the level of injury in well-defined regions of the body innervated by individual spinal segments.
The goal of the present study was to apply an advanced testing protocol for establishing normative values of CHEPs form cervical dermatomes. These values are imperative to accurately determine when a recording in a patient with suspected spinal cord disorder (traumatic and non-traumatic) can be deemed “pathological”, and to assess potential confounding variables, such as height, age, and psychological factors. To this end, we examined CHEPs following thermal stimulation of low and high intensity in a large cohort of healthy individuals using standard anatomical locations corresponding to cervical (C4, C6, and C8) spinal segments.
Material and Methods
Subjects
A total of 52 female and 53 male healthy control individuals were recruited. Inclusion criteria were (1) age between 18–80 years and (2) native language either English or German. We specifically recruited individuals of different age groups (group 1: 18–40 years, Group 2: 41–60years, Group 3: 61–80 years). Exclusion criteria comprised pregnancy, intake of any medication (except birth control), and any obvious neurological condition. All participants provided written informed consent and all procedures described below were in accordance with the Declaration of Helsinki and approved by the local ethics board ‘Kantonale Ethikkommission Zürich, KEK’ (ref. number: EK-04/2006, cinicaltrial.gov number: NCT02138344).
Study protocol
Prior to the measurement of CHEPs, all individuals were interviewed to assess pain catastrophizing using the German or English version of the pain catastrophizing scale (PCS)18. The PCS assesses individuals catastrophic thinking related to painful experiences. The overall reliability and validity of the PCS was demonstrated in earlier studies19,20,21,22.
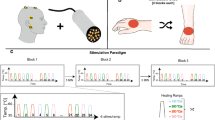
As illustrated in Fig. 1, the study protocol consisted of unilateral assessments of pinprick and light touch followed by the recording of CHEPs. Three body parts in the distribution of the 4th (C4, shoulder), 6th (C6, dorsum of hand), and 8th cervical segment (C8, dorsum of hand) were examined. The rationale for stimulating the dorsum of the hand was to stimulate the most distal part of the dermatome. Previous studies have shown that the separation of C6 and C8 dermatomes is best at the hand23. Furthermore, within the hairy skin of primates (i.e., dorsum) two distinct A-delta fibre receptor classes have been described and are almost equally distributed24. The type II AMH receptor mediates the first pain to heat stimuli, and is important for the fast transduction of contact heat stimuli. Evidence for the presence of type II-AMH in glabrous skin is sparse, and consequently it is difficult to elicit CHEPs stimulating these areas. Lastly, the contact surface of the chosen areas is sufficient allowing for a defined and evenly-distributed activation of a cutaneous area. The order of examination and body site tested was randomized for each subject25. Following light touch and pinprick testing, contact heat evoked potentials were recorded employing the conventional normal baseline protocol followed by the increased baseline protocol. The difference between the protocols was that the contact heat stimuli were delivered from either 35 °C (normal baseline protocol) or 42 °C (increased baseline protocol), respectively, to a peak temperature of 52 °C5,26. Contact heat evoked potentials (CHEPs) were recorded while subjects were lying in a supine position, with eyes open. In order to minimize ocular artefacts, subjects were asked to fix on a point on the ceiling, and to remain relaxed and quiet during testing. Prior to the testing, all individuals underwent a familiarization procedure that comprised of the presentation of two stimuli at the non-tested hand. A total of 15 contact heat stimuli per protocol (i.e., normal and increase baseline) were applied with an inter-stimulus time interval that randomly varied between 8 and 12 s. Traces contaminated with muscle or ocular artefacts were excluded in real-time and additional stimuli were applied in order to record 15 artefact-free traces. Cued by an auditory signal, individuals were asked to rate the perceived intensity of each stimulus from 0 (no pain) to 10 (most unbearable pain) two seconds after contact heat stimuli was delivered. The thermode was lightly repositioned subsequent to each stimulus within the dermatome tested to avoid peripheral sensitization/habituation (an area of approximately 4 × 4 cm).
Study design.
Prior to the recording of contact heat evoked potentials (CHEPs), all participants completed the pain catastrophizing questionnaire followed by pin prick and light touch testing. CHEPs were recorded from dermatomes C4, C6, and C8 (in a randomized order) employing normal followed by increased baseline stimulation.
