Abstract
Meta-analyses on coffee and cancer incidence mainly restricted to limited cancers. We carried out a more comprehensive meta-analysis of cohort studies to explore association between coffee and most cancer types. We conducted comprehensive search and summarized relative risk (RR) and 95% confidence intervals for the highest versus lowest coffee intake and cancer using STATA12. We conducted dose-analysis if result suggested significant association. The publication bias was evaluated with begg’s and egger’s test. Finally, 105 individual prospective studies were included. Inverse associations were observed on oral, pharyngeal, colon, liver, prostate, endometrial cancer and melanoma, with RR 0.69 (95% CI = 0.48–0.99, I2 = 73.4%, P = 0.044), 0.87 (95% CI = 0.78–0.96, I2 = 28.4%, P = 0.007), 0.46 (95% CI = 0.37–0.57, I2 = 0%, P = 0), 0.89 (95% CI = 0.84–0.93, I2 = 30.3%, P = 0.003), 0.73 (95% CI = 0.67–0.80, I2 = 0%, P = 0) and 0.89 (95% CI = 0.80–0.99, I2 = 0%, P = 0.031) respectively. However, the relative risk for lung cancer is 2.18 (95% CI = 1.26–3.75, I2 = 63.3%, P = 0.005). The summary relative risk for increment of 2 cups of coffee were RR = 0.73, 95% CI = 0.67–0.79 for liver cancer, RR = 0.97, 95% CI = 0.96–0.98 for prostate cancer and RR = 0.88, 95% CI = 0.85–0.92 for endometrial cancer. Accordingly, coffee intake was associated with reduced risk of oral, pharynx, liver, colon, prostate, endometrial cancer and melanoma and increased lung cancer risk.
Similar content being viewed by others
Introduction
Cancer is a major cause of morbidity and mortality worldwide. In 20121,2, there were 14.1 million new cancer cases and 8.2 million cancer deaths globally and the global burden from cancer has become significantly high. Although the strategy for cancer treatment has improved much, cancer is still the most difficult disease to overcome. Early diagnosis and treatment may provide good chance for cancer patients to improve overall prognosis. However, there is no promising survival for those who suffer from advanced cancer. Therefore, it is important to determine methods to prevent tumor occurrence. Nutrition, such as the consumption of citrus fruits, vegetables and coffee, has also been considered to play an important role in reducing cancer risk3,4,5.
Coffee is one of the most popular beverages worldwide and it has been speculated to decrease the risk of many types of cancers. Coffee is a complex mixture of many biologically active components, some of which may have anti-tumor effects. They include caffeine, cafestol, kahweol and chlorogenic acid. Although many standardized meta-analyses5,6,7,8,9,10,11,12,13,14,15,16,17,18 between coffee intake and various cancers have been conducted, most of analyses restricted to limited types of cancers. In addition, there is no relevant meta-analysis to explore relationships between coffee intake and some types of cancer including melanoma and lymphoma. Furthermore, dose-response meta-analyses were only performed for liver cancer and prostate cancer19,20, we endeavor to conduct such analysis for cancer as many as possible. Besides, most meta-analyses included prospective studies and case-control studies, which may lead to the unconvincing results and conclusions. However, we included prospective studies into our study. In order to present the relatively obvious associations between coffee intake and the incidence of most of cancer types, we carried out a more comprehensive systematic updated meta-analysis of cohort studies to explore the association between coffee consumption and most types of cancers. We try to provide a landscape of coffee and cancer incidence.
Methods
Search strategy
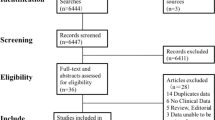
We conducted a computerized search of the literature on coffee and cancer from inception to July 2015. The search terms were (cancer OR tumor OR carcinoma) AND (coffee OR caffeine OR beverages OR diet OR drinking). Three major electronic databases (PubMed, EMBASE and The Cochrane Library) were used to search the relevant literature without language restriction. Then, we classified these studies into different groups according to cancer type and conducted repeated searches. Finally, we obtained a relatively complete literature record for every type of cancer. Moreover, we reviewed the references from retrieved articles for additional studies (Fig. 1).
Study selection
The included studies had to be prospective cohort, case-cohort, or nested case control studies and had to contain the association between coffee intake and cancer incidence. Case control studies and cross-sectional studies were excluded. Studies concerning the coffee intake and the mortality of cancer were also excluded. We also excluded the analysis about cancer if there was no enough to conduct meta-analysis or no updated prospective studies for previous meta-analysis. Each study had to provide estimates of hazard ratio (HR) or relative risk (RR) with 95% confidence intervals or numbers of cases and controls to calculate those values. For cancers for which a dose-response analysis could be conducted, the quantitative measure of intake and the total number of cases and person years or relative information to calculate them were necessary. Two independent reviewers evaluated the eligibility of every study in a standardized manner. We resolved disagreements by consensus.
Data abstraction
We extracted data from each study. These data included the first author’s name, year of publication, country of origin, number of cases and participants, sex, age, types of cancer, duration of follow-up and comparison of exposure level and adjusted confounding variables. The relative risks or hazard ratio and 95% confidence intervals for the highest versus the lowest intake were also extracted.
Statistical analysis
We summarized the overall relative risk and 95% confidence intervals for the highest versus lowest intake of coffee and various cancers using the fixed or random effects models of STATA12 (StataCorp, College Station, TX, USA). The command is metan logrr loglci loguci, eform label(namevar = study) fixed/random xlabel(), effect(RR). Metareg logrr study, wsse(se). The fixed and random effects models were conducted when there were no or low heterogeneity and medium or high heterogeneity, respectively. For studies that reported results by cancer type or sex, we combined them using fixed effects models. For studies that provided no relative risks and 95% confidence intervals, we calculated these values based on the number of cases and controls.
We conducted dose-response analyses for coffee and some types of cancers rather than all tumor types. When the summary RR indicates a significant association between coffee intake and cancer risk, we would conduct dose-response analysis for these types of cancers. Furthermore, the included studies for dose-response analysis had to be three at least. According to a previously described method21,22, information was required, such as the distribution of cases and person-years, variance of exposure levels and relative risks for at least three quantitative categories. The number of person-years was approximated from follow-up duration and number of subjects if the studies did not provide these values. We assigned the mid-point of the corresponding range of coffee intake as the exposure value in each category. When the highest category was open-ended, we assumed the width of the interval to be the same as the adjacent interval. When the lowest category was open-ended, we assigned zero as the lower boundary22. If coffee intake was reported as weeks or months instead of days, we computed the corresponding days. If coffee intake was not measured in cups, we used 150 ml23 as a cup to recalculate the intakes to a common scale. We present the dose-response results for a two-cup daily increment of coffee consumption. Using restricted cubic splines with four knots at percentiles 5%, 35%, 65% and 95% of the distribution; we evaluated the curve linear relation between coffee intake and risks of liver cancer, prostate cancer and endometrial cancer. The P value was calculated to test the null hypothesis that the coefficients of the second and third splines are equal to zero24,25. According to the result, we conducted linear or non-linear dose-response analysis.
