Abstract
P-glycoprotein (P-gp) is one of the best-known ATP-dependent efflux transporters, contributing to differences in pharmacokinetics and drug-drug interactions. Until now, studies on pig P-gp have been scarce. In our studies, the full-length porcine P-gp cDNA was cloned and expressed in a Madin-Darby Canine Kidney (MDCK) cell line. P-gp expression was then determined in tissues and its role in the pharmacokinetics of oral enrofloxacin in pigs was studied. The coding region of pig Abcb1 gene was 3,861 bp, encoding 1,286 amino acid residues (Mw = 141,966). Phylogenetic analysis indicated a close evolutionary relationship between porcine P-gp and those of cow and sheep. Pig P-gp was successfully stably overexpressed in MDCK cells and had efflux activity for rhodamine 123, a substrate of P-gp. Tissue distribution analysis indicated that P-gp was highly expressed in brain capillaries, small intestine, and liver. In MDCK-pAbcb1 cells, enrofloxacin was transported by P-gp with net efflux ratio of 2.48 and the efflux function was blocked by P-gp inhibitor verapamil. High expression of P-gp in the small intestine could modify the pharmacokinetics of orally administrated enrofloxacin by increasing the Cmax, AUC and Ka, which was demonstrated using verapamil, an inhibitor of P-gp.
Similar content being viewed by others
Introduction
P-glycoprotein (P-gp, encoded by the Abcb1 gene) is an important ATP-dependent efflux transporter, which was initially identified in Chinese hamster ovary cells in 19761. P-gp has been documented to be highly expressed in the intestine, liver, and kidney of humans and rodents and as such has essential roles in bioavailability and drug-drug interaction of substrate xenobiotics2,3. P-gp has been shown to recognize structurally and pharmacologically diverse compounds, including drugs widely used in veterinary medicine (e.g. ivermectin, macrolides, and fluoroquinoles)4,5; thus, the relevance of P-gp in veterinary medicine and drug development is significant. However, little information is available for veterinarians on oral absorption, elimination and drug-drug interactions related to P-gp.
The complete cDNA of P-gp from human6, mouse7,8, rat9,10, ovine11, dog12, feline13 and the Salmon louse14 have been cloned. Pig is an important model species for veterinary medicine, and it is commonly used in toxicological and pharmacological studies. Until now, information about porcine P-gp is lacking, and expression and function of P-gp in pharmacokinetically important tissue is scarce. Here, full-length cDNA for porcine P-gp was cloned and a Madin-Darby Canine Kidney (MDCK) cell line that stably expressing pig P-gp was established. Its expression in tissues, as well as its participation in enrofloxacin pharmacokinetics, was assessed in pigs.
Results
Sequence analysis of porcine P-gp
Based on sequence homology, three gene fragments of porcine Abcb1(971, 2480 and 712 bp) (Fig. 1A) were amplified (Fig. 1B) using primers depicted in Table 1. Fusion PCR was then performed to obtain full-length cDNA (Fig. 1C). The Abcb1 gene was confirmed by DNA sequencing and BLASTN analysis at NCBI. The cDNA was 4,060 bp, consisting of a 5′-untranslated region of 66 bp, an uninterrupted open reading frame of 3,861 bp, and a 3′-untranslated region of 133 bp. The sequence was submitted to GenBank and assigned an accession number (GenBank ID: KP233220). The nucleotide sequence shared 89, 88, 89 and 82% identities with that of cow (XM590317.7), sheep (NM001009790.1), human (NM000927.4) and mouse (NM011075.2), respectively. The deduced amino acid sequence of porcine P-gp was 1,286 amino acid residues in length and the estimated molecular weight was 141.966 kDa and the theoretical isoelectric point was 8.99. The protein sequence of porcine P-gp shared 89, 88 and 87% identity to that of human (NP000918.2), cow (XP590317.6) and sheep (CAM33439.1), respectively (Fig. 2A). Unique to the sequences of P-gp in ruminants (sheep and cow) is a 4-amino acids deletion at position 22–25 of the amino acid sequence.
(A) Three overlapping fragments (F1, F2 and F3) were initially PCR amplified. Fragments F1, F2 and F3 were designed and sub-cloned into pMD-18-T vector (TaKaRa, Japan) for sequencing. (B) Three fragments of the porcine Abcb1 amplified by PCR. M1: DNA Marker 2000, M2: DNA Marker 5000, Lanes 1, 2 - Fragment 1; Lanes 3, 4 - Fragment 2; Lanes 5, 6 - Fragment 3; The full-length gels are presented in Supplementary Figure 1. Gels were run under the same experimental conditions. (C) Full-length product amplified by fusion PCR using three fragments as templates. M: DNA Marker 5000, Lanes 1- Full-length cDNA of porcine Abcb1. Full-length gels appear in Supplementary Figure 1.
(A) Multiple sequence alignments of deduced amino acid sequence of pig Abcb1 with other species. Alignments were performed with Bio Edit. Walker A-, Walker B-, and the C-motif are boxed in red, which are characteristic of ABC-transporters. GenBank ID: Homo sapiens NP_000918.2, Bos Taurus XP_590317.6, Ovis aries NP_001009790.1. (B) Phylogenetic tree of Abcb1 coding sequences. Calculations were performed using the ClustalW algorithm and the evolutionary history was inferred using the Neighbor-Joining method in MEGA 4.0.
A phylogenetic tree was constructed using the neighbor-joining (NJ) method with a 1,000 bootstrap test based on the multiple alignments, which indicated that the selected 14 protein sequences of P-gp were clustered into two groups: avian and mammal. The porcine P-gp was located in vertebrates group and was closely related to cow and sheep, suggesting a close evolutionary relationship between them (Fig. 2B).