Contact Heat Evoked Potentials: Stimulating device and recording set up
To examine responses to noxious thermal stimulation, a contact heat stimulator was employed (Pathway, Medoc, RamatYishai, Israel). The thermode surface (diameter: 27 mm) consists of a heating thermo-foil covered with a layer of thermos-conductive plastic. The nominal heating rate of this device is 70 °C/s (thermo-foil), with a cooling rate of 40 °C/s (peltier element).
Cortical responses to the noxious heat stimuli were recorded with 9 mm Ag/AgCl surface disc electrodes filled with conductive adhesive gel. Scalp recording sites were prepared with Nuprep (D.O. Weaver & Co. Aurora, CO) and alcohol. Cortical recording electrodes were positioned in accordance with the International 10–20 system27. Both, N2 and P2, were acquired from an active vertex recording electrode (Cz) referenced to linked earlobes (A1-A2). The rationale for a reduced electrode set up arose from the fact that consistent negative and positive potentials, labelled N2 and P2, were reliably detected at Cz in previous studies10,28,29,30,31,32. All signals were sampled at 2000 Hz using a preamplifier (20000x, bandpass filter 1–300 Hz, ALEA Solutions, Zurich, Switzerland). Data were recorded with 100 ms pre-trigger and a one second post-trigger in a customized program based on LabView (V1.43 CHEP, ALEA Solutions, Zurich, Switzerland). The N2P2 waveform was visually detected based on the average of the 15-recorded trials. Waveforms had to be larger than 10 uV in order to be considered. The problem with waveforms below 10 uV is that the signal-to-noise ratio is too low to distinguish the signal from the noise.
Analysis and statistics
Statistical analyses were performed using the computing environment R version 2.14.0 for Windows. All data were tested for normal distribution using the Kolmogorov–Smirnov test. Bonferroni correction was used to account for multiple comparisons. Statistical significance was set at α = 0.05.
The means (±standard deviation, SD) of the N2 and P2 latency (ms), the N2P2 amplitude (μV), as well as the pain rating were determined for each stimulation protocol. A linear mixed model was used to examine the main effect of the different stimulation protocols and stimulation sites (i.e., dermatome C4, C6, and C8) as well as the interaction effect between the stimulation protocol and site. Random subject effects were included in this model.
Exploratory analysis of bivariate relationships between clinical (i.e., age, sex, height, and PCS) and CHEPs parameters (i.e., N2 and P2 latencies, N2P2 amplitude) were examined using Pearson correlation analyses. In a subsequent analysis, the effects of age, sex, and height on the N2P2 amplitude and N2 latencies of both stimulation protocols (i.e., normal and increased baseline) were examined using a multi-variate general linear mixed models (3 dependent variables, 3 levels). N2P2 amplitude or N2 latency of each dermatome (i.e., C4, C6, and C8) were set as dependent variables, while sex was included as a fixed factor and age and height as covariates.
Results
Subjects
Out of 105 healthy subjects enrolled in the study, four had to be excluded due to: (1) technical problems during data acquisition (n = 3) and (2) intolerance of the contact heat stimuli applied (n = 1). The remaining 101 subjects comprised of 49 men and 52 women (mean age 46.2 ± 16.5 years) with an average height of 172.8 ± 8.9 cm. Men were significantly taller than women (F = 61.2, p < 0.001). The mean score of the PCS questionnaire was 10.5 ± 9.3. There was no difference between male and females in PCS score.
Main effects of stimulation protocol
A representative example of averaged CHEPs (N2/P2) of each dermatome (C4, C6, C8) and different stimulation protocols are illustrated in Fig. 2. Summary amplitudes, latencies for N2 and P2, and pain ratings both protocols, normal and increased baseline, are shown in Table 1. With the aim to record 15 artefact-free EEG traces, the total number of applied stimuli ranged between 15 and 22.