We used the I2 value to evaluate if heterogeneity could be explained by study differences rather than by chance26. I2-values of approximately 25%, 50% and 75% indicate low, moderate and high heterogeneity. We did not use scores to assess the quality of the studies. Instead, we conducted subgroup analyses to evaluate the impact on overall RRs by cancer type, sex, follow-up period and some adjusted confounders, such as alcohol and BMI. Moreover, when the number of included studies was more than 10 and a substantial heterogeneity was observed, we would conduct subgroup analysis and meta-regression analysis to explore the potential source of heterogeneity. Furthermore, when the number of included studies was more than 10 and a substantial heterogeneity was observed, we would conduct subgroup analysis and meta-regression analysis to explore the potential source of heterogeneity.
We evaluated publication bias using Begg’s27 and Egger’s28 tests, with obvious publication bias suspected when P < 0.10. And we conducted a Trim and fill analysis29 to assess the stability of overall relative risk when the results suggested obvious publication bias. We conducted all of the statistical analyses using Stata Statistical Software, version 12.0.
Results
Digestive system cancer
Oral, pharynx cancer
Highest versus lowest intake: Six cohort studies30,31,32,33,34,35 were included in the analysis (1395309 samples) of the highest versus lowest intake of coffee and oral, pharynx cancer. The study characteristics are presented (Stable 1a). The summary RR was 0.69 (95% CI = 0.48–0.99, P = 0.044) with high heterogeneity (I2 = 73.4%, P = 0.002) (Fig. 2A). The results suggest no publication bias, with P = 1 for Begg’s test and P = 0.98 for Egger’s test. The subgroup analysis indicated that the inverse association was observed between coffee consumption and oral, pharynx cancer incidence in Asia and follow-up (>10 years) subgroup. The same relationship was observed in smoking adjustment subgroup, physical activity adjustment subgroup and total energy adjustment subgroup (Stable 1b).
Meta-analyses between coffee intake and risk of oral, pharynx cancer, colorectal cancer, lung cancer and melanoma.
Relative risks of oral, pharynx cancer (A), colorectal cancer (B), lung cancer (C) and melanoma (D) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Esophageal cancer
Highest versus lowest intake: Six cohort studies30,32,33,34,36,37 were included in the analysis (1395309 samples) of the highest versus lowest intake of coffee and esophageal cancer. The study characteristics are presented (Stable 1a). The summary RR was 0.86 (95% CI = 0.71–1.04, P = 0.124) with no heterogeneity (I2 = 0%, P = 0.64) (Fig. 3A). The results suggest no publication bias, with P = 1 for Begg’s test and P = 0.69 for Egger’s test. The subgroup analysis suggested that no significant association was observed between coffee intake and esophageal cancer incidence in each subgroup (Stable 1b).
Meta-analyses between coffee intake and risk of esophageal cancer and stomach cancer.
Relative risks of esophageal cancer (A) and stomach cancer (B) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Stomach cancer
Highest versus lowest intake: Twelve cohort studies30,31,33,38,39,40,41,42,43,44,45,46 were included in the analysis (1305447 samples) of the highest versus lowest intake of coffee and stomach cancer. The study characteristics are presented (Stable 1a). The summary RR was 1.15 (95% CI = 0.96–1.37, P = 0.121) with medium heterogeneity (I2 = 49.2%, P = 0.027) (Fig. 3B). The results suggest no publication bias, with P = 0.63 for Begg’s test and P = 0.85 for Egger’s test. The subgroup analysis indicated that coffee intake was associated with increased stomach cancer in USA and Asia. The same relationship was also observed in small number cases subgroup (<500 cases) and short follow-up subgroup (<10 years). The meta-regression analysis found no obvious to explain source of heterogeneity (Stable 1b).
Colorectal cancer
Highest versus lowest intake: Twenty-one cohort studies30,31,38,43,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63 were included in the analysis (2141185 samples) of the highest versus lowest intake of coffee and colorectal cancer. The study characteristics are presented (Stable 1a). The summary RR was 0.96 (95% CI = 0.91–1.02, P = 0.175) with low heterogeneity (I2 = 23.6%, P = 0.160) (Fig. 4A). The summary RRs for colon and rectal cancer were 0.87 (95% CI = 0.78–0.96, P = 0.007) and 0.94 (95% CI = 0.85–1.04, P = 0.236) (Fig. 2B). The results suggest no publication bias, with P = 0.70 for Begg’s test and P = 0.82 for Egger’s test. The subgroup analysis indicated that no significant association was observed between coffee intake and the risk of colorectal cancer in each subgroup. No substantial source of heterogeneity was found by meta-regression analysis (Stable 1b).
Meta-analyses between coffee intake and risk of colorectal cancer and pancreatic cancer.
Relative risks of colorectal cancer (A) and pancreatic cancer (B) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Pancreatic cancer
Highest versus lowest intake: Fifteen cohort studies30,31,38,44,64,65,66,67,68,69,70,71,72,73,74 were included in the analysis (1219019 samples) of the highest versus lowest intake of coffee and pancreatic cancer. The study characteristics are presented (Stable 1a). The summary RR was 1.02 (95% CI = 0.87–1.18, P = 0.832) with low heterogeneity (I2 = 16.2%, P = 0.27) (Fig. 4B). The results suggest no publication bias, with P = 0.43 for Begg’s test and P = 0.75 for Egger’s test. There was no significant association between coffee intake and the risk of pancreatic cancer in each subgroup through subgroup analysis. Meta-regression analysis found no factors explaining the source of heterogeneity (Stable 1b).
Liver cancer
Highest versus lowest intake: Nine cohort studies23,75,76,77,78,79,80,81,82 were included in the analysis (968517 samples) of the highest versus lowest intake of coffee and liver cancer. The study characteristics are presented (Stable 1a). The summary RR was 0.46 (95% CI = 0.37–0.57, P = 0) with no heterogeneity (I2 = 0%, P = 0.44) (Fig. 5A). The results suggest no publication bias, with P = 0.18 for Begg’s test and P = 0.23 for Egger’s test. The inverse association was observed between coffee consumption and liver cancer incidence in most subgroups through subgroup analysis. However, there was no significant relationship between coffee intake and the risk of liver cancer in women subgroup (Stable 1b).
Dose-response analyses between coffee intake and risk of liver cancer, prostate cancer and endometrial cancer.
Relative risks of liver cancer (A), prostate cancer (B) and endometrial cancer (C) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Dose-response analysis
We included seven studies23,75,76,77,78,79,80,82 for the dose-response analysis. There was no non-linear association between coffee intake and risk of liver cancer (P = 0.41 for non-linearity, Fig. 5A). A statistically significant inverse association was observed for liver cancer (RR = 0.73, 95% CI = 0.67–0.79) with an increased intake of 2 cups of coffee per day.
Tumors of the urinary system
Renal cancer
Highest versus lowest intake: Five cohort studies30,31,43,83,84 were included in the analysis (1036465 samples) of the highest group (samples/cases: 125065/144) versus lowest group (samples/cases: 232706/299) of coffee intake and renal cancer. The study characteristics are presented (Stable 2a). The summary RR was 0.79 (95% CI = 0.54–1.15, P = 0.226) with medium heterogeneity (I2 = 49.8%, P = 0.09) (Fig. 6A). The results suggest no publication bias, with P = 0.22 for Begg’s test and P = 0.14 for Egger’s test. There was no significant association between coffee intake and renal cancer risk in each subgroup through subgroup analysis (Stable 2bM). We did not found factors to explain the source of heterogeneity through subgroup analysis.