Protein structural model of porcine P-gp
Porcine P-gp was predicted to possess 12 transmembrane helices with extracellular N- and C-termini (Fig. 3A) and comprised four structural domains: two cytoplasmic domains containing the nucleotide-binding domains (NBD) and another two hydrophobic transmembrane domains (TMD), which was similar to human P-gp (see Fig. 3). The potential putative N-glycosylation sites of porcine and human P-gp was marked in red (Fig. 3), indicating seven in the porcine P-gp, whereas there were ten in human P-gp. The tertiary structure of porcine P-gp contained 42 α-helices and 21 β-strands (Fig. 4A). Fourteen binding site residues were found to be located in TM5 (Tyr-308), TM6 (Phe-337, Leu-340, Ile-341, Phe-344), TM7 (Gln-726, Phe-729, Ser-730, Phe-733), TM11 (Tyr-954) and TM12 (Phe-979, Ser-980, Val-983, Ala -986) in the porcine P-gp (Fig. 4B,C).
Establishment and characterization of an MDCK cell line stably-transfected with porcine Abcb1
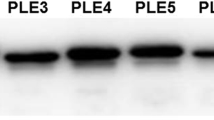
The pig Abcb1 gene was amplified using primers shown in Table 1 with XhoI and XbaI sites, respectively, and a recombinant pcDNA3.1-pAbcb1 plasmid was constructed. MDCK cells were transfected with pcDNA3.1-pAbcb1. A single cell colony resistant to G418 was selected as a stable transfectant (MDCK-pAbcb1). The presence of Abcb1 was confirmed with qRT-PCR and western blot (Fig. 5A,B). The accumulation of Rho123 (substrate of human P-gp) was measured to investigate whether MDCK-pAbcb1 cell line has enhanced porcine P-gp protein function. Figure 5C,D show that MDCK-pAbcb1 cells accumulated less Rho123 than MDCK cells (control), indicating a significantly enhanced extruding capacity of Rho123 in MDCK-pAbcb1 cells (p < 0.01). Furthermore, verapamil (100 μM), an inhibitor of human P-gp, significantly (p < 0.01) increased Rho123 accumulation in MDCK-pAbcb1 cells, demonstrating that such an accumulation could be reversed by verapamil.
(A) qRT-PCR analysis of pig Abcb1 mRNA levels in MDCK cells, IPEC-J2 cells and cells from three colonies of MDCK-pAbcb1. (B) Western blot of relative pig P-gp protein expression in MDCK, IPEC-J2 and cells from three colonies of MDCK-pAbcb1; assays run under the same experimental conditions and blots were cropped from original full-sized images (see Figure S2). Samples were derived from the same experiment and blots were processed simultaneously. (C) Accumulation of 5 μM Rho123 in MDCK and MDCK-pAbcb1 cells with/without verapamil (100 μM) during 2 h. Mean ± SEM; n = 3. (D) Rho123 accumulation at 2 h with flow cytometry. Histogram shows fluorescence (x-axis) representing Rho123 accumulation plotted as a function of the number of cells (y-axis) and is representative of three independent experiments.
Expression of P-gp in pigs
Porcine P-gp mRNA and protein were analyzed by qRT-PCR and Western blot, respectively. As shown in Fig. 6A, Abcb1 mRNA was detected in all tested tissues. In the CNS, the brain capillaries had the most Abcb1 mRNA and the cerebellum had the least. Abcb1 mRNA levels in the cerebral cortex, cerebellum, midbrain, hypothalamus and hippocampus was very low. In the peripheral tissues, Abcb1 were highly expressed in the jejunum, ileum, colon and liver. Abcb1 mRNA transcription in the ileum was greater than in the cecum and kidney (p = 0.005, p = 0.001) and significantly higher than that in the duodenum and rectum (p = 0.017, p = 0.014). Abcb1 mRNA in the jejunum, colon and liver was significantly higher than that in the kidney (p = 0.046, p = 0.018, p = 0.030, respectively).
(A) Relative Abcb1 mRNA levels; (B) Relative P-gp protein expression levels. Bars represent the mean ± SEM (n = 3). (C) Western blot of porcine P-gp protein; assays run under the same experimental conditions and blots were cropped from original full-sized images (see Figure S3). Samples were derived from the same experiment and blots were processed simultaneously.
P-gp protein (shown in Fig. 6C) was ~170 kDa as detected in different pig tissues, indicating that antibody Mdr-1 directed against the human isoform could also recognize porcine P-gp. Protein expression in tissues appears in Fig. 6B. The most P-gp protein was in the brain capillaries and the least was observed in the hypothalamus. The P-gp protein level was correlated to its mRNA level (r = 0.863, p < 0.05).
The effect of P-gp on in vitro transport and in vivo pharmacokinetics of enrofloxacin
Enrofloxacin was measured using HPLC, and the lowest limit of detection (LOD) of enrofloxacin was 0.02 μg/mL, based on a signal-to-noise ratio > 3. The lowest limit of quantification (LOQ) was 0.05 μg/mL with a signal-to-noise ratio > 10. The mean percent recovery of enrofloxacin exceeded 81%, and intra-assay and inter-assay reproducibility had a relative standard deviation (RSD) <11%. Assay linearity was good over 0.05–10 μg/mL with r2 = 0.9999.
MDCK and MDCK-pAbcb1 cells were grown to confluent monolayers on porous membrane filters, and vectorial transport of the enrofloxacin (12 μM) across the cells was determined. In the MDCK parental cell line, apically and basolaterally directed translocation of enrofloxacin were similar. However, in the pAbcb1 expressing MDCK cells, Papp(BL-AP) was significantly higher than Papp(AP-BL) (P < 0.01). The P-gp-mediated transport was completely inhibited by its inhibitor verapamil, resulting in a similar efflux ratio, compared to that of the MDCK parental cell line (Table 2). These results showed highly efficient transport of enrofloxacin by porcine P-gp.
