Abstract
Post-traumatic endocrine dysfunction is a complication of traumatic brain injury (TBI). However, there is lack of long-term follow-up and large sample size studies. This study included patients suffering from TBI registered in the Health Insurance Database. Endocrine disorders were identified using the ICD codes: 244 (acquired hypothyroidism), 253 (pituitary dysfunction), 255 (disorders of the adrenal glands), 258 (polyglandular dysfunction), and 259 (other endocrine disorders) with at least three outpatient visits within 1 year or one admission diagnosis. Overall, 156,945 insured subjects were included in the final analysis. The 1- and 5-year incidence rates of post-traumatic endocrinopathies were 0.4% and 2%, respectively. The risks of developing a common endocrinopathy (p < 0.001) or pituitary dysfunction (P < 0.001) were significantly higher in patients with a TBI history. Patients with a skull bone fracture had a higher risk of developing pituitary dysfunction at the 1-year follow up (p value < 0.001). At the 5-year follow up, the association between intracranial hemorrhage and pituitary dysfunction (p value: 0.002) was significant. The risk of developing endocrine dysfunction after TBI increased during the entire 5-year follow-up period. Skull bone fracture and intracranial hemorrhage may be associated with short and long-term post-traumatic pituitary dysfunction, respectively.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a common cause of disability and death among adults worldwide. The incidence is from 91/100,000 in Spain1 to 300/100,000 in Italy2. In the U.S., 230,000 patients are admitted with a head injury annually3. These figures demonstrate admitted cases; therefore, the actual number could be higher. Survivors of TBI face a variety of complications in the future, such as impaired movement, seizure, or hydrocephalus. Post-traumatic endocrinopathies have been reported among these complications. This phenomenon was first reported in 19184 and was originally thought to be a rare complication5. Subsequently, more and more studies reported a wide range of incidence rates from 2% to 90%6,7,8,9. An endocrinopathy can cause serious physical and mental effects in patients with TBI10,11,12. Therefore, the quality of life in these patients could be severely impaired. For example, depression and fatigue caused by hypopituitarism13, neuropsychiatric issues caused by thyroid hormone disorders10, electrolyte imbalance14, diabetes insipidus15, decreased cardiac function, and increased cardiovascular disease due to growth hormone deficiency16,17 are some of the possible effects impairing quality of life.
Numerous studies have described the risk of developing a post-traumatic endocrinopathy14,18,19,20. Several articles also suggest the need to screen patients with a TBI history at 3 and 12 months21,22,23,24, even if they present with nonspecific symptoms, such as fatigue, impaired concentration, or depression. However, several review articles have reported a number of limitations on the part of previous studies on post-traumatic endocrinopathies, including small sample size25 and choice of diagnostic criteria26. Besides, the risk and association has not been clarified27.
The other limitation in this respect is the lack of long-term follow up. Some studies have mentioned possible resolution of pituitary dysfunction 1 year after TBI28,29,30. Aimaretti et al. reported possible improvement or worsening over time23,31. Krahulik et al. reported recovery of hormonal function after 6 months32. Agha et al. described a patient who spontaneously recovered from hypopituitarism after 5 years33. Therefore, long-term follow up has been recommended by many review articles14,27. However, most studies have a median 1-year follow up, and only a few studies or a few patients had a follow-up time longer than 1 year29,34,35. Therefore, larger populations and longer follow-up periods are needed to confirm the association.
The National Health Insurance Research Database (NHIRD) was established by the National Health Research Institutes of Taiwan, and includes all medical claims data from 26 million enrollees from 1996 to 2009. This database covers >98% of the Taiwanese population over a period of 14 years. Due to the large population and long-term follow up period, this study used unique NHIRD Taiwanese data to explore the long-term risk of developing post-traumatic pituitary dysfunction in patients with TBI.
Materials and Methods
Data source
The Taiwanese government implemented the National Health Insurance program in March, 1995; this program provides general health insurance coverage to almost the entire Taiwanese population. The National Health Insurance Research Database (NHIRD) for this program contains the registration files and original reimbursement claims data maintained by the National Health Research Institutes (NHRI). The NHRI has provided these data to scientists for research purposes since 2000. The NHIRD contains medical information, including data on medical care facilities and specialties, information on prescriptions, including the names of prescribed drugs, dosages, prescription duration, and total expenditures, operations and examinations, patient sex and birth date, date of visit or hospitalization, transfer identification number, and diagnoses coded in the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) format. The NHRI extracted one million randomly sampled representative data from the registry of all enrollees and created the Longitudinal Health Insurance Database in 2005 (LHID 2005), which is representative of all beneficiaries.