Meta-analyses between coffee intake and risk of renal cancer and bladder cancer.
Relative risks of renal cancer (A) and bladder cancer (B) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Bladder cancer
Highest versus lowest intake: Ten cohort studies30,31,38,85,86,87,88,89,90,91 were included in the analysis (340544 samples) of the highest versus lowest intake of coffee and bladder cancer. The study characteristics are presented (Stable 2a). The summary RR was 1.12 (95% CI = 0.94–1.34, P = 0.192) with medium heterogeneity (I2 = 39.6%, P = 0.094) (Fig. 6B). The results suggest publication bias, with P = 0.016 for Begg’s test and P = 0.01 for Egger’s test. The summary RR was 1.04 (95% CI = 0.88–1.23, P = 0.669) after Trim and fill analysis. The subgroup analysis indicated that coffee intake was associated with increased bladder cancer in USA (Stable 2b). No factor could explain the source of heterogeneity through subgroup analysis and meta-regression analysis (Stable 2b).
Prostate cancer
Highest versus lowest intake: Fourteen cohort studies30,31,38,43,92,93,94,95,96,97,98,99,100,101 were included in the analysis (864012 samples) of the highest versus lowest intake of coffee and prostate cancer. The study characteristics are presented (Stable 2a). The summary RR was 0.89 (95% CI = 0.84–0.93, P = 0.003) with medium heterogeneity (I2 = 30.3%, P = 0.14) (Fig. 5B). The results suggest no publication bias, with P = 0.83 for Begg’s test and P = 0.84 for Egger’s test. Through subgroup analysis, we found the inverse association between coffee intake and prostate cancer incidence in most subgroups. However, there was no significant relationship between coffee intake and the risk of prostate cancer in Canada and Asia (Stable 2b). The same associations were observed in follow-up (<10 years) subgroup and some adjustment confounders including no physical activity adjustment subgroup and no BMI adjustment subgroup (Stable 2b). Meta-regression found no substantial factor to explain the source of heterogeneity.
Dose-response analysis: We included ten studies30,31,92,94,96,97,98,99,100,101 in the dose-response analysis. There was no non-linear association between coffee intake and risk of prostate cancer (P = 0.15 for non-linearity, Fig. 5B). A statistically significant inverse association was observed for prostate cancer (RR = 0.97, 95% CI = 0.96–0.98) with an increased intake of 2 cups of coffee per day.
Female genital system neoplasm
Breast cancer
Highest versus lowest intake: Seventeen cohort studies30,31,43,102,103,104,105,106,107,108,109,110,111,112,113,114,115 were included in the analysis (997482 samples) of the highest versus lowest intake of coffee and breast cancer. The study characteristics are presented (Stable 3a). The summary RR was 0.99 (95% CI = 0.94–1.04, P = 0.619) with no heterogeneity (I2 = 0%, P = 0.55) (Fig. 7A). The results suggest no publication bias, with P = 0.84 for Begg’s test and P = 0.75 for Egger’s test. No significant relationship was observed between coffee consumption and the risk of breast cancer in all subgroups through subgroup analysis (Stable 3b).
Meta-analyses between coffee intake and risk of breast cancer and ovarian cancer.
Relative risks of breast cancer (A) and ovarian cancer (B) associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Ovarian cancer
Highest versus lowest intake: Nine cohort studies9,31,43,116,117,118,119,120,121 were included in the analysis (687017 samples) of the highest versus lowest intake of coffee and ovarian cancer. The study characteristics are presented (Stable 3a). The summary RR was 1.04 (95% CI = 0.90–1.20, P = 0.582) with low heterogeneity (I2 = 23.7%, P = 0.23) (Fig. 7B). The results suggest publication bias, with P = 0.02 for Begg’s test and P = 0.009 for Egger’s test. The summary RR was 0.96 (95% CI = 0.84–1.09) after Trim and fill analysis. The subgroup analysis indicated that there was no significant association between coffee intake and ovarian cancer risk in each subgroup. No factor could explain the source of heterogeneity through subgroup analysis (Stable 3b).
Endometrial cancer
Highest versus lowest intake: Twelve cohort studies30,31,43,122,123,124,125,126,127,128,129,130 were included in the analysis (1114002 samples) of the highest versus lowest intake of coffee and endometrial cancer. The study characteristics are presented (Stable 3a). The summary RR was 0.73 (95% CI = 0.67–0.80, P = 0) with no heterogeneity (I2 = 0%, P = 0.58) (Fig. 5C). The results suggest no publication bias, with P = 0.19 for Begg’s test and P = 0.16 for Egger’s test. Subgroup analysis indicated that inverse significant association was observed between coffee consumption and endometrial cancer incidence in most subgroups. However, there was no significant relationship between coffee intake and the risk of endometrial cancer in no BMI adjustment subgroup (Stable 3b).
Dose-response analysis: We included eleven studies30,31,43,122,123,124,125,126,127,128,129 for the dose-response analysis. There was no non-linear association between coffee intake and risk of endometrial cancer (P = 0.69 for non-linearity, Fig. 5C). A statistically significant inverse association was observed for endometrial cancer (RR = 0.88, 95% CI = 0.85–0.92) with an increased intake of 2 cups of coffee per day.
Other cancers
Lung cancer
Highest versus lowest intake: Four cohort studies30,31,38,131 were included in the analysis (103137 samples) of the highest versus lowest intake of coffee and lung cancer. The study characteristics are presented (Stable 4a). The summary RR was 2.18 (95% CI = 1.26–3.75, P = 0.005) with high heterogeneity (I2 = 63.3%, P = 0.04) (Fig. 2C). The results suggest no publication bias, with P = 0.73 for Begg’s test and P = 0.39 for Egger’s test.
Melanoma
Highest versus lowest intake: Six cohort studies30,31,43,132,133,134 were included in the analysis (773536 samples) of the highest versus lowest intake of coffee and melanoma. The study characteristics are presented (Stable 4a). The summary RR was 0.89 (95% CI = 0.80–0.99, P = 0.031) with no heterogeneity (I2 = 0%, P = 0.42) (Fig. 2D). The results suggest no publication bias, with P = 0.71 for Begg’s test and P = 0.40 for Egger’s test. There was no significant association between coffee intake and melanoma risk through subgroup analysis. The subgroup analysis indicated no factor to explain the source of heterogeneity (Stable 4b).
Lymphoma
Highest versus lowest intake: Three cohort studies30,31,43 were included in the analysis (89897 samples) of the highest group (samples/cases: 32783/63) versus lowest group (samples/cases: 17229/22) coffee intake and lymphoma. The study characteristics are presented (Stable 4a). The summary RR was 1.23 (95% CI = 0.75–2.03, P = 0.415) with no heterogeneity (I2 = 0%, P = 0.769) (Fig. 8). The results suggest no publication bias, with P = 1 for Begg’s test and P = 0.18 for Egger’s test.
Meta-analyses between coffee intake and risk of lymphoma.