Effect of stimulation protocol, order, and site on CHEPs parameters
There was a significant main effect of stimulus protocol on N2P2 amplitude (F = 101.8, p < 0.001), N2 (F = 126.7, p < 0.001) and P2 latencies (F = 46.4, p < 0.001), as well as rating of perceived intensity (F = 81.7, p < 0.001). Notably, increasing the baseline temperature from 35 °C to 42 °C resulted in augmented N2P2 amplitudes, shortened N2 and P2 latencies, and elevated perceived intensity in all dermatomes. The main effect of stimulation site was significant (both stimulation protocols) for N2P2 amplitude (NB: F = 3.5, p = 0.44, IB: F = 0.127, p = 0.88), N2 (NB: F = 39.0, p < 0.001; IB: F = 33.5, p < 0.001) and P2 latencies (NB: F = 7.0, p = 0.001; IB: F = 8.5, p < 0.001) as well as rating of perceived intensity (NB: F = 7.9, p < 0.001: IB: F = 4.2, p = 0.16). Rating of perceived intensity (both stimulation protocols) and N2P2 amplitudes (normal baseline protocol) were significantly higher in dermatome C4 compared to dermatomes C6 and C8. For both stimulation protocols, N2 and P2 latencies gradually increased from dermatome C4 to C6 to C8 (Table 1 and Fig. 3). Group-wise comparisons revealed significant increased latencies for C6 and C8 when compared to C4 as well as an increased latency for C8 compared to C6.
Summary of latencies and amplitudes for each dermatome and both stimulation modalities.
Increased baseline resulted in (A) increased N2P2 amplitudes and (B) decreased N2 latencies across all dermatomes and age groups. The inter-subject variability of N2 can be minimized by employing increased baseline stimulation.
Effect of age, body height, and PCS score
Pearson correlation analyses yielded significant negative correlations for age and N2P2 amplitude (C4NB: r = −0.376, p < 0.001; C4IB: r = −0.445, p < 0.001; C6NB: r = −0.331, p = 0.001; C6IB: r = −0.439, p < 0.001; C8NB: r = −0.264, p = 0.013; C8IB: r = −0.396, p < 0.001), N2 amplitude (C4NB: r = −0.256, p = 0.011; C4IB: r = −0.382, p < 0.001; C6IB: r = −0.415, p < 0.001; C8NB: r = −0.233, p = 0.030; C8IB: r = −0.296, p = 0.004), and P2 amplitude (C4NB: r = −0.435. p < 0.001; C4IB: r = −0.456. p < 0.001; C6NB: r = −0.382, p < 0.001; C6IB: r = −0.376, p < 0.001; C8NB: r = −0.234, p = 0.029; C8IB: r = −0.442, p < 0.001) for all dermatomes in both stimulation protocols. Age was further positively correlated with the N2 (C4IB: r = 0.399, p < 0.001; C6IB: r = 0.307, p = 0.003; C8IB: r = 0.294, p = 0.005) and P2 (C4IB: r = 0.284, p = 0.007; C6IB: r = 0.329, p = 0.001) latencies of the increased baseline protocol. Sex correlated with N2 latency (C4IB: r = 0.357, p = 0.001; C6IB: r = 0.363, p < 0.001; C8IB: r = 0.362, p < 0.001) of all dermatomes only when the increased baseline stimulation was employed. Lastly, height was correlated with N2 latency (C4NB: r = −0.246, p = 0.015; C4IB: r = 0.219, p = 0.038; C6NB: r = 0.269, p = 0.009; C6IB: r = 0.190, p = 0.066; C8NB: r = 0.195, p = 0.071; C8IB: r = 0.211, p = 0.045). All correlations between demographics and CHEPs parameters are summarized in Fig. 4.
The multivariate general linear models revealed a significant effect of age on N2P2 amplitude of all dermatomes in both stimulation protocols (C4NB: F = 10.2, p = 0.02; C4IB: F = 24.2, p < 0.001; C6NB: F = 10.5, p = 0.002; C6IB: F = 20.0, p < 0.001; C8NB: F = 6.7, p = 0.012; C8IB: F = 16.0, p < 0.001). Age (C4IB: F = 22.0, p < 0.001; C6IB: F = 12.6, p = 0.001; C8IB: F = 12.3, p = 0.001) and sex (C4IB: F = 3.3, p = 0.025; C6IB: F = 3.3, p = 0.024; C8IB: F = 3.3, p = 0.024) had a significant effect on the N2 latency when the increased baseline temperature protocol was employed.