This study adhered to strict confidentiality guidelines, in accordance with regulations regarding personal electronic data protection, and was approved by the ethics review board of the Chang Gung Memorial Hospital, Chia-Yi Branch(No: 103-0504B). The data were analyzed anonymously and the requirement for informed consent was waived by institution of review board.
Study subjects and design
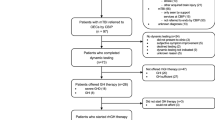
The flow diagram of this nationwide-based study is shown in Fig. 1. This study included patients who suffered from TBI (ICD9:800–804, 850–854) during 1996–2009. All medical records of the TBI cohort were extracted and analyzed, and all enrolled study subjects were followed up until death or the end of 2009. Endocrine disorders were identified using the following ICD codes: 244 (acquired hypothyroidism), 253 (pituitary dysfunction), 255 (adrenal gland disorders), 258 (polyglandular dysfunction), and 259 (other endocrine disorders) with at least three records of outpatient visits within 1 year or one admission diagnosis during the study period.
We excluded subjects with endocrine dysfunction, stroke (ICD9:430–438), or brain tumor (ICD9: 191, 225.0, 225.1, 225.2) diagnosed before the TBI event. Subjects with data errors or missing data were also excluded. The TBI subjects and non-TBI subjects were frequency matched randomly by age, sex, income, urbanization, diabetes, and hypertension at a ratio of 1:4 (TBI subjects vs. non-TBI user). Overall, 156,945 insured subjects (31,389 matched sets) were included in the final analysis (Fig. 1).
Statistical analysis
We used the Kaplan–Meier method to calculate survival curves and the log-rank test to detect differences in the survival curves. Finally, Cox proportional hazards models were used to compute the hazard ratios (HRs) and 95% confidence intervals (CIs) after adjustment for comorbidities and sociodemographic characteristics (age, sex, income, and level of urbanization). Urbanization levels in Taiwan are divided into four strata according to a Taiwan National Health Research Institute publication. Level 1 refers to the most urbanized community and level 4 refers to the least urbanized community. All analyses were conducted using SAS ver. 9.4 software (SAS Institute, Cary, NC, USA).
Results
Post-traumatic endocrinopathies
The demographic characteristics and selected morbidities are shown in Table 1. There were 31,389 patients in the TBI group and 125, 556 patients in the non-TBI group. Mean age and the sex distribution were similar because the non-TBI group was age- and sex-matched with the original cohort. In addition, we also decreased the effect of covariates, such as diabetes mellitus, hypertension, and heart disease; therefore, the proportions of these covariates are similar in these groups. Among them, 133 (0.4%) patients in the TBI group and 357 (0.3%) patients in the non-TBI group were newly diagnosed with an endocrine disorder at the 1-year follow up. The incidence of endocrine dysfunction was significantly higher in the TBI group than that in the non-TBI group (p value < 0.0001).
Patients in the TBI group seemed to have a higher risk of developing an endocrine disorder at the 1-year follow up compared with the non-TBI group, after adjusting for age, sex, and comorbidities (adjusted HR: 1.49; p < 0.001; Table 2).
Pituitary dysfunction
We suspected a higher risk of developing pituitary dysfunction, as opposed to another endocrinopathy, in patients with TBI because the pituitary gland is located intracranially. Therefore, we separated all endocrinopathies into two groups of pituitary and non-pituitary dysfunction according to their ICD-9 code.
Thirty-five (0.1%) patients in the TBI group and 80 (0.1%) patients in the non-TBI group were newly diagnosed with a pituitary disorder during the 1-year follow-up period (Table 1). Using multivariable regression analysis, patients in the TBI group demonstrated a significantly higher risk of developing a pituitary disorder during the 1-year follow up when compared with the non-TBI patients (adjusted HR: 2.06; p < 0.001; Table 3).