Relative risks of lymphoma associated with coffee intake. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weigh, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Subgroup, sensitivity and meta-regression analyses: We conducted subgroup analyses and meta-regression analyses based on sex, duration of follow-up, geographical location and adjusted confounders. Coffee intake was associated with a decreased risk of liver cancer in most subgroups, with no substantial heterogeneity between subgroups. However, the protective effect was absent in the female population (Stable 1b). Inverse association was observed for oral, pharynx cancer in Asia rather than Europe and USA (Stable 1b). Inverse associations were also observed for prostate cancer and endometrial cancer, with no substantial heterogeneity between subgroups (Stables 2b and 3b). Coffee intake was not significantly associated with colorectal cancer incidence in most subgroup analyses. However, an inverse relation was observed for colon cancer (Stable 1b). An increased risk was observed for lung cancer in most subgroups (Stable 4b). No association was observed for esophageal cancer, stomach cancer, pancreatic cancer, renal cancer, bladder cancer, breast cancer, ovarian cancer or melanoma in subgroup analyses (Stables 1b, 2b, 3b and 4b).
We conducted a sensitivity analysis by excluding studies that were not dose-response analyses to evaluate whether the results were stable. The summary relative risk for the highest versus lowest intake of coffee and liver cancer was 0.53 (95% CI = 0.43–0.65, I2 = 0%, P = 0.693). The results were 0.87 (95% CI = 0.82–0.92, I2 = 31.8%, P = 0.154), 0.70 (95% CI = 0.63–0.78, I2 = 0%, P = 0.663), 0.85 (95% CI = 0.71–1.03, I2 = 05%, P = 0.396), 0.62 (95% CI = 0.41–0.94, I2 = 64.3%, P = 0.024) and 0.89 (95% CI = 0.72–1.11, I2 = 0%, P = 0.882) for prostate cancer, endometrial cancer, colon cancer, oral, pharynx cancer and melanoma. The results were similar to those of the primary analysis except for colon cancer and melanoma.
Discussion
Our meta-analysis supports an inverse association between coffee intake and oral, pharynx cancer, liver cancer, colon cancer, prostate cancer, endometrial cancer and melanoma and increased association for lung cancer. Besides, the linear inverse associations were observed for liver cancer, prostate cancer and endometrial cancer. However, no significant association was found with esophageal cancer, stomach cancer, rectal cancer, pancreatic cancer, renal cancer, bladder cancer, prostate cancer, breast cancer, ovarian cancer, lung cancer, melanoma and lymphoma.
To date, our study is the most comprehensive to conduct meta-analyses on coffee intake and cancer. We can clearly determine the association between coffee and most types of cancers from our research. Our findings seem to be more stable because our analyses were based on prospective studies. We conducted subgroup analyses and sensitivity analyses to explore the source of heterogeneity and verify the stability of the results. We also quantified the association between coffee intake and liver cancer, prostate cancer and endometrial cancer by conducting linear and non-linear dose-response analyses. Begg’s and Egger’s methods were used to evaluate publication bias. When the results suggested publication bias, we conducted a trim and fill analysis to determine whether the results were different.
Some limitations of our study should be taken into consideration. First, not all of the included studies conducted analyses by potential confounding, which could affect the results. However, we conducted subgroup analyses by confounding factors and found no substantial factors influencing the results. Second, some eligible studies30,38,64 did not provide relative risks and confidence intervals; thus, we extracted raw data for further analysis, which may influence the accuracy of the overall results regardless of confounding factors. Third, some studies measured the level of coffee intake in milliliters and others reported as cups. Due to the ambiguity in the volume contained in a cup of coffee, it was difficult to precisely compare the amount of coffee intake, which could influence the results of dose-response analyses. Moreover, we excluded a few studies for dose-response analyses. However, the results are similar to those of the analyses of highest versus lowest intake when we conducted sensitivity analyses by excluding these same data. Fourth, some cancers such as oral, pharynx cancer, esophageal cancer, renal cancer, lung cancer, melanoma and lymphoma included relatively limited number of studies for meta-analysis, may cause problems for evaluation of heterogeneities and publication bias and finally reduce the confidence of the results. Finally, our study did not include all types of cancers such as skin cancer, laryngeal carcinoma and glioma.
In addition, we found high heterogeneities when we conducted meta-analyses on coffee intake and oral, pharynx cancer and lung cancer. Although an inverse association was found between coffee and oral and pharynx cancer, we did not conduct a further dose-response analysis because of substantial heterogeneity. The dose-response analyses were also not conducted for colon cancer, melanoma and lung cancer because of the null results of sensitivity analyses. Medium heterogeneities were found in the analyses of coffee intake and renal cancer, bladder cancer and stomach cancer. Heterogeneity refers to the inconsistency within included studies. The biology diversity and methodological diversity could bring about heterogeneity. To further explore the source of heterogeneity, we conducted subgroups analyses and meta-regression analyses based on many factors, such as cancer subtypes, geographical location, sex, duration of follow-up and potential confounding factors135. We found publication bias in meta-analyses on coffee intake and liver cancer, bladder cancer and ovarian cancer. We conducted trim and fill analyses and found that all of the results were stable. However, the evaluation for publication bias is not very reliable when the number of include studies was small. Therefore, we could not draw conclusion that there was no obvious publication bias for esophageal cancer, renal cancer, melanoma and lymphoma.
Coffee intake could reduce the incidence of colon cancer, liver cancer, prostate cancer, endometrial cancer, oral, pharyngeal cancer and melanoma. There are several mechanisms attempting to explain this phenomenon. Coffee contains many bioactive components, including caffeine, cafestol, kahweol and chlorogenic acid. Some studies indicate that caffeine can prevent oxidative DNA damage, modify the apoptotic response and reverse the cell cycle checkpoint function136,137. Moreover, some researchers138,139 have found that cafestol and kahweol are anticarcinogenic. Feng et al. hypothesized that chlorogenic acids can clear away reactive oxygen species and confer an anti-tumor effect140. Dong et al. demonstrated that caffeine suppresses the progression of HCC through the Akt signaling pathway141. Furthermore, caffeine and other compounds in coffee increased the clearance of estradiol and inhibited estradiol-mediated carcinogenesis in endometrial cells142. Coffee consumption decreased the exposure of epithelial cells to carcinogens in the colon by increasing colonic motility143. In addition, coffee has been reported to reduce the synthesis and secretion of bile acids, potential promoters of colon carcinogenesis144. The decreased risk of these types of cancers may attribute to some potential mechanism, however, there are many controversies about the mechanisms. Although a positive association was observed between coffee intake and lung cancer, it has been reported that high intakes of coffee are frequently associated with cigarette smoking145, which could contribute to the increased risk of lung cancer. However, we take a conservative responsibility for the results because of the limited study numbers and the absence of analysis between smokers and nonsmokers.
Our study demonstrates that coffee intake can reduce the risk of oral, pharynx cancer, colon cancer, liver cancer, prostate cancer endometrial cancer and melanoma by 31%, 13%, 54%, 11%, 27% and 11% respectively for the highest versus lowest coffee intake. Furthermore, coffee intake could reduce the risk of liver cancer, prostate cancer and endometrial cancer by 27%, 3% and 12% with an increment of 2 cups of coffee intake. Inversely, coffee intake seems could increase the risk of lung cancer by 118%. However, considering the shortcomings of our research, our conclusions should be carefully considered. Further studies are needed to clarify the potential underlying mechanisms by which coffee intake may reduce cancer risk. Perhaps add that further studies could assess the association among never smokers, particularly for smoking-related lung cancer.
