Abstract
Gamma-glutamyltransferase (GGT) is an established marker for proliferative/apoptotic balance and has been associated with cancer risk and prognosis. The aim of this study was to evaluate the value of pre-treatment GGT serum levels as prognostic biomarker in patients with primary uterine leiomyosarcoma (ULMS). Data of women with ULMS were extracted from a multi-center database. Pre-treatment GGT serum levels were measured and patients assigned to predefined GGT risk groups. GGT values were correlated with clinico-pathological parameters and univariate and multivariable survival analyses were performed. A total of 44 patients with ULMS were analyzed. Mean (SD) pre-therapeutic GGT serum level was 33.8 (39.8) U/L. In Figo Stage I versus II-IV mean (SD) GGT values were 28.8 (34.0) U/l and 43.5 (49.2) U/l, respectively (p = 0.25). Five-year overall survival (OS) rates in ULMS patients with normal low versus higher GGT levels were 70% and 37%, respectively (p = 0.043). Univariate and multivariable analyses revealed that higher GGT serum levels (p = 0.043, p = 0.005) and high histological grade (p = 0.029, p = 0.012) were independently associated with impaired OS, respectively. Higher pre-treatment GGT serum levels were independently associated with unfavorable prognosis in women with ULMS. Thus, GGT seems to be a useful novel biomarker in ULMS.
Similar content being viewed by others
Introduction
Gamma-glutamyltransferase (GGT) is a membrane bound enzyme essential in the glutathione (GSH) metabolism that protects cells from reactive oxygen species1. GGT is an established biomarker for apoptotic balance and promotes carcinogenesis, malignant transformation and tumor progression2,3. It has been described that pathologic states of oxidative stress, as found within carcinogenesis increase GSH and GGT levels1,4.
Serum GGT was shown to be associated with an increased risk of cancer development5,6 and is elevated in a variety of solid tumors7. Furthermore, GSH may play a relevant role in the development of chemo-resistance in malignancies including soft tissue sarcoma8. Recent studies investigated the prognostic role of serum GGT and GGT expression in various gynecologic malignancies including endometrial, cervical and ovarian cancer9,10,11.
ULMS can be considered as an orphan disease with distinctive clinical differences to non-uterine leiomyosarcoma and reports on valid pre-therapeutic prognostic biomarkers are limited12. The aim of the present multicenter study was to investigate the influence of pre-therapeutic GGT on survival of patients with ULMS.
Results
Patients’ characteristics are provided in Table 1. Mean (SD) pre-therapeutic GGT serum levels in patients with ULMS were 33.8 (39.8) U/L. Patients were assigned to previously defined GGT groups10,13. We observed a non-significant trend that higher pre-therapeutic GGT serum levels were associated with more advanced tumor stage and large tumor volume, when compared to patients with normal-low GGT (Table 2).
In univariate and multivariable survival analysis GGT serum levels, patients’ age, tumor stage and histological grading were analyzed as prognostic parameters for OS. Table 3 provides results of the univariate Kaplan-Meier analysis and the multivariable Cox regression model. Women with higher pre-treatment GGT serum levels showed impaired OS compared to women with normal low levels (<17.99 U/l) and 5-year OS rates of 37% and 70% were observed, respectively (p = 0.043). Kaplan-Meier survival curves, showing the association between pre-therapeutic GGT serum groups and OS are shown in Fig. 1.
In a sensitivity analysis we dichotomized GGT at the 25th percentile value of 17.5, which resulted in an adjusted hazard ratio of 2.77 (95% CI 0.93–10.8, p = 0.068). In multivariable analysis higher GGT serum levels, advanced patients’ age and high-grade histology were associated with unfavorable prognosis. Using age as a continuous variable in the Cox model resulted in a hazard ratio for age (per decade) of 0.62 (95% CI 0.33–1.24, p = 0.168), but did not materially change the hazard ratio for GGT (HR 1.80 per each doubling of GGT, 95% CI 1.19–2.68, p = 0.006).