Types of TBI
Previous studies have reported an association between different types of brain injury and the risk of developing a post-traumatic endocrinopathy. We separated the patients with TBI into mild head injury (ICD-9: 850), head injury with intracranial hemorrhage (ICD-9: 851–854), and skull bone fracture (ICD-9: 800–804). Table 1 shows that 11,063 (35.2%) patients had mild head injury, 14,940 (47.6%) had head injury and intracranial hemorrhage, and 5,386 (17.2%) had a skull bone fracture.
The association between mild head injury and the risk of developing an endocrinopathy was not significant in the multivariate analysis (p value: 0.064, HR: 1.34; Table 2), but the association is significant in the group with hemorrhagic head injury (p value: 0.007, HR: 1.44). Moreover, the association was more significant in the skull bone fracture group (p value < 0.001, HR: 2.05).
The association between mild head injury and a pituitary disorder remained insignificant (p value: 0.065, HR: 1.78; Table 3). In addition, the association between intracranial hemorrhage and pituitary dysfunction was not significant. However, the association was well demonstrated in the skull bone fracture group of patients (p value < 0.001, HR: 3.77).
Five-year follow-up
According to Fig. 2a,b, the incidence of post-traumatic endocrinopathy and pituitary dysfunction increased linearly over the 5-year period, and the gap between patients with and without TBI increased gradually. Because previous studies have described possible resolution or worsening of an endocrinopathy during a long-term follow up, we extended our follow up to 5 years to explore whether there was any change in the association between TBI and endocrinopathies.
The association between post-traumatic endocrinopathies and TBI remained at the 5-year follow up (p value < 0.001, HR: 1.27; Table 2). However, the association between a pituitary disorder and skull bone fracture was not significant at 5 years (p value 0.134, HR: 1.41; Table 3), but the association between pituitary dysfunction and intracranial hemorrhage became significant (p value: 0.002, HR: 1.46).
Discussion
This is the first study to describe long-term follow-up results of the incidence of post-traumatic endocrinopathies using a nationwide population. We noted that the incidence of endocrinopathies and pituitary dysfunction increased gradually during the 5-year follow up after TBI. Patients with traumatic brain injury had a higher risk of developing an endocrinopathy (p value: < 0.0001) and pituitary dysfunction (HR: 2.06 at 1-year, HR: 1.27 at 5-year) at the 1- and 5-year follow-ups. We also explored the different effects among the three types of brain injury. Among the three types, a skull bone fracture had the most significant association with pituitary dysfunction during the 1-year follow-up period. However, intracranial hemorrhage had a stronger association with pituitary dysfunction than that of skull bone fracture during the 5-year follow-up period.
Post-traumatic endocrinopathy was first reported in 19184, and many studies have reported follow ups for decades. However, incidence has varied from 5% to 90%36. A lower incidence (5.4%) was reported by Kokshoorn8 et al. A systematic review article by Lauzier et al. reported that about one third of TBI cases developed later pituitary dysfunction25 The incidence of pituitary injury at autopsy is 26–86%37,38,39.
In our study, the incidence of post-traumatic endocrinopathies at the 1-year follow up was 0.4%, which is far lower than most studies. Some of the reasons for this low incidence rate are, initially, that clinicians tend to attribute non-specific symptoms such as headache, dizziness, and extremity weakness to head injury sequelae rather than to an endocrinopathy. Consequently, patients seldom receive a hormonal evaluation to confirm the diagnosis.
Second, patients with severe head injury usually present with lethargy, stupor, or even coma. It is difficult for clinicians to assess subtle changes in consciousness and to investigate the possibility of changes in hormone levels in patients with low coma scale scores. In addition, we were unable to obtain clinical data, such as the coma scale score and duration of the ICU stay, from the NHIRD. The combined lack of severity data and assessments of hormonal changes in patients with extremely severe head injury likely contributed to the low incidence of post-traumatic endocrinopathy identified in our study.
Third, selecting a reliable test is difficult, particularly when the patient is in the intensive care unit31. A variety of tests have been used to assess hormone levels, and there is a wide range of diagnostic cut-off values40,41,42,43. Additionally, Klose et al. have pointed to the unreliability of hormone assessments during the acute phase of brain injury27.