Additional Information
How to cite this article: Wang, A. et al. Coffee and cancer risk: A meta-analysis of prospective observational studies. Sci. Rep. 6, 33711; doi: 10.1038/srep33711 (2016).
References
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, 10.3322/caac.21262 (2015).
Jemal, A. et al. Global cancer statistics. CA: a cancer journal for clinicians 61, 69–90, 10.3322/caac.20107 (2011).
Bae, J. M., Lee, E. J. & Guyatt, G. Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Pancreas 38, 168–174, 10.1097/MPA.0b013e318188c497 (2009).
Aune, D. et al. Dietary fibre, whole grains and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ (Clinical research ed.) 343, d6617, 10.1136/bmj.d6617 (2011).
Sang, L. X., Chang, B., Li, X. H. & Jiang, M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC gastroenterology 13, 34, 10.1186/1471-230x-13-34 (2013).
Lee, J. E. et al. Intakes of coffee, tea, milk, soda and juice and renal cell cancer in a pooled analysis of 13 prospective studies. International journal of cancer. Journal international du cancer 121, 2246–2253, 10.1002/ijc.22909 (2007).
Tang, N., Wu, Y., Ma, J., Wang, B. & Yu, R. Coffee consumption and risk of lung cancer: a meta-analysis. Lung cancer (Amsterdam, Netherlands) 67, 17–22, 10.1016/j.lungcan.2009.03.012 (2010).
Turati, F., Galeone, C., La Vecchia, C., Garavello, W. & Tavani, A. Coffee and cancers of the upper digestive and respiratory tracts: meta-analyses of observational studies. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 22, 536–544, 10.1093/annonc/mdq603 (2011).
Braem, M. G. et al. Coffee and tea consumption and the risk of ovarian cancer: a prospective cohort study and updated meta-analysis. The American journal of clinical nutrition 95, 1172–1181, 10.3945/ajcn.111.026393 (2012).
Je, Y. & Giovannucci, E. Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. International journal of cancer. Journal international du cancer 131, 1700–1710, 10.1002/ijc.27408 (2012).
Turati, F. et al. A meta-analysis of coffee consumption and pancreatic cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 23, 311–318, 10.1093/annonc/mdr331 (2012).
Jiang, W., Wu, Y. & Jiang, X. Coffee and caffeine intake and breast cancer risk: an updated dose-response meta-analysis of 37 published studies. Gynecologic oncology 129, 620–629, 10.1016/j.ygyno.2013.03.014 (2013).
Zheng, J. S. et al. Effects of green tea, black tea and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutrition and cancer 65, 1–16, 10.1080/01635581.2013.741762 (2013).
Green, C. J. et al. Tea, coffee and milk consumption and colorectal cancer risk. Journal of epidemiology/Japan Epidemiological Association 24, 146–153 (2014).
Huang, T. B. et al. Coffee consumption and urologic cancer risk: a meta-analysis of cohort studies. International urology and nephrology 46, 1481–1493, 10.1007/s11255-014-0699-9 (2014).
Xie, F., Wang, D., Huang, Z. & Guo, Y. Coffee consumption and risk of gastric cancer: a large updated meta-analysis of prospective studies. Nutrients 6, 3734–3746, 10.3390/nu6093734 (2014).
Liu, H. et al. Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutrition and cancer 67, 392–400, 10.1080/01635581.2015.1004727 (2015).
Xie, Y. et al. Coffee consumption and the risk of lung cancer: an updated meta-analysis of epidemiological studies. European journal of clinical nutrition, 10.1038/ejcn.2015.96 (2015).
Zhong, S. et al. Coffee consumption and risk of prostate cancer: an up-to-date meta-analysis. European journal of clinical nutrition 68, 330–337, 10.1038/ejcn.2013.256 (2014).
Bravi, F., Bosetti, C., Tavani, A., Gallus, S. & La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 11, 1413–1421 e1411, 10.1016/j.cgh.2013.04.039 (2013).
Greenland, S. & Longnecker, M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American journal of epidemiology 135, 1301–1309 (1992).
Moskal, A., Norat, T., Ferrari, P. & Riboli, E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. International journal of cancer. Journal international du cancer 120, 664–671, 10.1002/ijc.22299 (2007).
Shimazu, T. et al. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. International journal of cancer. Journal international du cancer 116, 150–154, 10.1002/ijc.20989 (2005).
Royston, P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Statistics in medicine 19, 1831–1847 (2000).
Rong, Y. et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ (Clinical research ed.) 346, e8539, 10.1136/bmj.e8539 (2013).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, 10.1002/sim.1186 (2002).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634 (1997).
Sena, E. S., van der Worp, H. B., Bath, P. M., Howells, D. W. & Macleod, M. R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS biology 8, e1000344, 10.1371/journal.pbio.1000344 (2010).
Jacobsen, B. K., Bjelke, E., Kvale, G. & Heuch, I. Coffee drinking, mortality and cancer incidence: results from a Norwegian prospective study. Journal of the National Cancer Institute 76, 823–831 (1986).
Stensvold, I. & Jacobsen, B. K. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer causes & control: CCC 5, 401–408 (1994).
Naganuma, T. et al. Coffee consumption and the risk of oral, pharyngeal and esophageal cancers in Japan: the Miyagi Cohort Study. American journal of epidemiology 168, 1425–1432, 10.1093/aje/kwn282 (2008).
Ren, J. S. et al. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. European journal of cancer (Oxford, England: 1990) 46, 1873–1881, 10.1016/j.ejca.2010.03.025 (2010).
Tverdal, A., Hjellvik, V. & Selmer, R. Coffee intake and oral-oesophageal cancer: follow-up of 389,624 Norwegian men and women 40-45 years. British journal of cancer 105, 157–161, 10.1038/bjc.2011.192 (2011).
Hildebrand, J. S. et al. Coffee, tea and fatal oral/pharyngeal cancer in a large prospective US cohort. American journal of epidemiology 177, 50–58, 10.1093/aje/kws222 (2013).
Ishikawa, A. et al. Smoking, alcohol drinking, green tea consumption and the risk of esophageal cancer in Japanese men. Journal of epidemiology/Japan Epidemiological Association 16, 185–192 (2006).
Zamora-Ros, R. et al. Tea and coffee consumption and risk of esophageal cancer: the European prospective investigation into cancer and nutrition study. International journal of cancer. Journal international du cancer 135, 1470–1479, 10.1002/ijc.28789 (2014).
Nomura, A., Heilbrun, L. K. & Stemmermann, G. N. Prospective study of coffee consumption and the risk of cancer. Journal of the National Cancer Institute 76, 587–590 (1986).
Galanis, D. J., Kolonel, L. N., Lee, J. & Nomura, A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. International journal of epidemiology 27, 173–180 (1998).
van Loon, A. J., Goldbohm, R. A. & van den Brandt, P. A. Socioeconomic status and stomach cancer incidence in men: results from The Netherlands Cohort Study. Journal of epidemiology and community health 52, 166–171 (1998).
Tsubono, Y. et al. Green tea and the risk of gastric cancer in Japan. The New England journal of medicine 344, 632–636, 10.1056/nejm200103013440903 (2001).
Larsson, S. C., Giovannucci, E. & Wolk, A. Coffee consumption and stomach cancer risk in a cohort of Swedish women. International journal of cancer. Journal international du cancer 119, 2186–2189, 10.1002/ijc.22105 (2006).