To further confirm whether P-gp affects the pharmacokinetics of enrofloxacin in pigs, verapamil (an inhibitor of P-gp) was administrated prior to enrofloxacin, and the plasma concentration-time curve and pharmacokinetics of enrofloxacin are summarized in Fig. 7 and Table 3, respectively. Compared with the control group, Cmax, AUC and Ka of enrofloxacin were increased, whereas Tpeak and T1/2ka of enrofloxacin were shortened in animals when co-administrated with verapamil. In summary, enrofloxacin pharmacokinetics was altered by P-gp inhibition, indicating that porcine P-gp can influence enrofloxacin behavior in vivo.
Discussion
Oral drug administration is convenient intake route for humans, as well as for animals. The effects of P-gp on drug pharmacokinetics are important for the evaluation of the capacity of oral drug intake in pigs. The localization of P-gp in different tissues in pigs was studied in our previous work15; however, how P-gp functions in pigs is unclear. To address this deficit, we cloned full-length cDNA of porcine P-gp, and characterized the transport activity of P-gp by exogenously expressing the Abcb1 gene in MDCK cells and measuring the porcine P-gp at the protein and transcriptional levels. Then, we assessed its role in the pharmacokinetics of oral enrofloxacin in pigs. This represents the first cloning of full-length porcine cDNA of P-gp.
The porcine P-gp shared high similarities with P-gps from cow, sheep, human and mouse, suggesting a similar physiological role in these animals. Drug-binding pockets are predicted to reside in transmembrane segments, which formed a translocation path for drug substrates as they exit the membranes16. The nucleotide binding domain may bind ATP or its analogs and can hydrolyze ATP17,18,19,20. There is “cross-talk” between nucleotide binding and transmembrane domains as evidenced by cross-linking studies21, and transmembrane domains of porcine P-gp were generated here with Protter protein display software. The prediction showed that porcine P-gp contains twelve transmembrane domains which was similar to human P-gp, but glycosylation site prediction revealed seven potential putative N-linked glycosylation sites on porcine P-gp, fewer than that in human P-gp. Some studies have shown that N-glycosylation can participate in many biological processes such as regulation of intracellular targeting, protein folding and maintenance of protein stability22,23. However, other studies have reported that N-linked glycosylation is not essential for protein expression, plasma membrane localization or overall function24,25. Further studies are necessary to clarify whether glycosylation affects the function of porcine P-gp.
To better understand how porcine P-gp transports various hydrophobic compounds, we predicted residues that participate in drug binding. Our results indicated that amino acids participating in substrate binding were located at TMD1 sites 5 and 6 and TMD2 sites 7, 11 and 12 of porcine P-gp, whereas previous studies have shown that human P-gp substrate binding sites were located in TMD1 sites 5 and 6 and TMD2 sites 11 and 1226. This may influence substrate types. Site-directed mutagenesis studies of Abcb1 can be performed to prove whether these residues in porcine P-gp participate in ligand binding. Knowing substrate binding sites may enable development of drugs that bypass recognition of P-gp.
Human colorectal adenocarcinoma cell line (Caco-2) has been approved by the US Food and Drug Administration (FDA) for in vitro transport studies of P-gp27,28. However, pharmacokinetics might be different because of the specific functions of P-gp across different species29. An alternative approach was to establish a porcine P-gp expression system. The MDCK cell line is an approved model for overexpressing Abcb1 originating from other species due to its negligible expression of endogenous transporters30,31. MDCK cell line overexpression porcine P-gp could be used to screen substrate drugs and potential inhibitors or inducers.
The efflux efficiency of P-gp substrate is determined by the P-gp number or density on the cell membrane32. Differences expression pattern of P-gp in tissues may influence therapeutic outcomes by affecting the accumulation of drugs in specific tissues. Transcription of Abcb1 had been measured using RT-PCR in the liver and brain capillary endothelial cells (pBCECs)33 and in the kidneys34. We measured Abcb1 mRNA level in the CNS, intestine, liver and kidneys. The data demonstrated that its expression in intestine was not uniform. In the peripheral tissues, the greatest expression was in the ileum. The porcine liver also expressed relatively high Abcb1 mRNA, which was consistent with previous data from human and canines35,36. Renal Abcb1 mRNA is lower in the pig, compared to that in humans36,37. P-gp in intestinal epithelial cells may prevent pharmacotherapeutic agents from entering the systemic circulation and therefore change their pharmacokinetics38. Hepatic metabolism is significant, and high expression level of P-gp is vital for biliary drug, toxicant, and metabolite excretion39. More Abcb1 mRNA in the intestine and liver suggests that porcine P-gp may limit absorption and facilitate secretion of substrates into the intestines. In addition, we measured P-gp protein expression level in 60-day old piglets and found that this was coincident with mRNA levels, except for the ileum and jejunum, suggesting a tissue-specific post-transcriptional mode of regulation. Hence, RNA analysis of pig tissue samples should be done in conjunction with protein analysis, because RNA and protein may differ among certain organs.