Some differences were noted between the populations with and without TBI. The incidence of endocrinopathies differed between the groups, as patients with TBI had a higher risk of developing an endocrinopathy (p value: 0.0001). However, the ICD-9 codes we used included pituitary dysfunction, hypothyroidism, adrenal gland and cortisol-secreting diroders, and others. Many studies have discussed the effects of TBI on different hormone abnormalities22,23,25. In particular, the pituitary gland is more vulnerable to injury because it is located intracranially.
The differences between the TBI and non-TBI groups still existed when we evaluated pituitary dysfunction. Patients with a TBI history had a higher chance of developing pituitary dysfunction. In contrast, the difference in the risk of developing a non-pituitary abnormality was less significant between the TBI and non-TBI groups.
This result shows that TBI may have a greater effect on pituitary than on other non-pituitary hormone tissues because the pituitary gland is located inside the cranial vault and could face direct impact from the injury, whereas other endocrine glands, located outside the cranial vault, would not have the same level of risk. As a result, a patient with a TBI injury history would have a higher chance of developing pituitary dysfunction compared with a non-pituitary abnormality.
Wachter et al. reported that the increased intracranial pressure caused by brain edema is a mechanism for pituitary dysfunction after TBI44. Salehi et al. also stated that brain injury from a shearing force could result in a hypothalamic-pituitary injury45. Hypoxia following brain injury also causes pituitary dysfunction6.
Previous studies have reported a variety of effects from different types of brain injuries27,46,47,48. Aimaretti et al. reported that subarachnoid hemorrhage is a risk factor for pituitary dysfunction31. In our study, we subgrouped patients with TBI into three ICD-9 code groups of mild head injury, intracranial hemorrhage, and skull bone fracture.
According to our results, the risk of developing a future endocrinopathy was less significant in patients with only mild head injury, (HR: 1.34); however, if a patient had an intracranial hemorrhage or a skull bone fracture, risk increased. In particular, the HR was 2.05 in the skull bone fracture group. Furthermore, the risk of developing pituitary dysfunction in patients with mild head injury was not as significant (HR: 1.78). Intracranial hemorrhage and skull bone fracture both increased the risk of developing pituitary dysfunction, and the association was the strongest in the skull bone fracture group (HR: 3.77).
Several reasons could explain the strong effect of skull bone fracture on pituitary dysfunction. First, the pituitary gland is situated above the sphenoid sinus where it is in close contact with the surrounding skull bone. The impact causing a head injury and skull bone fracture could be transmitted to the pituitary gland by direct contact. However, if an intracranial hemorrhage is located remotely to the pituitary gland, less injury is likely to be caused. The direct force could cause vessel injury, particularly the long hypophyseal vessel and portal capillaries in the stalk that supply the pituitary gland39. Second, impact power differs, as the energy needed to cause a skull bone fracture is probably greater than the energy required to cause a mild head injury. Therefore, a higher impact would result in a greater chance of pituitary injury and later dysfunction.
Furthermore, according to a review by Kokshoorn et al., most studies have a median 1-year follow-up time43. However, some studies have reported on the long-term effect of TBI7,21,29,39. Aimaretti et al. reported possible improvement or worsening in pituitary function after a 3-month follow-up31. Other studies have reported the possible resolution of pituitary dysfunction after 1 year.
Therefore, patients with brain injury need a long-term follow up. We noted a linear pattern of cumulative incidence of post-traumatic endocrinopathies during 1996–2009, and a gradual widening of the gap between the groups with and without TBI. The incidence of pituitary dysfunction after TBI also showed a similar pattern. This result reveals the possibility of long-term, persistent effects of TBI at the endocrine level.
When we evaluated the risk of pituitary dysfunction among the three types of brain injury at the 5-year follow-up, the association between skull bone fracture and pituitary dysfunction became less significant compared with the association at the 1-year follow up. In contrast, the association between intracranial hemorrhage and pituitary dysfunction became stronger. However, the reasons for these differences are unknown.
There are some possible explanations. First, the impact that causes a skull bone fracture lasts for only a short period, which would only cause short-term pituitary dysfunction. Short-term hormone dysfunction could also be explained by acute phase inflammation and stress49,50,51,52.