Interestingly, in our cohort 29 patients had FIGO stage I ULMS. In eight of these patients cancer-related death was observed and seven (87.5%) of these patients had GGT serum levels of ≥17.99 U/L. A total of 10 (47.6%) of the remaining 21 patients with FIGO stage I were still alive within the observation period had GGT serum levels of ≥17.99 U/L. With respect to tumor grade all 8 patients that died from FIGO I ULMS revealed grade 3 histology. Within this cohort, 4 and 3 patients had a maximal tumor size ≤5 cm and >5 cm, respectively. The tumor size of one patient was not exactly documented. Out of 4 patients with a tumour size ≤5 cm and fatal outcome 3 (75%) patients had high serum GGT levels (≥17.99 U/L).
Discussion
The present study shows that pre-therapeutic serum GGT is a novel prognostic biomarker for patients with ULMS, with higher levels being associated with worse outcomes. Multivariable analysis revealed prognostic relevance of GGT independent of established parameters such as age, FIGO stage and tumor size. Results of previous studies indicate that GGT reflects a valid prognostic biomarker for different solid tumors, including gynecologic malignancies but no data for ULMS have been published so far9,10,14. From a clinical view it seems plausible that GGT, a marker of oxidative stress, provides prognostic information. By revealing the amount of pathologic oxidative stress in cancer patients, GGT most likely reflects the extent of malignant cell transformation and cell turnover caused not only by the extent of tumor load, but also by the tumor’s aggressiveness and biological behavior11. In our study, GGT serum levels were only slightly (non-significantly) higher in patients with large tumors (>10 cm) and advanced stage (FIGO II-IV). On the other hand, 7 out of 8 patients that died from early stage disease had elevated GGT serum levels. In the past years, several studies investigated the biology of GGT and its’ impact on cancer development, progression and drug resistance15. GGT and GSH play an important role in the cellular antioxidant defense mechanism by binding reactive oxygen species1,16. Interestingly, several studies showed that GGT influences the cellular proliferative-apoptotic balance by exerting pro-oxidant effects at the membrane surface level and in the extracellular microenvironment7,15. By providing cysteine, GSH has been linked with rapid tumor growth and cancer survival2. Since expression of GGT provides a growth advantage for the tumor tissue, it seems reasonable that elevated GGT serum levels are associated with rapidly growing and large tumors. This would explain that patients with small tumors but high GGT serum levels, as seen in this cohort, were associated with poor prognosis.
Enhancing the capacity of GSH and its associated enzymes, in order to protect cells from redox-related changes or environmental toxins, represents a persistent aim in the search for cyto-protective strategies against cancer. Novel compounds that act as uncompetitive inhibitors of human GGT with low toxicity and a large therapeutic window were already tested in preclinical models. New potent agents are currently in the process of further development with GGT as a potential therapeutic target17.
Due to the low prevalence of the disease, patients with ULMS are often studied together with non-uterine LMS or soft tissue sarcoma (STS) patients12. Glutathione concentration and GGT activity were previously investigated in extremity STS8. It was found that glutathione and GGT were significantly elevated in patients with aggressive disease, especially in high-grade and metastatic STS. In addition, increased glutathione levels were found in pretreated tumors previously exposed to doxorubicin-based chemotherapy. Therefore it was postulated that glutathione might play a role in the development of resistance to chemotherapeutic agents in STS that might subsequently influence patients’ prognosis. The mechanism underlying the correlation between expression of GGT and drug resistance seems to be the ability of GGT to cleave extracellular GSH and thereby, provide cells with an additional source of cysteine with which to increase intracellular GSH levels18.