Discussion
The present study provides a protocol and normative data for the segmental assessment of cervical dermatomes with CHEPs. CHEP parameters were significantly associated with age and sex, but not psychological factors (i.e., PCS). Height, which was significantly associated with CHEPs according to a bivariate analysis, had no effect on CHEPs after accounting for sex and age. The use of an increased baseline stimulation protocol improved the acquisition of CHEPS by increasing N2P2 amplitudes and reducing N2 latency variability between subjects. The segmental approach described herein is well suited for the measurement of sensory deficits associated with a distinct level of damage in the cervical spinal cord.
Correlations of age, sex, and height with CHEPs parameters
In the present study, age was negatively correlated with the N2P2 amplitude and positively associated with the N2 and P2 latencies. This confirms a number of previous LEP/CHEPs studies11,17,33,34. Changes of N2P2 amplitude as a function of age were evident in both stimulation protocols, normal and increased baseline. Demonstrating the sensitivity of our approach, the effect of age on N2 and P2 latencies was only evident using the increased baseline stimulation. Physiologically, age-related attenuation of amplitudes and prolongation of latencies have been attributed to a dysfunction of peripheral nociceptive mechanisms, alteration in CNS processing, or de-synchronization of the ascending nociceptive volleys35,36,37.
Also in agreement with previous studies, sex-related effects were observed for N2 and P2 latencies, and rating of perceived intensity11,16,34. Owing to longer afferent conduction distances, height is a plausible explanation for the sex-differences considering that male participants were significantly taller than females in our study. However, a multi-variable analysis indicates that sex, not height accounts for differences in CHEP latency. Previous findings regarding the effect of height on N2 latency are rather inconsistent, ranging from strong correlations to no effect3,16,17. Most studies, however, suggest that females report higher levels of perceived intensity to noxious heat stimulation than males38. Various biologic (e.g., skin thickness, brain structure), psychologic (e.g., anxiety and catastrophizing), and social factors (e.g., social expectations) have been proposed to account for sex related differences39,40,41,42,43. In the present cohort of healthy subjects, pain catastrophizing was comparable across age groups and sex. As such, the sex-related difference in ratings of perceived intensity is unlikely the result of greater catastrophizing in females.
No association between PCS and any of the CHEPs parameters (i.e., N2P2 amplitude, N2 and P2 latencies, and NRS) were identified. This contrasts with what has been reported for pain thresholds, which are significantly related to pain catastrophizing (i.e., higher PCS scores, lower pain thresholds)44,45,46,47. The lack of a relationship with PCS potentially indicates that CHEPs reflect underlying nociceptive processes that are independent of psychological factors.
Mechanism of improved CHEP acquisition with increased baseline temperature
In line with our earlier investigations5,48, an increased baseline protocol (42 °C) notably improved the acquisition of CHEPs. Adopting a higher baseline temperature results in shorter stimulus duration and higher synchronization of peripheral small-diameter afferents48. The clear benefit of this protocol is larger amplitude N2P2 waveforms. Using a higher baseline temperature has previously revealed preserved sensory sparing in individuals with absent pinprick and/or no sensation to conventional contact heat stimulation26,49. Thus, in addition to defining a level of injury, this protocol is more sensitive to assess the severity of damage in cutaneous areas with reduced sensation.
In order to decrease latency jitter (i.e., increase synchronization), previous studies have applied contact heat close to the midline11,50,51. This approach, which decreases peripheral conduction distances, has two major limitations in terms of segmental assessment of patients with cervical spinal cord disorders. First, from an anatomical perspective, cutaneous areas corresponding to single spinal levels are less well defined at the midline23. Comparatively, dermatomal overlap is less pronounced distally23. Thus, testing cervical segments distally (i.e., C4 on the shoulder and C6/C8 on the hands) is more ideal for the segmental assessment of damage in the spinal cord. Second, stimulating along the midline, particularly along the back, may be difficult for some patients with limited mobility. For example, positioning an individual with a severe spinal cord injury on their stomach can be uncomfortable and require a considerable amount of assistance. Comparatively, testing the arm and hands allows a patient to be comfortably and safely positioned in a sitting or supine position. Taken together, incorporating the increase baseline stimulation in the segmental assessment provides an elegant and clinically feasible alternative to improve the acquisition of CHEPs without the need to test at the midline.