Of note, the P-gp expression pattern in tissues affects pharmacokinetics of enrofloxacin, a fluoroquinolone (FQ) widely used in veterinary medicine either orally or parenterally. Sorgel’s group40 and others41,42,43 reported that the concentrations of ciprofloxacin and danofloxacin-mesylate in the gut lumen are higher than those in plasma. Other studies proved that ciprofloxacin and danofloxacin-mesylate were substrates of multiple human ABC transporters44,45,46, indicating that intestinal efflux is the underlying mechanism for danofloxacin-mesylate and ciprofloxacin secretion into the lumen. In this study, the net efflux ratio of enrofloxacin across MDCK-pAbcb1 cells was more than 2 and basolateral-to-apical transport of enrofloxacin could be counteracted by the P-gp inhibitor verapamil. Although the concentration of enrofloxacin was not measured in the luminal compartment, its concentration in plasma was increased when animals received verapamil. These results suggest that enrofloxacin might be a substrate of porcine P-gp, similar to the data obtained in chickens47. Initially, we treated piglets with the P-gp inhibitor PSC-833, but some animals began vomiting. Thus, PSC-833 was replaced with verapamil, which could inhibit P-gp function and be monitored in an MDCK-pAbcb1 cell line.
Our data also suggested that co-administration of P-gp inhibitors may be an alternative strategy to improve oral bioavailability and therapeutic efficacy of enrofloxacin when used to treat systemic bacterial infection in the swine industry. Three generations of P-gp inhibitors have been identified but none of them show improved therapeutic efficacy due to their broad activity and toxicity48. Berberine can significantly inhibit P-gp (data not shown) so it may be a potential P-gp inhibitor as it is not absorbed after oral administration and only inhibits P-gp in the gastrointestinal tract.
In conclusion, the complete cDNA of porcine P-gp was cloned for the first time. The protein structure was modeled and analyzed. The phylogenetic analysis on P-gp proteins from different species was performed, and its protein expression and mRNA levels in different tissues were studied. Also, the effect of P-gp on enrofloxacin transmembrane transport and in vivo pharmacokinetics was evaluated. More studies are required to fully elucidate the complex epigenetic regulation of porcine P-gp during development, tissue-specific expression patterns, and the contribution of epigenetics to species variability in drug disposition and therapeutic response.
Materials and Methods
Cells
MDCK (Madin-Darby canine kidney) and IPEC-J2 cells were obtained from Shanghai Institute of Cell Biology, the Chinese Academy of Sciences (Shanghai, China) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, HyClone Laboratories Inc.). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and sub-cultured once 80% confluent.
Animals
Sixty-day-old healthy crossbred pigs (large white x Landrace x Duroc, 20 ± 2 kg) were bought from Jiangsu Agricultural Academy and reared under standard conditions of light and temperature. Feed and water were provided ad libitum during the study. All pigs were fed according to the breeding standards of the Chinese Local Pigs and National Research Council (NRC). Animal use and handling protocols were approved by the regional Animal Ethics Committee and Nanjing Agricultural University. The protocol of the study was conducted in accordance with guidelines of the regional Animal Ethics Committee and Nanjing Agricultural University.
Total RNA extraction and cDNA synthesis
All pigs were anesthetized and killed by decapitation. Samples of liver, kidney, intestines and brain tissues were taken immediately (within 5–10 min after death). Brain capillaries were isolated from ~10 g of fresh cerebral cortices according to published methods with minor modification49. In brief, meninges and choroid plexus from the brains were removed and cerebral matter was homogenized in 20 volumes of cold Ringer’s solution containing 10 mM HEPES (Santa Cruz, Heidelberg, Germany) at pH 7.4 in a glass homogenizer. Ten upwards and downwards strokes were applied during the homogenization. The homogenates were collected and filtered initially through a 150 μm nylon mesh, the filtrate was then re-filtered through a 60 μm nylon mesh. The brain capillaries are trapped on the 60 μm nylon meshes and were collected in the tube. All samples were frozen in liquid nitrogen and then stored at −80 °C until RNA and protein extraction.
Total RNA was extracted from tissues using TRIZOL (TaKaRa, Japan) following the manufacturer’s instructions. Quality and concentration were measured using a photometer (Eppendorf Biophotometer, Germany). RNA integrity was assessed with RNA electrophoresis. cDNA templates were synthesized from 1 μg of total RNA using a HISCRIPT 1st strand cDNA synthesis kit (Vazyme, Nanjing, China).
Cloning and sequencing of full-length cDNA of porcine Abcb1
PCR primers (Table 1) toward three overlapping cDNA fragments of porcine Abcb1 (F1, F2, F3, shown in Fig. 1A) were designed based on sequence alignments of conserved regions from various species (Human, NM_000927.4; sheep, NM_001009790.1; dog, NM_001003215.1; mouse, NM_011075.2). Three overlapping fragments of porcine Abcb1 were amplified by touchdown PCR using PrimeSTAR GXL DNA Polymerase (TaKaRa, Japan) under these conditions: 95 °C for 5 min, 36 cycles of 95 °C for 30 s, 65–55 °C for 30 s (initial annealing temperature of 65 °C was reduced by 2 °C after every six cycles to 55 °C for the final six cycles), 72 °C for 1 min, and an extra extension of 15 min at 72 °C. PCR products were verified using published methods50. After the three products were confirmed to be part of the Abcb1 gene by sequencing, full-length cDNA was obtained from the three fragments using fusion PCR as follows: 95 °C for 5 min, 30 cycles of 95 °C for 30 s, 62.5 °C for 30 s, 72 °C for 1 min, and 72 °C for 15 min. Final PCR products were confirmed by sequencing and the sequence was submitted to GenBank to obtain an accession number.
Bioinformatics analysis of porcine Abcb1 and its protein structural model
cDNA sequence analysis was conducted using NCBI BLAST (http://www.ncbi.nlm.nih.gov/blast). Calculated molecular weights and predicted isoelectric points were obtained with the ExPASy online server (http://www.expasy.org/tools/). Multiple alignments of P-gp protein sequences were generated using the Bio Edit program. A phylogenetic analysis was used to determine relatedness of porcine P-gp to other mammalian P-gp sequences, and alignment data were imported into MEGA version 4.1. The phylogenetic tree was constructed using the neighbor-joining method with Poisson correction51. Bootstrap analysis was performed using 1,000 replicates.