Second, although the impact causing an intracranial hemorrhage lasts for a short time, once the hemorrhage occurs, it can mean long-term neuron functional loss. Shallice et al. reported that disrupting brain networks could cause altered cognition and behavior53. Therefore, it is reasonable to consider that the risk of developing pituitary dysfunction increases in patients with long-term cerebral dysfunction.
Some limitations of our study should be mentioned. First, selection bias could not be avoided completely, although patients diagnosed with endocrine dysfunction before the TBI event were excluded. However, patients with a pre-existing endocrinopathy but with subclinical symptoms would probably not have undergone endocrine testing. These patients would not have been recognized as such and therefore would not have been excluded from our study. However, this error would have occurred randomly, since the same definitions/criteria were used for the two groups, likely resulting in a similar incidence of endocrine dysfunction in both groups. Second, the lack of actual laboratory data in the NHIRD databases, the kind of hormone measurements that could be extracted, and some examination results were not recorded. Consequently, we were unable to further assess the types of hormone dysfunction. Third, we could not differentiate TBI severity among patients with intracranial hemorrhage and skull bone fracture. For example, no data are available in the database regarding the coma scale score, pupil size, or muscle power. In addition, imaging information is unavailable. Therefore, we were unable to further explore the association between TBI severity and the risk of developing an endocrinopathy. In addition, details of the TBI mechanism are not recorded in the NHIRD, such as falls and pedestrian-related, motor vehicle-related, sports, and work injuries. As a result, we were unable to analyze the potential relationship between the TBI mechanism and the risk of endocrinopathy. The fourth limitation was that some patients with TBI may not have been recorded in the database because patients with mild head injury do not always seek medical help and would not be admitted to a hospital. This population could further decrease the risk of post-traumatic endocrinopathy and cause an over-estimate in our study.
Conclusion
In conclusion, we report long-term follow-up results on the incidence of post-traumatic endocrinopathies using nationwide population. Patients with TBI had a higher risk of developing an endocrinopathy and pituitary dysfunction at the 1- and 5-year follow-ups. Among the three brain injuries, skull bone fracture was most significantly associated with pituitary dysfunction during the 1-year follow-up period. However, intracranial hemorrhage had a stronger association with pituitary dysfunction than that of skull bone fracture during the 5-year follow-up period. Long-term monitoring of pituitary function is recommended in patients with a TBI history due to the long-term effects of brain injury on the pituitary.
Additional Information
How to cite this article: Yang, W.-H. et al. Endocrine dysfunction following traumatic brain injury: a 5-year follow-up nationwide-based study. Sci. Rep. 6, 32987; doi: 10.1038/srep32987 (2016).
References
Servadei, F., Verlicchi, A., Soldano, F., Zanotti, B. & Piffer, S. Descriptive epidemiology of head injury in Romagna and Trentino. Neuroepidemiology 21, 297–304 (2002).
Vazquez-Barquero, A. et al. The epidemiology of head injury in Cantabria. European Journal of Epidemiology 8, 832–837 (1992).
Thurman, D. J., Alverson, C., Dunn, K. A., Guerrero, J. & Sniezek, J. E. Traumatic brain injury in the United States: a public health perspective. The Journal of head trauma rehabilitation 14, 602–615 (1999).
Cyran, E. Hypophysenschädigung durch schädelbasisfraktur. Dtsch Med Wschr 44, 1261 (1918).
Escamilla, R. F. & Lisser, H. Simmonds’ Disease. The Journal of Clinical Endocrinology & Metabolism 2, 65–96 (1942).
Kelly, D. F. et al. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a preliminary report. Journal of neurosurgery 93, 743–752 (2000).
Lieberman, S. A., Oberoi, A. L., Gilkison, C. R., Masel, B. E. & Urban, R. J. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. Journal of Clinical Endocrinology & Metabolism 86, 2752–2756 (2001).
Kokshoorn, N. et al. Low prevalence of hypopituitarism after traumatic brain injury: a multicenter study. European journal of endocrinology 165, 225–231 (2011).
Schneider, H. J., Kreitschmann-Andermahr, I., Ghigo, E., Stalla, G. K. & Agha, A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. Jama 298, 1429–1438 (2007).
Agha, A. et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. Journal of Clinical Endocrinology & Metabolism 89, 4929–4936 (2004).
