Abstract
During pregnancy a variety of immunological changes occur to accommodate the fetus. It is unknown whether these changes continue to affect humoral immunity postpartum or how quickly they resolve. IgG levels were measured to P. falciparum and P. vivax antigens in 201 postpartum and 201 controls over 12 weeks. Linear mixed-effects models assessed antibody maintenance over time and the effect of microscopically confirmed Plasmodium spp. infection on antibody levels, and whether this was different in postpartum women compared with control women. Postpartum women had reduced Plasmodium spp. antibody levels compared to controls at baseline. Over 12 weeks, mean antibody levels in postpartum women increased to levels observed in control women. Microscopically confirmed P. falciparum and P. vivax infections during follow-up were associated with an increase in species-specific antibodies with similar magnitudes of boosting observed in postpartum and control women. Antibodies specific for pregnancy-associated, VAR2CSA-expressing parasites did not rapidly decline postpartum and did not boost in response to infection in either postpartum or control women. After pregnancy, levels of malaria-specific antibodies were reduced, but recovered to levels seen in control women. There was no evidence of an impaired ability to mount a boosting response in postpartum women.
Similar content being viewed by others
Introduction
Malaria in pregnancy, caused by Plasmodium falciparum and Plasmodium vivax, is a major global public health problem. Over 125 million pregnancies are at risk of malaria annually1. Pregnant women are at increased risk of infection with negative consequences for the mother and baby including maternal anaemia, low birth weight and neonatal mortality2. The increased risk of P. falciparum and P. vivax infection during pregnancy may be partly due to immunological changes to accommodate the fetus during pregnancy3. The increased risk of P. falciparum infection can also be attributed to the ability of P. falciparum infected erythrocytes to bind and sequester in the placenta4,5, a pathology that does not occur often in P. vivax infections6. Antibody-mediated immunity against P. falciparum variants that sequester during pregnancy develops over successive pregnancies, conferring a degree of protection in future pregnancies6. Postpartum women may also be at differential risk of P. falciparum7,8,9 and P. vivax9 infection compared to non-postpartum women. This suggests that the altered risk of malaria during pregnancy may continue after delivery, however, little is known about humoral immunity to malaria in the postpartum period.
Individuals living in malaria endemic areas develop naturally acquired immunity to Plasmodium spp. infections with repeated infections10. Naturally acquired antibodies to sporozoites can protect against liver-stage infection and antibodies targeting blood-stage antigens can suppress high parasite densities and progression to symptomatic disease10. Breadth and magnitude of antibody responses are important, with responses to a repertoire of antigens associated with increased protection against disease11,12,13,14, while also acting as biomarkers of past exposure15.
Pregnant women in malaria endemic settings may possess antibodies to a broad range of antigens but are still susceptible to P. falciparum and P. vivax infection in pregnancy16. In the case of P. falciparum, this is largely because they lack antibodies to placental binding P. falciparum isolates that express VAR2CSA, a variant surface antigen expressed on the surface of the infected erythrocyte involved in placental sequestration17,18. As VAR2CSA is primarily encountered by the immune system during pregnancy, VAR2CSA antibodies are typically absent or at low levels prior to first pregnancy and increase with exposure through multiple pregnancies19. Antibody responses to other blood-stage antigens may also boost upon exposure in pregnancy20. There is currently a lack of consensus as to how antibody responses, particularly VAR2CSA antibodies, are maintained or decline postpartum. Previous studies have reported increases21,22, no change23,24 and decreases22,23,24,25,26 in P. falciparum antibody levels postpartum compared to pregnancy depending on study site and antigen. However, no study included a control group of non-pregnant women to enable comparisons, nor had available data on the presence of Plasmodium infection between serological measurements21,22,23,24,25,26. Importantly, most studies assessed antibodies at one time-point postpartum21,22,23,24,26, thereby not taking into account potential fluctuations and limiting inferences about postpartum antibody persistence. Additionally, past research has only considered P. falciparum targets in African settings.
To address the paucity of data and investigate whether the postpartum period comprises a period of humoral immunity transitioning to a non-pregnant state, we determined P. falciparum and P. vivax antibody levels at multiple time points in postpartum and control women living at the Thai-Myanmar border. We sought to determine whether there are differences in boosting and maintenance of Plasmodium spp. specific antibodies in postpartum women compared with control women.
Methods
Ethics statement
The study was performed in accordance with the guidelines approved by The Alfred Hospital Human Research and Ethics Committee, Melbourne, Australia (88/13); The Faculty of Tropical Medicine Ethics Committee, Mahidol University Bangkok, Thailand (MUTM 2007-023) and Oxford Tropical Medicine Ethical Committee, Oxford University, England (Code 002-07). All participants gave informed consent prior to enrolment.
Study design and population
This study investigated 201 postpartum cases and 201 non-postpartum (and non-pregnant) controls over a 12-week period of study. Individuals with at least 3 sera samples available, or who experienced a microscopically confirmed P. falciparum infection, were selected from a larger case-control study of malaria in the postpartum period (described previously9). Briefly, pregnant women attending Shoklo Malaria Research Unit (SMRU) antenatal clinics located in North-Western Thailand from November 2007 to September 2009 were invited to participate and were asked to find a non-pregnant female of similar age and location to act as a control. During the follow-up period all women were tested for the presence of Plasmodium infection via weekly light microscopy of blood smears and completed questionnaires on behavior. Monthly blood samples (~200 μl) were collected to assess haematocrit and serology. The first measurement (baseline) was obtained at first postpartum visit (median days since delivery: 13, interquartile range: 9–16). Microscopically confirmed P. falciparum infections were treated with mefloquine and artesunate. Microscopically confirmed P. vivax infections were treated with chloroquine.