Owing to the retrospective design of this study several limitations have to be taken into account. Firstly, this retrospective study recruited patients from a long treatment period of more than 15 years. In this period of time therapeutic regimes for patients with ULMS have changed profoundly and the influence of this change could not be reflected in this trial. Secondly, the number of patients included into this study is relatively small. On the other hand, ULMS is an orphan disease for which only few prognostic parameters are established yet. This is the first study that evaluated pre-therapeutic serum GGT levels as a potential prognostic parameter in this population. Therefore, we think that these findings might be interesting from a clinical point of view and seem biologically plausible. Undoubtedly, the results found retrospectively need to be confirmed in future studies. At present, GGT is widely used in clinical routine mainly to monitor liver function and is a cost-effective and readily available prognostic parameter. Especially due to the lack of reliable prognostic serum biomarkers for women diagnosed with ULMS, serum GGT levels might be clinically useful, when included in future trials.
An interesting aspect is the potential role of GGT serum levels for screening purposes that has been investigated in several recent studies. The population-based AMORIS study showed an association between elevated serum GGT levels and overall cancer risk19. A similar study showed an association between elevated GGT serum levels and increased cervical cancer risk6. However, in both studies no GGT treshold for a population based screening has been established6,19. ULMS is considered an orphan disease, therefore population based screening for ULMS might not be reasonable. However, in clinical practice patients with rapidly growing uterine myoma, or post-menopausal women with growing myoma are often suspected to have underlying malignancies of the uterus in clinical practice. One might argue that in these patients GGT measurements could be useful to differentiate between myoma and ULMS. However, to the best of our knowledge there are no data supporting this strategy and future studies are necessary to investigate the potential role of GGT for differential diagnosis in this cohort.
In summary, we identified GGT as promising independent prognostic biomarker in women with ULMS. If the current results can be validated in an independent prospective trial, it might be reasonable to divide ULMS patients into subgroups according to GGT-levels to facilitate patient counseling and treatment surveillance. Until then, our results may be considered when planning future clinical trials.
Further research is needed to address the biological mechanisms linking GGT to ULMS progression and prognosis.
Materials and Methods
Patients
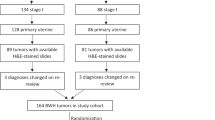
A total of 44 consecutive patients diagnosed with ULMS were treated between 1996 and 2014 at the Comprehensive Cancer Center Vienna, Vienna, Department of Obstetrics and Gynecology of the Medical University of Graz and the Department of Gynecology, Barmherzige Schwestern Hospital Linz, Austria and enrolled in the present study. Clinical data were obtained using available tumor databases and by electronic chart review. The 2009 International Federation of Gynecology and Obstetrics (FIGO) classification system was used20. Primary tumor assessment was performed by clinical examination, transvaginal sonography and in some cases with magnetic resonance imaging (MRI) and/or computed tomography (CT). Treatment consisted of surgery including hysterectomy and bilateral salpingo-oophorectomy. Pelvic and/or paraaortic lymphadenectomy was carried out in the case of intraoperative palpably enlarged lymph nodes. Surgical cytoreduction was performed in women with extrauterine disease as described previously12. If clinically indicated, radiation therapy, adjuvant and/or palliative chemotherapy was given based on the physicians’ choice. In all patients included in this study a pre-therapeutic physical examination by a specialist in internal medicine was performed in order to rule out the presence of liver diseases. Patients with liver diseases or liver metastasis were not eligible for analysis. All patients were included into the institutions’ follow-up care program. The latter included clinical examination. Patients were seen every three to four months for the first three years, every six months for the following two years and afterwards annually up to ten years. If recurrent disease was suspected imaging methods based on the physicians choice were conducted.
Ethics
The study was approved by the Ethics Committee of the Medical University of Vienna (IRB approval number: 1520/2012). All patients consented to treatment according to institutional guidelines and all patients had consented to anonymized assessments and analysis of data and outcome of therapy. Since this study was a retrospective analysis the ethics committee waived the requirement to obtain distinct informed consent from patients. All patient records were anonymized and de-identified prior to analysis. This study was performed in accordance to the ICH Harmonized Tripartite Guideline for Good Clinical Practice, the Declaration of Helsinki and the guidelines of the Ethics Committee of the Medical University of Vienna.