Zihl, J. & Almeida, O. F. Neuropsychology of Neuroendocrine Dysregulation after Traumatic Brain Injury. Journal of Clinical Medicine 4, 1051–1062 (2015).
High Jr, W. M. et al. Effect of growth hormone replacement therapy on cognition after traumatic brain injury. Journal of neurotrauma 27, 1565–1575 (2010).
Ulfarsson, T. et al. Pituitary function and functional outcome in adults after severe traumatic brain injury: the long-term perspective. Journal of neurotrauma 30, 271–280 (2013).
Bondanelli, M. et al. Occurrence of pituitary dysfunction following traumatic brain injury. Journal of neurotrauma 21, 685–696 (2004).
Agha, A. et al. Posterior pituitary dysfunction after traumatic brain injury. The Journal of Clinical Endocrinology & Metabolism 89, 5987–5992 (2004).
Merola, B. et al. Cardiac structural and functional abnormalities in adult patients with growth hormone deficiency. The Journal of Clinical Endocrinology & Metabolism 77, 1658–1661 (1993).
Beshyah, S. A. & Johnston, D. G. Cardiovascular disease and risk factors in adults with hypopituitarism. Clinical endocrinology 50, 1–15 (1999).
Aimaretti, G. & Ghigo, E. Traumatic brain injury and hypopituitarism. The Scientific World Journal 5, 777–781 (2005).
Bavisetty, S. et al. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery 62, 1080–1094 (2008).
Agha, A., Sherlock, M., Phillips, J., Tormey, W. & Thompson, C. J. The natural history of post-traumatic neurohypophysial dysfunction. European Journal of Endocrinology 152, 371–377 (2005).
Masel, B. E. & Urban, R. Chronic Endocrinopathies in Traumatic Brain Injury Disease. Journal of neurotrauma 32, 1–9 (2015).
Powner, D. J., Boccalandro, C., Alp, M. S. & Vollmer, D. G. Endocrine failure after traumatic brain injury in adults. Neurocritical Care 5, 61–70 (2006).
Aimaretti, G. et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. The Journal of Clinical Endocrinology & Metabolism 90, 6085–6092 (2005).
Ghigo, E. et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Injury 19, 711–724 (2005).
Lauzier, F. et al. Clinical outcomes, predictors, and prevalence of anterior pituitary disorders following traumatic brain injury: a systematic review. Critical care medicine 42, 712–721 (2014).
Klose, M. et al. Prevalence and predictive factors of post‐traumatic hypopituitarism. Clinical endocrinology 67, 193–201 (2007).
Klose, M. & Feldt-Rasmussen, U. Does the type and severity of brain injury predict hypothalamo–pituitary dysfunction? Does post-traumatic hypopituitarism predict worse outcome? Pituitary 11, 255–261 (2008).
Kaulfers, A.-M. D. et al. Endocrine dysfunction following traumatic brain injury in children. The Journal of pediatrics 157, 894–899 (2010).
Tanriverdi, F. et al. Three years prospective investigation of anterior pituitary function after traumatic brain injury: a pilot study. Clinical endocrinology 68, 573–579 (2008).
Giordano, G., Aimaretti, G. & Ghigo, E. Variations of pituitary function over time after brain injuries: the lesson from a prospective study. Pituitary 8, 227–231 (2005).
Aimaretti, G. et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clinical endocrinology 61, 320–326 (2004).
Krahulik, D., Zapletalova, J., Frysak, Z. & Vaverka, M. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults: Clinical article. Journal of neurosurgery 113, 581–584 (2010).
Agha, A., Ryan, J., Sherlock, M. & Thompson, C. J. Spontaneous recovery from posttraumatic hypopituitarism. American journal of physical medicine & rehabilitation 84, 381–385 (2005).
Popovic, V. et al. Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. Journal of endocrinological investigation 27, 1048–1054 (2004).
Leal‐Cerro, A. et al. Prevalence of hypopituitarism and growth hormone deficiency in adults long‐term after severe traumatic brain injury. Clinical endocrinology 62, 525–532 (2005).
Richmond, E. & Rogol, A. D. Traumatic brain injury: endocrine consequences in children and adults. Endocrine 45, 3–8 (2014).