Potential strategies to examine pathology
The goal of establishing normative CHEPs values in cervical dermatomes was primary but not limited for clinical applications in traumatic spinal cord injury and cervical spondylotic myelopathy. While traumatic spinal cord injury is a relatively rare event, diagnosing level of injury and monitoring changes near the level of lesion (i.e., syringomyelia) is of paramount importance6,8,26,52. Diagnosing the level of impairment and monitoring deteriorating neurological function is equally important in the case of cervical spondylotic myelopathy, for which surgical intervention may be indicated. Conventionally, sensory function following damage to the spinal cord is evaluated by testing light touch and pinprick sensation (i.e., International Standards for the Neurological Classification of SCI, ISNCSCI)53. However, the subjective nature (one has to rely on the patient’s appreciation) and classification with an ordinal scale limits their sensitivity to detect pathology. Sensory evoked potentials, such as CHEPs, constitute a complementary strategy to overcome these limitations based on objective and quantitative information (i.e., amplitudes and latencies). The overall reliability and validity of CHEPs was demonstrated previously6. Assessing multiple dermatomes along the spinal axis spanning the anatomical area of damage allows the precise determination of the level of lesion and potentially provides information about the injury severity. Moreover, clinical meaningful changes (recovery or aggravation of sensory function) occurring close to the injury level can be disclosed and rigorously tracked over time.
The question now turns to how CHEPs can be interpreted in a clinical setting. The difficulty with interpreting impairments based on amplitude is two-fold. First, there is a prominent effect of arousal and attention, well established in the literature54,55,56. Second, between-subject variability in amplitude is high, and this persists to for increased baseline temperature. For example, an objective cut-off to classify CHEPs as pathological would require, particularly in older male subjects, N2P2 amplitudes that are difficult to distinguish from background “noise”. Testing a contralateral side would overcome this limitation; however, spinal cord damage is often bilateral, so there may not always be a “normal” matching dermatome for comparison.
To overcome these problems, latency has been proposed as a more sensitive measure of pathology17. Compared to LEPs, however, the conventional acquisition of CHEPs remains disadvantaged by higher between-subject N2 latency variability57. Presumably, this is related to longer stimulus durations, which introduce heterogeneity in peripheral recruitment. Based on our observations, increasing the baseline temperature preceding contact heat stimulation has the significant potential to improve the clinical interpretation of evoked potentials. Indeed, adopting this protocol resulted in between-subject variance (Fig. 3) comparable to reported LEPs17. Therefore, this protocol not only improves the acquisition of CHEPs at the single subjects (i.e., increases amplitude and the likelihood to detect afferent sparing)5,6,48, but also interpretation at the group level.
Limitations
A major limitation of our study is that normative values are limited to studies or clinical sites using the same CHEP acquisition equipment. Centers interested in adopting CHEPs will still need to develop their own normative data. Also, increased baseline stimulation may not be tolerated for some individuals. This was the case for two individuals in our study. However, we have previously demonstrated that increasing the baseline temperature and adjusting the peak stimulation temperature results in improved CHEP acquisition48. This approach could then be utilized in subjects that report high pain ratings to a fixed 52 °C stimulation.
Conclusion
The current study provides normative CHEPs data in a large cohort of healthy subjects from segmentally examined cervical dermatomes (C4, C6, and C8). Age and sex were identified as important factors contributing to N2 latency and N2P2 amplitude variability. Employing an increased baseline stimulation protocol reduced latency jitter within subjects, yielding larger N2P2 amplitudes, and substantially decreased inter-subject N2 latency variability. The latter is important in terms of generalizing our findings to the interpretation of pathological CHEPs in patient populations. The normative data can be used to diagnose a variety of spinal cord pathologies, as well as track changes in sensory function emerging close to the level of lesion. The recovery of a patient but also the success of therapeutic intervention (e.g., surgery or drugs) can be monitored by the segmental assessment approach.