The secondary structure of porcine and human P-gp was predicted and compared with Protter1.0 servers (http://wlab.ethz.ch/protter/start/)52 and 3D structures and precise substrate binding sites were predicted using I-TASSER (http://zhanglab.ccmb.med.umich.edu/)53.
Establishment of an MDCK cell line stably-transfected with porcine Abcb1
Primers used to amplify pig Abcb1 complementary DNAs (cDNAs) were designed based on our previously submitted sequence (GenBank ID: KP233220) containing an XhoI site within the sense primer and an Xba I site within the antisense primer (See Table 1 for primers). PCR amplifications were performed as previously described in the Methods. Pig Abcb1 full cDNA was cloned into the plasmid pcDNA3.1 and transformed into Escherichia coli DH5α cells (Vazyme, China). Plasmid was purified using an Omega Endo-Free Plasmid Mini Kit and was sequenced using vector primers to confirm inserted genes (Invitrogen, Shanghai, China).
To generate cell lines stably expressing porcine P-gp, an MDCK cell line was transfected with pcDNA3.1-pAbcb1 plasmid using LipofectAMINE 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Stable transfectants were selected with G418 (600 μg/mL, Gibco). When single-cell stable colonies resistant to G418 were observed, cells were sub-cultured again and distinct colonies were isolated using cloning cylinders. Cells were grown in the presence of G418 to maintain expression of transfected genes. MDCK -pAbcb1 cells were propagated and sub-cultured in the same medium with the addition of G418 (300 μg/mL). MDCK, IPEC-J2 and three colonies of MDCK -pAbcb1 were used to measure exogenous expression of porcine P-gp. qRT-PCR and western blot were used according to published methods54, with different primers (Table 1) and antibodies (Mdr-1(Santa Cruz, Heidelberg, Germany), dilution 1:200).
Rhodamine 123 accumulation assay
MDCK and MDCK-pAbcb1 cells were seeded in six-well plates, and cells were washed with PBS and rhodamine 123 (Rho123; 5 μM) (Sigma-Aldrich, Castle Hill, Australia) was added in the presence and absence of verapamil (100 μM) to identify P-gp mediated Rho123 accumulation. To study the time-course of Rho123 accumulation, cells were harvested at 15, 30, 60 and 120 min after the addition of Rho123 and accumulation was measured using a FACS Calibur (BD Biosciences, Bedford, MA) with CellQuest Prosoftware. Data were collected for a minimum of 10,000 gated events per sample as geometric mean fluorescent intensity for all samples.
Tissues distribution of P-gp (Abcb1) in pigs
Pigs (N = 6) were used to measure constitutive P-gp (Abcb1) expression in different tissues with real time quantitative PCR and western blot. Abcb1 mRNA was measured with qRT-PCR and gene-specific primers for PCR were designed according to the cloned sequence of porcine Abcb1 (KP233220 in GenBank). GAPDH was as internal control for each sample. Primer pairs for genes appear in Table 1. qRT-PCR was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad, USA), and SYBR Green was the dye (Toyobo, Japan). mRNA was measured using 12.5 μL SYBR Green real-time PCR Master Mix in a 25 μL volume with 2 μL cDNA. Each run consisted of an initial 1 min activation cycle at 95 °C, followed by 40 cycles of denaturation for 20 s at 95 °C, annealing for 30 s at 60 °C, and elongation for 31 s at 72 °C. Uniform amplification of products was analyzed according to melting curves of the amplified products. Abcb1 mRNA relative expression in different tissues was measured using the 2−ΔΔCt method55.
P-gp relative expression was measured with standard western blot with minor modifications50. Tissue protein was extracted using a membrane and cytosol protein extraction kit (Beyotime, Haimen, China). Briefly, equal amounts of membrane proteins (5 μg/lane) were fractionated with SDS-PAGE and then transferred onto PVDF membranes (BIO-RAD, USA). Membranes were blocked with 5% bovine serum albumin and incubated with primary antibody at the appropriate dilution (Mdr-1, Santa Cruz, Germany, 1:200; β-actin, TransGen, China, 1:5,000). Membranes were incubated overnight at 4 °C, followed washing three times with PBST for 15 min. Thereafter, membranes were incubated with horseradish peroxidase-labeled goat anti-mouse IgG secondary antibody (1:5,000, Boster, Wuhan, China) for 1 h at 37 °C, then washed three times with PBST for 15 min and two times with PBS for 10 min. Interactive bands were visualized with ECL (Vazyme, China) and scanned with a Tanon 5200 chemiluminescent imaging system (Tanon, China). Relative protein was quantified by calculating densitometric values of target bands using Image J software.
Experimental design of enrofloxacin bidirectional transport assay and pharmacokinetic studies in pigs
Transport assays were carried out as described45 with minor modifications. MDCK and MDCK-pAbcb1 with a density of 6.6 × 104 cells/insert were seeded on a polyethylene terephthalate membrane insert (Millicell cell culture inserts, 1.0 μm pore size, 6.5 mm diameter) in 24-well culture plates. Cell monolayers with TEER values > 150 Ω.cm2 were used in the study56. Two hours before the experiment, medium in both apical and basolateral sides of the monolayer was replaced by HBSS transport buffer, either with or without 100 μM verapamil. The experiment was started (t = 0) by replacing the medium in either the apical (AP) or basolateral (BL) compartment with fresh HBSS buffer containing 12 μM enrofloxacin in the presence or absence of 100 μM verapamil. Transwells were shaken gently at 50 rpm. At 120 min, 0.20 ml samples were withdrawn from the receiver chambers. Samples were stored at −20 °C. All experiments were conducted in triplicate.