Nilsson, L. M., Johansson, I., Lenner, P., Lindahl, B. & Van Guelpen, B. Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer causes & control: CCC 21, 1533–1544, 10.1007/s10552-010-9582-x (2010).
Bidel, S. et al. Coffee consumption and risk of gastric and pancreatic cancer–a prospective cohort study. International journal of cancer. Journal international du cancer 132, 1651–1659, 10.1002/ijc.27773 (2013).
Ainslie-Waldman, C. E. et al. Coffee intake and gastric cancer risk: the Singapore Chinese health study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23, 638–647, 10.1158/1055-9965.epi-13-0886 (2014).
Sanikini, H. et al. Total, caffeinated and decaffeinated coffee and tea intake and gastric cancer risk: results from the EPIC cohort study. International journal of cancer. Journal international du cancer 136, E720–730, 10.1002/ijc.29223 (2015).
Wu, A. H., Paganini-Hill, A., Ross, R. K. & Henderson, B. E. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. British journal of cancer 55, 687–694 (1987).
Klatsky, A. L., Armstrong, M. A., Friedman, G. D. & Hiatt, R. A. The relations of alcoholic beverage use to colon and rectal cancer. American journal of epidemiology 128, 1007–1015 (1988).
Hartman, T. J. et al. Tea and coffee consumption and risk of colon and rectal cancer in middle-aged Finnish men. Nutrition and cancer 31, 41–48, 10.1080/01635589809514676 (1998).
Terry, P., Bergkvist, L., Holmberg, L. & Wolk, A. Coffee consumption and risk of colorectal cancer in a population based prospective cohort of Swedish women. Gut 49, 87–90 (2001).
Michels, K. B., Willett, W. C., Fuchs, C. S. & Giovannucci, E. Coffee, tea and caffeine consumption and incidence of colon and rectal cancer. Journal of the National Cancer Institute 97, 282–292, 10.1093/jnci/dji039 (2005).
Larsson, S. C., Bergkvist, L., Giovannucci, E. & Wolk, A. Coffee consumption and incidence of colorectal cancer in two prospective cohort studies of Swedish women and men. American journal of epidemiology 163, 638–644, 10.1093/aje/kwj067 (2006).
Mucci, L. A., Adami, H. O. & Wolk, A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. International journal of cancer. Journal international du cancer 118, 169–173, 10.1002/ijc.21309 (2006).
Oba, S. et al. The relationship between the consumption of meat, fat and coffee and the risk of colon cancer: a prospective study in Japan. Cancer letters 244, 260–267, 10.1016/j.canlet.2005.12.037 (2006).
Lee, K. J. et al. Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. International journal of cancer. Journal international du cancer 121, 1312–1318, 10.1002/ijc.22778 (2007).
Naganuma, T. et al. Coffee consumption and the risk of colorectal cancer: a prospective cohort study in Japan. International journal of cancer. Journal international du cancer 120, 1542–1547, 10.1002/ijc.22505 (2007).
Bidel, S. et al. Coffee consumption and risk of colorectal cancer. European journal of clinical nutrition 64, 917–923, 10.1038/ejcn.2010.103 (2010).
Peterson, S. et al. Coffee intake and risk of colorectal cancer among Chinese in Singapore: the Singapore Chinese Health Study. Nutrition and cancer 62, 21–29, 10.1080/01635580903191528 (2010).
Simons, C. C. et al. Fluid intake and colorectal cancer risk in the Netherlands Cohort Study. Nutrition and cancer 62, 307–321, 10.1080/01635580903407098 (2010).
Sinha, R. et al. Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. The American journal of clinical nutrition 96, 374–381, 10.3945/ajcn.111.031328 (2012).
Dominianni, C., Huang, W. Y., Berndt, S., Hayes, R. B. & Ahn, J. Prospective study of the relationship between coffee and tea with colorectal cancer risk: the PLCO Cancer Screening Trial. British journal of cancer 109, 1352–1359, 10.1038/bjc.2013.434 (2013).
Dik, V. K. et al. Coffee and tea consumption, genotype-based CYP1A2 and NAT2 activity and colorectal cancer risk-results from the EPIC cohort study. International journal of cancer. Journal international du cancer 135, 401–412, 10.1002/ijc.28655 (2014).
Yamada, H. et al. Coffee consumption and risk of colorectal cancer: the Japan Collaborative Cohort Study. Journal of epidemiology/Japan Epidemiological Association 24, 370–378 (2014).
Nomura, A., Stemmermann, G. N. & Heilbrun, L. K. Coffee and pancreatic cancer. Lancet (London, England) 2, 415 (1981).
Whittemore, A. S., Paffenbarger, R. S. Jr., Anderson, K. & Halpern, J. Early precursors of pancreatic cancer in college men. Journal of chronic diseases 36, 251–256 (1983).
Hiatt, R. A., Klatsky, A. L. & Armstrong, M. A. Pancreatic cancer, blood glucose and beverage consumption. International journal of cancer. Journal international du cancer 41, 794–797 (1988).
Shibata, A., Mack, T. M., Paganini-Hill, A., Ross, R. K. & Henderson, B. E. A prospective study of pancreatic cancer in the elderly. International journal of cancer. Journal international du cancer 58, 46–49 (1994).
Harnack, L. J. et al. Smoking, alcohol, coffee and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women’s Health Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 6, 1081–1086 (1997).
Michaud, D. S., Giovannucci, E., Willett, W. C., Colditz, G. A. & Fuchs, C. S. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 10, 429–437 (2001).
Isaksson, B. et al. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. International journal of cancer. Journal international du cancer 98, 480–482 (2002).
Stolzenberg-Solomon, R. Z., Pietinen, P., Taylor, P. R., Virtamo, J. & Albanes, D. Prospective study of diet and pancreatic cancer in male smokers. American journal of epidemiology 155, 783–792 (2002).
Khan, M. M. et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pacific journal of cancer prevention: APJCP 5, 58–65 (2004).
Luo, J. et al. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study). European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 16, 542–548, 10.1097/CEJ.0b013e32809b4d30 (2007).
Bhoo-Pathy, N. et al. Intake of coffee, decaffeinated coffee, or tea does not affect risk for pancreatic cancer: results from the European Prospective Investigation into Nutrition and Cancer Study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 11, 1486–1492, 10.1016/j.cgh.2013.05.029 (2013).
Inoue, M., Yoshimi, I., Sobue, T. & Tsugane, S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. Journal of the National Cancer Institute 97, 293–300, 10.1093/jnci/dji040 (2005).
Hu, G. et al. Joint effects of coffee consumption and serum gamma-glutamyltransferase on the risk of liver cancer. Hepatology (Baltimore, Md.) 48, 129–136, 10.1002/hep.22320 (2008).
Ohishi, W. et al. Risk factors for hepatocellular carcinoma in a Japanese population: a nested case-control study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 17, 846–854, 10.1158/1055-9965.epi-07-2806 (2008).
Inoue, M. et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 18, 1746–1753, 10.1158/1055-9965.epi-08-0923 (2009).
Johnson, S. et al. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer causes & control: CCC 22, 503–510, 10.1007/s10552-010-9725-0 (2011).
Lai, G. Y. et al. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. British journal of cancer 109, 1344–1351, 10.1038/bjc.2013.405 (2013).