GGT Measurement
As part of clinical routine, blood samples for evaluation of serum GGT levels were obtained by peripheral venous puncture 24–48 hours prior to primary surgery. GGT concentrations were analyzed with an enzyme kinetic assay (Modular Hitachi 747 and Hitachi 917, Roche Diagnostics), as described previously21. Patients were assigned to the previously described GGT groups as follows: GGT < 17.99 U/L: group A (normal low), 18.00 to 35.99 U/L: group B (normal high), 36.00 to 71.99 U/L: group C (elevated) and >72.00 U/L: group D (highly elevated)10,13.
Statistical Analysis
Values are given as mean (standard deviation [SD]). Students’ T- tests and one-way ANOVA tests were applied to compare mean GGT serum levels and clinico-pathological findings. P-values of <0.05 were considered statistically significant. Survival probabilities were calculated by the product limit method of Kaplan and Meier. Differences between groups were tested using the log-rank test. The results were analyzed for the endpoint of overall survival (OS). Survival times of patients that were still alive at the last follow up visit were censored with the last follow-up date. Univariate survival analysis was performed using log-rank test and Cox Regression analysis. Kaplan-Meier survival curves were computed. Multivariable analysis was conducted using Cox regression including as independent variables log2-transformed GGT and the clinically relevant variables age (dichotomized at the median value of 55.3), tumor stage (FIGO II-IV vs. FIGO I) and grading (G2-G3 vs. G1). Because there were no events in the G1 group, ordinary maximum likelihood estimation could not be performed and was replaced by the penalized likelihood method recommended for such situations by22. Statistical analysis was performed using the commercially available statistical software SPSS 23.0 for MAC (SPSS 23.0, IBM Inc., Armonk, NY). All analyzed data are available as Supplementary Database 1.
Additional Information
How to cite this article: Schwameis, R. et al. Gamma-glutamyltransferase as novel biomarker in patients with uterine leiomyosarcoma. Sci. Rep. 6, 33757; doi: 10.1038/srep33757 (2016).
References
Whitfield, J. B. Gamma glutamyl transferase. Critical reviews in clinical laboratory sciences 38, 263–355, doi: 10.1080/20014091084227 (2001).
Hanigan, M. H. Expression of gamma-glutamyl transpeptidase provides tumor cells with a selective growth advantage at physiologic concentrations of cyst(e)ine. Carcinogenesis 16, 181–185 (1995).
Hanigan, M. H., Gallagher, B. C., Townsend, D. M. & Gabarra, V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20, 553–559 (1999).
Pompella, A., De Tata, V., Paolicchi, A. & Zunino, F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochemical pharmacology 71, 231–238, doi: 10.1016/j.bcp.2005.10.005 (2006).
Mok, Y., Son, D. K., Yun, Y. D., Jee, S. H. & Samet, J. M. gamma-Glutamyltransferase and cancer risk: The Korean cancer prevention study. International journal of cancer. Journal international du cancer 138, 311–319, doi: 10.1002/ijc.29659 (2016).
Strasak, A. M. et al. Prospective study of the association of serum gamma-glutamyltransferase with cervical intraepithelial neoplasia III and invasive cervical cancer. Cancer research 70, 3586–3593, doi: 10.1158/0008-5472.CAN-09-3197 (2010).
Corti, A., Franzini, M., Paolicchi, A. & Pompella, A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer research 30, 1169–1181 (2010).
Hochwald, S. N., Rose, D. M., Brennan, M. F. & Burt, M. E. Elevation of glutathione and related enzyme activities in high-grade and metastatic extremity soft tissue sarcoma. Annals of surgical oncology 4, 303–309 (1997).