To evaluate the effect of P-gp on the pharmacokinetics of enrofloxacin, animals (N = 8 total) were randomized to 2 groups. Group 1 animals received enrofloxacin (10 mg/kg, po), and each animal in group 2 was pre-treated with verapamil (10 mg/kg, po) and then enrofloxacin (10 mg/kg, po). Blood samples were collected from the jugular vein 20 min before and at 0.083, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 12 and 24 h after enrofloxacin administration. Samples were centrifuged at 1,500 × g for 10 min and plasma was harvested and aliquoted for storage at −80 °C before HPLC analysis.
HPLC analysis of enrofloxacin
HPLC analysis of enrofloxacin was performed as published with minor modification47,57. Concentrations of enrofloxacin were measured using an Agilent 1200 HPLC system. In brief, plasma was thawed at room temperature and centrifuged at 2,000 × g for 5 min. The supernatant (0.5 mL) was mixed with acetonitrile and separated into organic and water phases by centrifugation. The organic phase was evaporated to dryness under a nitrogen stream and residue was resuspended with mobile phase solution. The samples from the transport assays were directly evaporated to dryness under a nitrogen stream and 40 μL of the mobile phase solution was used to concentrate the samples for 5 times. Twenty microliters of the mixture were injected into the HPLC column (Kromasil C18 columns, 5 mm particle size, 250 × 4.6 mm). The mobile phase composition was 0.1 M phosphoric acid (adjusted pH to 3.5 with triethylamine)/acetonitrile (84:16, v/v) and the flow rate of mobile phase was set to 0.85 mL/min. UV absorbance was measured at 278 nm. Assay validation including recovery rate, inter- and intra-assay precision, accuracy and assay linearity of enrofloxacin were assessed using published methods58.
Papp Calculation and Pharmacokinetic analysis
Apparent permeability coefficients (Papp) were calculated using the following equation: Papp = (dQ/dt)/(A × C0), where A is the area of filter membrane, C0 is the initial concentration of the test drug, dQ is the amount of transported drug, and dt is time elapsed. The efflux ratio (ER) was calculated from (Papp B → A)/(Papp A → B)59. Where Papp B → A and Papp A → B are BL to AP and AP to BL apparent permeability coefficients, respectively. The net efflux ratio was calculated using the following equation: Net efflux ratio = efflux ratio in MDCK-pAbcb1/efflux ratio in MDCK.
Pharmacokinetics were calculated for individual data using 3p97 practical pharmacokinetic software (Version97, Chinese Pharmacologic Association, Beijing, China). The best fit compartment model was assessed according to Akaike’s information criterion.
Statistical analysis
All data were analyzed for statistical significance using SPSS Statistics 17.0 (version 17.0, SPSS Inc., Chicago, IL, USA). The differences between means were considered significant at p < 0.05 and very significant at p < 0.01. Data were shown as means ± SEM.
Additional Information
How to cite this article: Guo, T. et al. Abcb1 in Pigs: Molecular cloning, tissues distribution, functional analysis, and its effect on pharmacokinetics of enrofloxacin. Sci. Rep. 6, 32244; doi: 10.1038/srep32244 (2016).
References
Juliano, R. L. & Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et biophysica acta 455, 152–162 (1976).
Wang, J. S. et al. Brain penetration of methadone (R)- and (S)-enantiomers is greatly increased by P-glycoprotein deficiency in the blood-brain barrier of Abcb1a gene knockout mice. Psychopharmacology 173, 132–138, doi: 10.1007/s00213-003-1718-1 (2004).
Xie, R., Hammarlund-Udenaes, M., de Boer, A. G. & de Lange, E. C. The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. British journal of pharmacology 128, 563–568, doi: 10.1038/sj.bjp.0702804 (1999).
Bugyei, K., Black, W. D. & McEwen, S. Pharmacokinetics of enrofloxacin given by the oral, intravenous and intramuscular routes in broiler chickens. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 63, 193–200 (1999).
Greiner, B. et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. The Journal of clinical investigation 104, 147–153, doi: 10.1172/JCI6663 (1999).
Chen, C. J. et al. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. The Journal of biological chemistry 265, 506–514 (1990).
Raymond, M., Rose, E., Housman, D. E. & Gros, P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Molecular and cellular biology 10, 1642–1651 (1990).
Kirschner, L. S. et al. Biochemical and genetic characterization of the multidrug resistance phenotype in murine macrophage-like J774.2 cells. Biochemical pharmacology 43, 77–87 (1992).
Deuchars, K. L., Duthie, M. & Ling, V. Identification of distinct P-glycoprotein gene sequences in rat. Biochimica et biophysica acta 1130, 157–165 (1992).
Silverman, J. A., Raunio, H., Gant, T. W. & Thorgeirsson, S. S. Cloning and characterization of a member of the rat multidrug resistance (mdr) gene family. Gene 106, 229–236 (1991).
Zahner, D., Alber, J. & Petzinger, E. Cloning and heterologous expression of the ovine (Ovis aries) P-glycoprotein (Mdr1) in Madin-Darby canine kidney (MDCK) cells. Journal of veterinary pharmacology and therapeutics 33, 304–311, doi: 10.1111/j.1365-2885.2009.01141.x (2010).
Steingold, S. F. et al. Characterization of canine MDR1 mRNA: its abundance in drug resistant cell lines and in vivo . Anticancer research 18, 393–400 (1998).
Okai, Y. et al. Molecular analysis of multidrug resistance in feline lymphoma cells. American journal of veterinary research 61, 1122–1127 (2000).