Bamia, C. et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. International journal of cancer. Journal international du cancer 136, 1899–1908, 10.1002/ijc.29214 (2015).
Setiawan, V. W. et al. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology 148, 118–125; quiz e115, 10.1053/j.gastro.2014.10.005 (2015).
Washio, M. et al. Risk factors for kidney cancer in a Japanese population: findings from the JACC Study. Journal of epidemiology/Japan Epidemiological Association 15 Suppl 2, S203–211 (2005).
Allen, N. E., Balkwill, A., Beral, V., Green, J. & Reeves, G. Fluid intake and incidence of renal cell carcinoma in UK women. British journal of cancer 104, 1487–1492, 10.1038/bjc.2011.90 (2011).
Mills, P. K., Beeson, W. L., Phillips, R. L. & Fraser, G. E. Bladder cancer in a low risk population: results from the Adventist Health Study. American journal of epidemiology 133, 230–239 (1991).
Chyou, P. H., Nomura, A. M. & Stemmermann, G. N. A prospective study of diet, smoking and lower urinary tract cancer. Annals of epidemiology 3, 211–216 (1993).
Michaud, D. S. et al. Fluid intake and the risk of bladder cancer in men. The New England journal of medicine 340, 1390–1397, 10.1056/nejm199905063401803 (1999).
Nagano, J. et al. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. International journal of cancer. Journal international du cancer 86, 132–138 (2000).
Zeegers, M. P., Dorant, E., Goldbohm, R. A. & van den Brandt, P. A. Are coffee, tea and total fluid consumption associated with bladder cancer risk? Results from the Netherlands Cohort Study. Cancer causes & control: CCC 12, 231–238 (2001).
Tripathi, A., Folsom, A. R. & Anderson, K. E. Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women’s Health Study. Cancer 95, 2316–2323, 10.1002/cncr.10975 (2002).
Kurahashi, N., Inoue, M., Iwasaki, M., Sasazuki, S. & Tsugane, S. Coffee, green tea and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: a prospective study in Japan. Cancer science 100, 294–291, 10.1111/j.1349-7006.2008.01027.x (2009).
Severson, R. K., Nomura, A. M., Grove, J. S. & Stemmermann, G. N. A prospective study of demographics, diet and prostate cancer among men of Japanese ancestry in Hawaii. Cancer research 49, 1857–1860 (1989).
Le Marchand, L., Kolonel, L. N., Wilkens, L. R., Myers, B. C. & Hirohata, T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology (Cambridge, Mass) 5, 276–282 (1994).
Ellison, L. F. Tea and other beverage consumption and prostate cancer risk: a Canadian retrospective cohort study. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 9, 125–130 (2000).
Allen, N. E. et al. A prospective study of diet and prostate cancer in Japanese men. Cancer causes & control: CCC 15, 911–920, 10.1007/s10552-004-1683-y (2004).
Shafique, K. et al. Coffee consumption and prostate cancer risk: further evidence for inverse relationship. Nutrition journal 11, 42, 10.1186/1475-2891-11-42 (2012).
Wilson, K. M., Giovannucci, E. L. & Mucci, L. A. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian journal of andrology 14, 365–374, 10.1038/aja.2011.142 (2012).
Bosire, C. et al. Coffee consumption and the risk of overall and fatal prostate cancer in the NIH-AARP Diet and Health Study. Cancer causes & control: CCC 24, 1527–1534, 10.1007/s10552-013-0229-6 (2013).
Discacciati, A. et al. Coffee consumption and risk of localized, advanced and fatal prostate cancer: a population-based prospective study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 24, 1912–1918, 10.1093/annonc/mdt105 (2013).
Li, Q. et al. Coffee consumption and the risk of prostate cancer: the Ohsaki Cohort Study. British journal of cancer 108, 2381–2389, 10.1038/bjc.2013.238 (2013).
Tverdal, A. Boiled coffee consumption and the risk of prostate cancer: follow-up of 224,234 Norwegian men 20–69 years. British journal of cancer 112, 576–579, 10.1038/bjc.2014.645 (2015).
Vatten, L. J., Solvoll, K. & Loken, E. B. Coffee consumption and the risk of breast cancer. A prospective study of 14,593 Norwegian women. British journal of cancer 62, 267–270 (1990).
Hoyer, A. P. & Engholm, G. Serum lipids and breast cancer risk: a cohort study of 5,207 Danish women. Cancer causes & control: CCC 3, 403–408 (1992).
Folsom, A. R., McKenzie, D. R., Bisgard, K. M., Kushi, L. H. & Sellers, T. A. No association between caffeine intake and postmenopausal breast cancer incidence in the Iowa Women’s Health Study. American journal of epidemiology 138, 380–383 (1993).
Key, T. J. et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. British journal of cancer 81, 1248–1256, 10.1038/sj.bjc.6690837 (1999).
Michels, K. B., Holmberg, L., Bergkvist, L. & Wolk, A. Coffee, tea and caffeine consumption and breast cancer incidence in a cohort of Swedish women. Annals of epidemiology 12, 21–26 (2002).
Hirvonen, T. et al. Consumption of antioxidant-rich beverages and risk for breast cancer in French women. Annals of epidemiology 16, 503–508, 10.1016/j.annepidem.2005.09.011 (2006).
Ganmaa, D. et al. Coffee, tea, caffeine and risk of breast cancer: a 22-year follow-up. International journal of cancer. Journal international du cancer 122, 2071–2076, 10.1002/ijc.23336 (2008).
Ishitani, K., Lin, J., Manson, J. E., Buring, J. E. & Zhang, S. M. Caffeine consumption and the risk of breast cancer in a large prospective cohort of women. Archives of internal medicine 168, 2022–2031, 10.1001/archinte.168.18.2022 (2008).
Larsson, S. C., Bergkvist, L. & Wolk, A. Coffee and black tea consumption and risk of breast cancer by estrogen and progesterone receptor status in a Swedish cohort. Cancer causes & control: CCC 20, 2039–2044, 10.1007/s10552-009-9396-x (2009).
Wilson, K. M. et al. Dietary acrylamide intake and risk of premenopausal breast cancer. American journal of epidemiology 169, 954–961, 10.1093/aje/kwn421 (2009).
Bhoo Pathy, N. et al. Coffee and tea intake and risk of breast cancer. Breast cancer research and treatment 121, 461–467, 10.1007/s10549-009-0583-y (2010).
Boggs, D. A. et al. Tea and coffee intake in relation to risk of breast cancer in the Black Women’s Health Study. Cancer causes & control: CCC 21, 1941–1948, 10.1007/s10552-010-9622-6 (2010).
Fagherazzi, G., Touillaud, M. S., Boutron-Ruault, M. C., Clavel-Chapelon, F. & Romieu, I. No association between coffee, tea or caffeine consumption and breast cancer risk in a prospective cohort study. Public health nutrition 14, 1315–1320, 10.1017/s1368980011000371 (2011).
Gierach, G. L. et al. Coffee intake and breast cancer risk in the NIH-AARP diet and health study cohort. International journal of cancer. Journal international du cancer 131, 452–460, 10.1002/ijc.26372 (2012).
Snowdon, D. A. & Phillips, R. L. Coffee consumption and risk of fatal cancers. American journal of public health 74, 820–823 (1984).