Additional Information
How to cite this article: Jutzeler, C. R. et al. Normative data for the segmental acquisition of contact heat evoked potentials in cervical dermatomes. Sci. Rep. 6, 34660; doi: 10.1038/srep34660 (2016).
References
Chen, A. C., Niddam, D. M. & Arendt-Nielsen, L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci Lett 316, 79–82 (2001).
Chen, A. C., Niddam, D. M., Crawford, H. J., Oostenveld, R. & Arendt-Nielsen, L. Spatial summation of pain processing in the human brain as assessed by cerebral event related potentials. Neurosci Lett 328, 190–194 (2002).
Chen, I. A. et al. Contact heat evoked potentials in normal subjects. Acta Neurol Taiwan 15, 184–191 (2006).
Iannetti, G. D. et al. Simultaneous recording of laser-evoked brain potentials and continuous, high-field functional magnetic resonance imaging in humans. Neuroimage 28, 708–719, 10.1016/j.neuroimage.2005.06.060 (2005).
Kramer, J. L., Haefeli, J., Curt, A. & Steeves, J. D. Increased baseline temperature improves the acquisition of contact heat evoked potentials after spinal cord injury. Clin Neurophysiol 123, 582–589, 10.1016/j.clinph.2011.08.013 (2012).
Kramer, J. L. et al. Test-retest reliability of contact heat-evoked potentials from cervical dermatomes. J Clin Neurophysiol 29, 70–75, 10.1097/WNP.0b013e318246ada2 (2012).
Roberts, K. et al. Contact Heat Evoked Potentials Using Simultaneous Eeg And Fmri And Their Correlation With Evoked Pain. BMC Anesthesiol 8, 8, 10.1186/1471-2253-8-8 (2008).
Ulrich, A., Haefeli, J., Blum, J., Min, K. & Curt, A. Improved diagnosis of spinal cord disorders with contact heat evoked potentials. Neurology 80, 1393–1399, 10.1212/WNL.0b013e31828c2ed1 (2013).
Valeriani, M., Le Pera, D., Niddam, D., Chen, A. C. & Arendt-Nielsen, L. Dipolar modelling of the scalp evoked potentials to painful contact heat stimulation of the human skin. Neurosci Lett 318, 44–48 (2002).
Wydenkeller, S., Wirz, R. & Halder, P. Spinothalamic tract conduction velocity estimated using contact heat evoked potentials: what needs to be considered. Clin Neurophysiol 119, 812–821, 10.1016/j.clinph.2007.12.007 (2008).
Granovsky, Y. et al. Normative data for Adelta contact heat evoked potentials (CHEPs) in adult population: A multicenter study. Pain, 10.1097/j.pain.0000000000000495 (2016).
Truini, A. et al. Trigeminal small-fibre function assessed with contact heat evoked potentials in humans. Pain 132, 102–107, 10.1016/j.pain.2007.01.030 (2007).
Chao, C. C. et al. Pathophysiology of neuropathic pain in type 2 diabetes: skin denervation and contact heat-evoked potentials. Diabetes Care 33, 2654–2659, 10.2337/dc10-1135 (2010).
Wong, M. C. & Chung, J. W. Feasibility of contact heat evoked potentials for detection of diabetic neuropathy. Muscle Nerve 44, 902–906, 10.1002/mus.22192 (2011).
Truini, A. et al. Laser-evoked potentials in post-herpetic neuralgia. Clin Neurophysiol 114, 702–709 (2003).
Lagerburg, V. et al. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle Nerve 51, 743–749, 10.1002/mus.24465 (2015).
Truini, A. et al. Laser-evoked potentials: normative values. Clin Neurophysiol 116, 821–826, 10.1016/j.clinph.2004.10.004 (2005).
Sullivan, M. B., S. R. & Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess 7, 524–532 (1995).
Meyer, K., Sprott, H. & Mannion, A. F. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res 64, 469–478, 10.1016/j.jpsychores.2007.12.004 (2008).
D’Eon, J. L., Harris, C. A. & Ellis, J. A. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med 27, 361–372 (2004).
Osman, A. et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 23, 351–365 (2000).
Osman, A. et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 20, 589–605 (1997).