Heumann, J., Carmichael, S., Bron, J. E., Tildesley, A. & Sturm, A. Molecular cloning and characterisation of a novel P-glycoprotein in the salmon louse Lepeophtheirus salmonis. Comparative biochemistry and physiology . Toxicology & pharmacology: CBP 155, 198–205, doi: 10.1016/j.cbpc.2011.08.004 (2012).
Dong, L., Guo, L., Dai, X., Sun, Y. & Wang, L. Distribution and Relative mRNA Transcription Level of P-gp in Liver, Kidney and Intestines of Piglet. Acta Veterinaria et Zootechnica Sinica 44, 1454–1461 (2013).
Loo, T. W. & Clarke, D. M. The transmembrane domains of the human multidrug resistance P-glycoprotein are sufficient to mediate drug binding and trafficking to the cell surface. The Journal of biological chemistry 274, 24759–24765 (1999).
Shimabuku, A. M., Nishimoto, T., Ueda, K. & Komano, T. P-glycoprotein. ATP hydrolysis by the N-terminal nucleotide-binding domain. The Journal of biological chemistry 267, 4308–4311 (1992).
Baubichon-Cortay, H., Baggetto, L. G., Dayan, G. & Di Pietro, A. Overexpression and purification of the carboxyl-terminal nucleotide-binding domain from mouse P-glycoprotein. Strategic location of a tryptophan residue. The Journal of biological chemistry 269, 22983–22989 (1994).
Sharma, S. & Rose, D. R. Cloning, overexpression, purification, and characterization of the carboxyl-terminal nucleotide binding domain of P-glycoprotein. The Journal of biological chemistry 270, 14085–14093 (1995).
Dayan, G. et al. Binding of steroid modulators to recombinant cytosolic domain from mouse P-glycoprotein in close proximity to the ATP site. Biochemistry 36, 15208–15215, doi: 10.1021/bi9718696 (1997).
Loo, T. W. & Clarke, D. M. Inhibition of oxidative cross-linking between engineered cysteine residues at positions 332 in predicted transmembrane segments (TM) 6 and 975 in predicted TM12 of human P-glycoprotein by drug substrates. The Journal of biological chemistry 271, 27482–27487 (1996).
Urquhart, P., Pang, S. & Hooper, N. M. N-glycans as apical targeting signals in polarized epithelial cells. Biochemical Society symposium, 39–45 (2005).
Lee, T. K., Koh, A. S., Cui, Z., Pierce, R. H. & Ballatori, N. N-glycosylation controls functional activity of Oatp1, an organic anion transporter. American journal of physiology. Gastrointestinal and liver physiology 285, G371–G381, doi: 10.1152/ajpgi.00358.2002 (2003).
Diop, N. K. & Hrycyna, C. A. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry 44, 5420–5429, doi: 10.1021/bi0479858 (2005).
Mohrmann, K., van Eijndhoven, M. A., Schinkel, A. H. & Schellens, J. H. Absence of N-linked glycosylation does not affect plasma membrane localization of breast cancer resistance protein (BCRP/ABCG2). Cancer chemotherapy and pharmacology 56, 344–350, doi: 10.1007/s00280-005-1004-5 (2005).
Wang, R. B., Kuo, C. L., Lien, L. L. & Lien, E. J. Structure-activity relationship: analyses of p-glycoprotein substrates and inhibitors. Journal of clinical pharmacy and therapeutics 28, 203–228 (2003).
Balimane, P. V., Patel, K., Marino, A. & Chong, S. Utility of 96 well Caco-2 cell system for increased throughput of P-gp screening in drug discovery. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 58, 99–105, doi: 10.1016/j.ejpb.2004.02.014 (2004).
Madgula, V. L., Avula, B., Reddy, V. L. N., Khan, I. A. & Khan, S. I. Transport of decursin and decursinol angelate across Caco-2 and MDR-MDCK cell monolayers: in vitro models for intestinal and blood-brain barrier permeability. Planta medica 73, 330–335, doi: 10.1055/s-2007-967137 (2007).
Takeuchi, T. et al. Establishment and characterization of the transformants stably-expressing MDR1 derived from various animal species in LLC-PK1. Pharmaceutical research 23, 1460–1472, doi: 10.1007/s11095-006-0285-7 (2006).
Liu, Y. & Zeng, S. [Advances in the MDCK-MDR1 cell model and its applications to screen drug permeability]. Yao xue xue bao = Acta pharmaceutica Sinica 43, 559–564 (2008).
Tang, F., Horie, K. & Borchardt, R. T. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharmaceutical research 19, 765–772 (2002).
Tang, H., Pak, Y. & Mayersohn, M. Protein expression pattern of P-glycoprotein along the gastrointestinal tract of the Yucatan micropig. Journal of biochemical and molecular toxicology 18, 18–22, doi: 10.1002/jbt.20001 (2004).
Childs, S. & Ling, V. Duplication and evolution of the P-glycoprotein genes in pig. Biochimica et biophysica acta 1307, 205–212 (1996).
Goh, L. B. et al. Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochemical pharmacology 64, 1569–1578 (2002).
Conrad, S. et al. Sequencing and tissue distribution of the canine MRP2 gene compared with MRP1 and MDR1. Toxicology 156, 81–91 (2001).
Langmann, T. et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clinical chemistry 49, 230–238 (2003).
Nishimura, M. & Naito, S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug metabolism and pharmacokinetics 20, 452–477 (2005).
Takano, M., Yumoto, R. & Murakami, T. Expression and function of efflux drug transporters in the intestine. Pharmacology & therapeutics 109, 137–161, doi: 10.1016/j.pharmthera.2005.06.005 (2006).