Larsson, S. C. & Wolk, A. Coffee consumption is not associated with ovarian cancer incidence. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 14, 2273–2274, 10.1158/1055-9965.epi-05-0280 (2005).
Silvera, S. A., Jain, M., Howe, G. R., Miller, A. B. & Rohan, T. E. Intake of coffee and tea and risk of ovarian cancer: a prospective cohort study. Nutrition and cancer 58, 22–27, 10.1080/01635580701307945 (2007).
Steevens, J., Schouten, L. J., Verhage, B. A., Goldbohm, R. A. & van den Brandt, P. A. Tea and coffee drinking and ovarian cancer risk: results from the Netherlands Cohort Study and a meta-analysis. British journal of cancer 97, 1291–1294, 10.1038/sj.bjc.6604008 (2007).
Lueth, N. A., Anderson, K. E., Harnack, L. J., Fulkerson, J. A. & Robien, K. Coffee and caffeine intake and the risk of ovarian cancer: the Iowa Women’s Health Study. Cancer causes & control: CCC 19, 1365–1372, 10.1007/s10552-008-9208-8 (2008).
Tworoger, S. S., Gertig, D. M., Gates, M. A., Hecht, J. L. & Hankinson, S. E. Caffeine, alcohol, smoking and the risk of incident epithelial ovarian cancer. Cancer 112, 1169–1177, 10.1002/cncr.23275 (2008).
Shimazu, T. et al. Coffee consumption and risk of endometrial cancer: a prospective study in Japan. International journal of cancer. Journal international du cancer 123, 2406–2410, 10.1002/ijc.23760 (2008).
Friberg, E., Orsini, N., Mantzoros, C. S. & Wolk, A. Coffee drinking and risk of endometrial cancer–a population-based cohort study. International journal of cancer. Journal international du cancer 125, 2413–2417, 10.1002/ijc.24543 (2009).
Giri, A. et al. Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: a prospective cohort study among US postmenopausal women. Nutrients 3, 937–950, 10.3390/nu3110937 (2011).
Je, Y., Hankinson, S. E., Tworoger, S. S., De Vivo, I. & Giovannucci, E. A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 20, 2487–2495, 10.1158/1055-9965.epi-11-0766 (2011).
Gunter, M. J. et al. A prospective investigation of coffee drinking and endometrial cancer incidence. International journal of cancer. Journal international du cancer 131, E530–536, 10.1002/ijc.26482 (2012).
Uccella, S. et al. Intake of coffee, caffeine and other methylxanthines and risk of Type I vs Type II endometrial cancer. British journal of cancer 109, 1908–1913, 10.1038/bjc.2013.540 (2013).
Gavrilyuk, O. et al. High coffee consumption and different brewing methods in relation to postmenopausal endometrial cancer risk in the Norwegian women and cancer study: a population-based prospective study. BMC women’s health 14, 48, 10.1186/1472-6874-14-48 (2014).
Weiderpass, E. et al. Endometrial cancer in relation to coffee, tea and caffeine consumption: a prospective cohort study among middle-aged women in Sweden. Nutrition and cancer 66, 1132–1143, 10.1080/01635581.2014.948214 (2014).
Merritt, M. A. et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 24, 466–471, 10.1158/1055-9965.epi-14-0970 (2015).
Takezaki, T. et al. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutrition and cancer 45, 160–167, 10.1207/s15327914nc4502_04 (2003).
Veierod, M. B., Thelle, D. S. & Laake, P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. International journal of cancer. Journal international du cancer 71, 600–604 (1997).
Loftfield, E. et al. Coffee drinking and cutaneous melanoma risk in the NIH-AARP diet and health study. Journal of the National Cancer Institute 107, 10.1093/jnci/dju421 (2015).
Wu, H., Reeves, K. W., Qian, J. & Sturgeon, S. R. Coffee, tea and melanoma risk among postmenopausal women. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 24, 347–352, 10.1097/cej.0000000000000093 (2015).
Wang, A. et al. Citrus Fruit Intake Substantially Reduces the Risk of Esophageal Cancer: A Meta-Analysis of Epidemiologic Studies. Medicine 94, e1390, 10.1097/md.0000000000001390 (2015).
Asaad, N. A., Zeng, Z. C., Guan, J., Thacker, J. & Iliakis, G. Homologous recombination as a potential target for caffeine radiosensitization in mammalian cells: reduced caffeine radiosensitization in XRCC2 and XRCC3 mutants. Oncogene 19, 5788–5800, 10.1038/sj.onc.1203953 (2000).
Joerges, C., Kuntze, I. & Herzinger, T. Induction of a caffeine-sensitive S-phase cell cycle checkpoint by psoralen plus ultraviolet A radiation. Oncogene 22, 6119–6128, 10.1038/sj.onc.1206613 (2003).
Cavin, C. et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 40, 1155–1163 (2002).
Majer, B. J. et al. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2). Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 43, 433–441, 10.1016/j.fct.2004.11.009 (2005).
Feng, R. et al. Inhibition of activator protein-1, NF-kappaB and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. The Journal of biological chemistry 280, 27888–27895, 10.1074/jbc.M503347200 (2005).
Dong, S. et al. Low Concentration of Caffeine Inhibits the Progression of the Hepatocellular Carcinoma via Akt Signaling Pathway. Anti-cancer agents in medicinal chemistry 15, 484–492 (2015).
Fotsis, T. et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 368, 237–239, 10.1038/368237a0 (1994).
Higdon, J. V. & Frei, B. Coffee and health: a review of recent human research. Critical reviews in food science and nutrition 46, 101–123, 10.1080/10408390500400009 (2006).
Potter, J. D. Reconciling the epidemiology, physiology and molecular biology of colon cancer. Jama 268, 1573–1577 (1992).
Leviton, A. & Cowan, L. A review of the literature relating caffeine consumption by women to their risk of reproductive hazards. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 40, 1271–1310 (2002).
Acknowledgements
We thank for their help Nature Publishing Group Language Editing in English language revision of this manuscript. We also thank for the support of International Science and Technology Cooperation Projects (2015DFA30650 and 2010DFB33720), Capital Special Research Project for Health Development (2014-2-4012), Capital research project for the characteristics clinical application (Z151100004015170) and Program for New Century Excellent Talents in University (NCET-11-0288).
Author information
Authors and Affiliations
Contributions
A.W. designed the study and wrote this manuscript. S.W. and C.Z. searched database and reviewed studies. C.H., C.W., X.W., X.Y., H.Z., Y.M. and L.H. collected and analyzed data. X.S. and H.Z. coordinated and provided financial support for this work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, A., Wang, S., Zhu, C. et al. Coffee and cancer risk: A meta-analysis of prospective observational studies. Sci Rep 6, 33711 (2016). https://doi.org/10.1038/srep33711
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33711
This article is cited by
-
Coffee consumption and breast cancer risk: a narrative review in the general population and in different subtypes of breast cancer
European Journal of Nutrition (2021)
-
Association between dietary intake and risk of ovarian cancer: a systematic review and meta-analysis
European Journal of Nutrition (2021)
-
Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population
European Journal of Nutrition (2020)
-
Dietary compounds and cutaneous malignant melanoma: recent advances from a biological perspective
Nutrition & Metabolism (2019)
-
Coffee consumption by type and risk of digestive cancer: a large prospective cohort study
British Journal of Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.