Lee, M. W., McPhee, R. W. & Stringer, M. D. An evidence-based approach to human dermatomes. Clin Anat 21, 363–373, 10.1002/ca.20636 (2008).
Treede, R. D., Meyer, R. A. & Campbell, J. N. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 80, 1082–1093 (1998).
Haefeli, J., Kramer, J. L., Blum, J. & Curt, A. Heterotopic and homotopic nociceptive conditioning stimulation: distinct effects of pain modulation. Eur J Pain 18, 1112–1119, 10.1002/j.1532-2149.2014.00454.x (2014).
Haefeli, J., Kramer, J. L., Blum, J. & Curt, A. Assessment of Spinothalamic Tract Function Beyond Pinprick in Spinal Cord Lesions: A Contact Heat Evoked Potential Study. Neurorehabil Neural Repair 28, 494–503, 10.1177/1545968313517755 (2013).
Cruccu, G. et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol 119, 1705–1719, 10.1016/j.clinph.2008.03.016 (2008).
Kakigi, R. et al. Human brain processing and central mechanisms of pain as observed by electro- and magneto-encephalography. J Chin Med Assoc 67, 377–386 (2004).
Treede, R. D., Kief, S., Holzer, T. & Bromm, B. Late somatosensory evoked cerebral potentials in response to cutaneous heat stimuli. Electroencephalogr Clin Neurophysiol 70, 429–441 (1988).
Bromm, B. & Chen, A. C. Brain electrical source analysis of laser evoked potentials in response to painful trigeminal nerve stimulation. Electroencephalogr Clin Neurophysiol 95, 14–26 (1995).
Albu, S. et al. Modulation of thermal somatosensory thresholds within local and remote spinal dermatomes following cervical repetitive magnetic stimulation. Neurosci Lett 555, 237–242, 10.1016/j.neulet.2013.06.067 (2013).
Jutzeler, C. R., Curt, A. & Kramer, J. L. Effectiveness of High-Frequency Electrical Stimulation Following Sensitization With Capsaicin. J Pain 16, 595–605, 10.1016/j.jpain.2015.03.005 (2015).
Cruccu, G. et al. Assessment of trigeminal small-fiber function: brain and reflex responses evoked by CO2-laser stimulation. Muscle Nerve 22, 508–516 (1999).
Chao, C. C., Hsieh, S. T., Chiu, M. J., Tseng, M. T. & Chang, Y. C. Effects of aging on contact heat-evoked potentials: the physiological assessment of thermal perception. Muscle Nerve 36, 30–38, 10.1002/mus.20815 (2007).
Gagliese, L. & Melzack, R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev 24, 843–854 (2000).
Gibson, S. J. & Helme, R. D. Age-related differences in pain perception and report. Clin Geriatr Med 17, 433–456, v-vi (2001).
Chakour, M. C., Gibson, S. J., Bradbeer, M. & Helme, R. D. The effect of age on A delta- and C-fibre thermal pain perception. Pain 64, 143–152 (1996).
Derbyshire, S. W., Nichols, T. E., Firestone, L., Townsend, D. W. & Jones, A. K. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain 3, 401–411 (2002).
Lautenbacher, S. & Strian, F. Sex differences in pain and thermal sensitivity: the role of body size. Percept Psychophys 50, 179–183 (1991).
Croft, P. R. & Rigby, A. S. Socioeconomic influences on back problems in the community in Britain. J Epidemiol Community Health 48, 166–170 (1994).
Otto, M. W. & Dougher, M. J. Sex differences and personality factors in responsivity to pain. Percept Mot Skills 61, 383–390, 10.2466/pms.1985.61.2.383 (1985).
Robinson, M. E., Wise, E. A., Gagnon, C., Fillingim, R. B. & Price, D. D. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain 5, 77–82, 10.1016/j.jpain.2003.11.004 (2004).
Sullivan, M. J. L. T. D. A. & Santor, A. Gender Differences in Pain and Pain Behavior: The Role of Catastrophizing. Cognitive Therapy and Research 24, 121–134 (2000).
Bartley, E. J. & Fillingim, R. B. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111, 52–58, 10.1093/bja/aet127 (2013).