Chandra, P. & Brouwer, K. L. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharmaceutical research 21, 719–735 (2004).
Sorgel, F. et al. Gastrointestinal secretion of ciprofloxacin. Evaluation of the charcoal model for investigations in healthy volunteers. The American journal of medicine 87, 62S–65S (1989).
Lindecrona, R. H., Friis, C. & Nielsen, J. P. Pharmacokinetics and penetration of danofloxacin into the gastrointestinal tract in healthy and in Salmonella typhimurium infected pigs. Research in veterinary science 68, 211–216, doi: 10.1053/rvsc.1999.0361 (2000).
McKellar, Q. A., Gibson, I. F. & McCormack, R. Z. Pharmacokinetics and tissue disposition of danofloxacin in sheep. Biopharmaceutics & drug disposition 19, 123–129 (1998).
Shem-Tov, M. et al. Pharmacokinetics and penetration of danofloxacin from the blood into the milk of cows. Journal of veterinary pharmacology and therapeutics 21, 209–213 (1998).
Merino, G. et al. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug metabolism and disposition: the biological fate of chemicals 34, 690–695, doi: 10.1124/dmd.105.008219 (2006).
Pulido, M. M. et al. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. Journal of veterinary pharmacology and therapeutics 29, 279–287, doi: 10.1111/j.1365-2885.2006.00744.x (2006).
Schrickx, J. A. & Fink-Gremmels, J. Danofloxacin-mesylate is a substrate for ATP-dependent efflux transporters. British journal of pharmacology 150, 463–469, doi: 10.1038/sj.bjp.0706974 (2007).
Guo, M. et al. Age-related P-glycoprotein expression in the intestine and affecting the pharmacokinetics of orally administered enrofloxacin in broilers. PloS one 8, e74150, doi: 10.1371/journal.pone.0074150 (2013).
Palmeira, A., Sousa, E., Vasconcelos, M. H. & Pinto, M. M. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Current medicinal chemistry 19, 1946–2025 (2012).
Mrsulja, B. B., Mrsulja, B. J., Fujimoto, T., Klatzo, I. & Spatz, M. Isolation of brain capillaries: a simplified technique. Brain research 110, 361–365 (1976).
Guo, T. T. et al. Cloning of Neuromedin B and its receptor in the rabbit and generating a polyclonal antibody to the Neuromedin B protein. Gene 564, 21–28, doi: 10.1016/j.gene.2015.03.038 (2015).
Haws, D. C., Hodge, T. L. & Yoshida, R. Optimality of the neighbor joining algorithm and faces of the balanced minimum evolution polytope. Bulletin of mathematical biology 73, 2627–2648, doi: 10.1007/s11538-011-9640-x (2011).
Omasits, U., Ahrens, C. H., Muller, S. & Wollscheid, B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30, 884–886, doi: 10.1093/bioinformatics/btt607 (2014).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nature protocols 5, 725–738, doi: 10.1038/nprot.2010.5 (2010).
Huan, C. C. et al. Porcine epidemic diarrhea virus uses cell-surface heparan sulfate as an attachment factor. Archives of virology 160, 1621–1628, doi: 10.1007/s00705-015-2408-0 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, doi: 10.1006/meth.2001.1262 (2001).
Lo, C. M., Keese, C. R. & Giaever, I. Cell-substrate contact: another factor may influence transepithelial electrical resistance of cell layers cultured on permeable filters. Experimental cell research 250, 576–580, doi: 10.1006/excr.1999.4538 (1999).
Guo, M. et al. E. coli infection modulates the pharmacokinetics of oral enrofloxacin by targeting P-glycoprotein in small intestine and CYP450 3A in liver and kidney of broilers. PloS one 9, e87781, doi: 10.1371/journal.pone.0087781 (2014).
Sunderland, J. et al. A reverse-phase HPLC assay for the simultaneous determination of enrofloxacin and ciprofloxacin in pig faeces. International journal of antimicrobial agents 23, 390–393, doi: 10.1016/j.ijantimicag.2003.07.014 (2004).
Karlsson, J., Kuo, S. M., Ziemniak, J. & Artursson, P. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. British journal of pharmacology 110, 1009–1016 (1993).
Acknowledgements
The authors are grateful for the manuscript correction from Prof. Shile Huang from Louisiana State University Health Sciences Center in USA and Prof. Xiang Mao from Chinese Academy of Agricultural Sciences. The study was supported in part by Qinlan Project of Jiangsu Province, National Natural Science Foundation of China (No. 31572567), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Program for Student Innovation through Research and Training (No. 201510307052).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: T.G. and L.W. Performed the experiments: T.G., J.H. and L.D. Contributed material/analysis tools: D.G., L.G., F.H., H.Z. and Z.A.B. Analyzed the data: T.G., L.D. and L.W. Statistical analyses: T.G. and L.D. Writing of manuscript: T.G. and L.W. Preparation of tables and figures: T.G. and L.D. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, T., Huang, J., Zhang, H. et al. Abcb1 in Pigs: Molecular cloning, tissues distribution, functional analysis, and its effect on pharmacokinetics of enrofloxacin. Sci Rep 6, 32244 (2016). https://doi.org/10.1038/srep32244
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32244
This article is cited by
-
Use of quercetin in animal feed: effects on the P-gp expression and pharmacokinetics of orally administrated enrofloxacin in chicken
Scientific Reports (2018)
-
Reproducible Molecularly Imprinted QCM Sensor for Accurate, Stable, and Sensitive Detection of Enrofloxacin Residue in Animal-Derived Foods
Food Analytical Methods (2018)
-
Using the lentiviral vector system to stably express chicken P-gp and BCRP in MDCK cells for screening the substrates and studying the interplay of both transporters
Archives of Toxicology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.