Seebacher, V. et al. Prognostic significance of gamma-glutamyltransferase in patients with endometrial cancer: a multi-centre trial. British journal of cancer 106, 1551–1555, doi: 10.1038/bjc.2012.16 (2012).
Polterauer, S. et al. Relevance of gamma-glutamyltransferase–a marker for apoptotic balance–in predicting tumor stage and prognosis in cervical cancer. Gynecol Oncol 122, 590–594, doi: 10.1016/j.ygyno.2011.05.027 (2011).
Grimm, C. et al. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. British journal of cancer 109, 610–614, doi: 10.1038/bjc.2013.323 (2013).
Lamm, W. et al. Distinctive outcome in patients with non-uterine and uterine leiomyosarcoma. BMC cancer 14, 981, doi: 10.1186/1471-2407-14-981 (2014).
Franzini, M. et al. Modulation of cell growth and cisplatin sensitivity by membrane gamma-glutamyltransferase in melanoma cells. European journal of cancer 42, 2623–2630, doi: 10.1016/j.ejca.2006.04.016 (2006).
Staudigl, C. et al. Prognostic relevance of pretherapeutic gamma-glutamyltransferase in patients with primary metastatic breast cancer. Plos One 10, e0125317, doi: 10.1371/journal.pone.0125317 (2015).
Hofbauer, S. L. et al. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. British journal of cancer 111, 1526–1531, doi: 10.1038/bjc.2014.450 (2014).
Pastore, A., Federici, G., Bertini, E. & Piemonte, F. Analysis of glutathione: implication in redox and detoxification. Clinica chimica acta; international journal of clinical chemistry 333, 19–39 (2003).
Wickham, S. et al. Inhibition of human gamma-glutamyl transpeptidase: development of more potent, physiologically relevant, uncompetitive inhibitors. The Biochemical journal 450, 547–557, doi: 10.1042/BJ20121435 (2013).
Hanigan, M. H. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Advances in cancer research 122, 103–141, doi: 10.1016/B978-0-12-420117-0.00003-7 (2014).
Van Hemelrijck, M. et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. European journal of cancer 47, 2033–2041, doi: 10.1016/j.ejca.2011.03.010 (2011).
Prat, J. FIGO staging for uterine sarcomas. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 104, 177–178, doi: 10.1016/j.ijgo.2008.12.008 (2009).
Kazemi-Shirazi, L. et al. Gamma glutamyltransferase and long-term survival: is it just the liver? Clinical chemistry 53, 940–946, doi: 10.1373/clinchem.2006.081620 (2007).
Heinze, G. & Schemper, M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 57, 114–119 (2001).
Acknowledgements
This study was supported by the Department of General Gynecology and Gynecologic Oncology, Medical University Vienna and the Karl Landsteiner Institute for General Gynecology and Experimental Gynecologic Oncology, Vienna, Austria.
Author information
Authors and Affiliations
Contributions
R.S. conceived the manuscript, performed data acquisition, analyzed data, C.G. analyzed data, T.B. performed data acquisition, E.P. performed data acquisition, K.H.-F. performed data acquisition, C.S. performed data acquisition, A.R. conceived manuscript, performed data analysis, G.H. helped with data analysis, S.P. conceived manuscript, performed data analysis, wrote ethics protocol, M.P. conceived manuscript, performed data acquisition, analyzed data. All authors have reviewed the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Schwameis, R., Grimm, C., Brodowicz, T. et al. Gamma-glutamyltransferase as novel biomarker in patients with uterine leiomyosarcoma. Sci Rep 6, 33757 (2016). https://doi.org/10.1038/srep33757
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33757
This article is cited by
-
Circulating Transcripts and Biomarkers in Uterine Tumors: Is There a Predictive Role?
Current Oncology Reports (2020)
-
Prognostic role of plasma fibrinogen in patients with uterine leiomyosarcoma – a multicenter study
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.