Fillingim, R. B., King, C. D., Ribeiro-Dasilva, M. C., Rahim-Williams, B. & Riley, J. L. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10, 447–485, 10.1016/j.jpain.2008.12.001 (2009).
Racine, M. et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain 153, 602–618, 10.1016/j.pain.2011.11.025 (2012).
Paulson, P. E., Minoshima, S., Morrow, T. J. & Casey, K. L. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76, 223–229 (1998).
Kramer, J. L., Haefeli, J., Jutzeler, C. R., Steeves, J. D. & Curt, A. Improving the acquisition of nociceptive evoked potentials without causing more pain. Pain 154, 235–241, 10.1016/j.pain.2012.10.027 (2013).
Haefeli, J. S., Blum, J., Steeves, J. D., Kramer, J. L. & Curt, A. E. Differences in spinothalamic function of cervical and thoracic dermatomes: insights using contact heat evoked potentials. J Clin Neurophysiol 30, 291–298, 10.1097/WNP.0b013e31827ed9ee (2013).
Cruccu, G. et al. Conduction velocity of the human spinothalamic tract as assessed by laser evoked potentials. Neuroreport 11, 3029–3032 (2000).
Iannetti, G. D. et al. Evidence of a specific spinal pathway for the sense of warmth in humans. J Neurophysiol 89, 562–570, 10.1152/jn.00393.2002 (2003).
Ulrich, A., Min, K. & Curt, A. High sensitivity of contact-heat evoked potentials in “snake-eye” appearance myelopathy. Clin Neurophysiol 126, 1994–2003, 10.1016/j.clinph.2014.12.020 (2015).
Maynard, F. M., Jr. et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 35, 266–274 (1997).
Beydoun, A., Morrow, T. J., Shen, J. F. & Casey, K. L. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol 88, 173–181 (1993).
Qiu, Y., Inui, K., Wang, X., Tran, T. D. & Kakigi, R. Effects of attention, distraction and sleep on CO(2) laser evoked potentials related to C-fibers in humans. Clin Neurophysiol 113, 1579–1585 (2002).
Garcia-Larrea, L., Peyron, R., Laurent, B. & Mauguiere, F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport 8, 3785–3789 (1997).
Baumgartner, U., Greffrath, W. & Treede, R. D. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin 42, 267–280, 10.1016/j.neucli.2012.06.002 (2012).
Acknowledgements
We would like to thank all of the subjects participating in our study. The study was supported by the Swiss National Science Foundation (SNF; Grant No. 320030 135558) and the Clinical Research Priority Program “Neurorehab” of the University of Zurich, Switzerland. Catherine Jutzeler is funded by a postdoctoral research fellowship from the International Foundation for Research in Paraplegia (IRP). John Kramer is supported by a Michael Smith Foundation for Health Research and Rick Hansen Scholar Award.
Author information
Authors and Affiliations
Contributions
C.R.J. contributed substantially to the conception and design of the study, the data acquisition, analysis, and interpretation. Furthermore, she drafted the research article. J. Rosner, was substantially involved in the data collection, data analysis, and revising the research article. J. Rinert, was involved in the data collection, data analysis, and revising the research article. J.L.K.K. contributed substantially to the data analysis and interpretation, and was involved in drafting the research article. A.C. made substantial contributions to study conception and design as well as participated in revising the research article critically for important intellectual content.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jutzeler, C., Rosner, J., Rinert, J. et al. Normative data for the segmental acquisition of contact heat evoked potentials in cervical dermatomes. Sci Rep 6, 34660 (2016). https://doi.org/10.1038/srep34660
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34660
This article is cited by
-
EEG-based sensory testing reveals altered nociceptive processing in elite endurance athletes
Experimental Brain Research (2023)
-
Improved acquisition of contact heat evoked potentials with increased heating ramp
Scientific Reports (2022)
-
An intensity matched comparison of laser- and contact heat evoked potentials
Scientific Reports (2021)
-
Exploring the effect of capsaicin-induced central sensitization on the upper limb nociceptive withdrawal reflex threshold
Experimental Brain Research (2021)
-
Degenerative cervical myelopathy — update and future directions
Nature Reviews Neurology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.