Ceballos, R. Pituitary changes in head trauma (analysis of 102 consecutive cases of head injury). The Alabama journal of medical sciences 3, 185–198 (1966).
Crompton, M. R. Hypothalamic lesions following closed head injury. Brain 94, 165–172 (1971).
Benvenga, S., CampennÍ, A., Ruggeri, R. M. & Trimarchi, F. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab 85, 1353–1361 (2000).
Niederland, T. et al. Abnormalities of pituitary function after traumatic brain injury in children. Journal of neurotrauma 24, 119–127 (2007).
Norwood, K. W. et al. Traumatic brain injury in children and adolescents: surveillance for pituitary dysfunction. Clinical pediatrics (2010).
Khadr, S. N. et al. Evaluation of pituitary function after traumatic brain injury in childhood. Clinical endocrinology 73, 637–643 (2010).
Kokshoorn, N. E. et al. Hypopituitarism following traumatic brain injury: prevalence is affected by the use of different dynamic tests and different normal values. European Journal of Endocrinology 162, 11–18 (2010).
Wachter, D., Gündling, K., Oertel, M. F., Stracke, H. & Böker, D.-K. Pituitary insufficiency after traumatic brain injury. Journal of Clinical Neuroscience 16, 202–208 (2009).
Salehi, F., Kovacs, K., Scheithauer, B. W., Pfeifer, E. A. & Cusimano, M. Histologic study of the human pituitary gland in acute traumatic brain injury. Brain Injury 21, 651–656 (2007).
Prasanna, K., Mittal, R. & Gandhi, A. Neuroendocrine dysfunction in acute phase of moderate-to-severe traumatic brain injury: A prospective study. Brain injury 29, 336–342 (2015).
Barton, R., Stoner, H. & Watson, S. Relationships among plasma cortisol, adrenocorticotrophin, and severity of injury in recently injured patients. Journal of Trauma and Acute Care Surgery 27, 384–392 (1987).
Feibel, J., Kelly, M., Lee, L. & Woolf, P. Loss of Adrenocortical Suppression after Acute Brain Injury: Role of Increased Intracranial Pressure and Brain Stem Function. The Journal of Clinical Endocrinology & Metabolism 57, 1245–1250 (1983).
Van den Berghe, G. & de Zegher, F. & Bouillon, R. Clinical review 95: acute and prolonged critical illness as different neuroendocrine paradigms. Journal of clinical endocrinology and metabolism 83, 1827–1834 (1998).
Beishuizen, A., Thijs, L. G. & Vermes, I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive care medicine 27, 1584–1591 (2001).
Annane, D. et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. Jama 283, 1038–1045 (2000).
Hamrahian, A. H., Oseni, T. S. & Arafah, B. M. Measurements of serum free cortisol in critically ill patients. New England Journal of Medicine 350, 1629–1638 (2004).
Shallice, T. & Cooper, R. The organisation of mind. (Oxford University Press, 2011).
Acknowledgements
We thank the Center of Excellence for Chang Gung Research Datalink (CORPG6D0161) for the comments and assistance with the data analysis. This study was supported by a grant from Chang Gung Memorial Hospital, Chia-yi Branch, and was based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Author information
Authors and Affiliations
Contributions
Conception of the article: W.-H.Y., T.-C.W. and C.-Y.C. Drafting and Design: W.-H.Y. Analysis and interpretation of the data: P.-C.C., Y.-H.Y. and T.-Y.K. Critical revision of the article: P.-C.C.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, WH., Chen, PC., Wang, TC. et al. Endocrine dysfunction following traumatic brain injury: a 5-year follow-up nationwide-based study. Sci Rep 6, 32987 (2016). https://doi.org/10.1038/srep32987
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32987
This article is cited by
-
Coverage of education and training of traumatic brain injury-induced growth hormone deficiency in US residency and fellowship programs: a cross-sectional study
BMC Medical Education (2024)
-
Neuroendocrine Disruptions Following Head Injury
Current Neurology and Neuroscience Reports (2023)
-
Tanshinone-IIA mediated neuroprotection by modulating neuronal pathways
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Association between carbon monoxide poisoning and adrenal insufficiency: a nationwide cohort study
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.