Antibody determination
We measured antibodies to eight P. falciparum antigens (merozoite (PfEBA140RIII-V, PfEBA175RII, PfEBA175RIII-V, PfAMA-1, PfMSP2, PfRh2); P. falciparum infected erythrocyte: PfDBLα; sporozoite (PfCSP), all 3D7 alleles) and four P. vivax antigens (merozoite (PvAMA-1 (Palo Alto), PvMSP119 (SalI), PvDBP (SalI)); sporozoite (PvCSP (Belem and PNG)) and one VAR2CSA expressing P. falciparum parasite strain (CS2)27 (Supplementary Table 1). These antibodies represent different lifecycle stages and are biomarkers of exposure and protective immunity28,29.
1462 sera samples from 402 women were assayed by high-throughput ELISA and flow cytometry. Nine seroreactive pools from Thailand and Papua New Guinea individuals were included as positive controls for standardisation and 37 non-exposed Melbourne donors acted as negative controls. ELISA was conducted as described previously20. The locations of samples on plates were randomised across the entire cohort and for each assay all samples were processed on the same day to minimise batch effects. Reactivity from the nine positive controls (in quadruplicate) was used to adjust for any inter-plate batch variability. Antigen and sera concentration are provided in Supplementary Table 1. Testing for IgG binding to the surfaces of CS2 infected erythrocytes was conducted as described previously30. Data were acquired by flow cytometry (FACSVerse, BD Biosciences) and analysed using FlowJo (FlowJo LLC, OR, USA). Assay output was derived by subtracting the mean fluorescence intensity (MFI) of uninfected erythrocytes from the MFI of trophozoite-infected erythrocytes. Seropositivity was defined as an OD or MFI exceeding the mean plus three standard deviations of negative controls.
Statistical analysis
Statistical analyses were performed using Stata Version 13.1 (StataCorp, College Station, TX, USA). Correlations of antibodies at baseline were assessed using Spearman’s rank correlation coefficients. Antibody levels were analysed as log transformed continuous variables centred on the median value (log2([OD or MFI] + 0.001) − median(log transformed value)). Overall P. falciparum and P. vivax merozoite immunity scores were derived by calculating the average of the log transformed (centred) values for each antibody targeting species-specific merozoite antigens. To investigate if postpartum status was associated with species-specific antibody responses, linear mixed-effects modeling (with random intercept and slope) of log2 antibody levels versus time was performed with interaction terms between postpartum status and time (weeks). This enabled us to compare the slope of antibodies over time in postpartum women with control women.
Potential confounders, selected a priori using a causal diagram30, included clinic attended (Mawker Thai/Wang Pha/Walley/Mu Ler Chai), age (years) and history of working outdoors. In the case of PfVAR2CSA expressing parasites, a binary variable of gravidity ≥3 (note, gravidity of 0, 1 and 2 had similar PfVAR2CSA antibody levels) was included as a confounder instead of age, as gravidity is more predictive than age for placenta-adherent parasite immunity, and age and gravidity were highly collinear. In a subgroup analysis of postpartum women, detectable (by light microscopy) species-specific infection during pregnancy (yes/no) was included as a covariate. To assess the impact of changing haematocrit on antibodies over time, an interaction term between time and haematocrit change (≥4%) was assessed. To assess whether antibodies were boosted in response to infection, species-specific infection during follow-up was included as a time-varying variable with presence of infection (yes or no) at any visit prior to the corresponding antibody level measurement. The effect of heterologous species infection was assessed for each antibody response but only incorporated into the final model if p < 0.05. Given that we have reported all statistical comparisons conducted, p values were presented unadjusted for multiple comparisons31. Associations were interpreted based on the magnitude and direction of effect in addition to confidence intervals and p values.
Results
Characteristics of postpartum and control women
This study comprised 201 postpartum women and 201 controls living in North West Thailand (Table 1). Bednet use was high in both postpartum and control women (>93%); postpartum women were less likely to report a recent history of working outdoors than control women (60% versus 72%). During follow-up, 22 postpartum and 32 control women experienced a microscopically confirmed P. falciparum infection; 67 postpartum and 50 control women experienced a microscopically confirmed P. vivax infection. One postpartum woman and four control women experienced a co-infection.
P. falciparum and P. vivax antibodies in postpartum and control women at baseline
At baseline, seroprevalence and levels of antibodies against P. falciparum and P. vivax antigens were reduced in postpartum compared with control women, with varying degrees of magnitude depending on antigen (Fig. 1). Median (interquartile range) seroprevalence of the antibodies investigated was 15% (9%, 25%) lower in control women than postpartum women. Antibody levels against all Plasmodium spp. targets investigated were positively correlated with each other similarly in both postpartum and control women (median (IQR) pairwise correlations 0.40 (0.29, 0.48) and 0.42 (0.33, 0.53) respectively). In all women, antibody responses specific for P. falciparum merozoite antigens were more strongly correlated with each other (median: 0.65 (IQR: 0.60, 0.71)) than other P. falciparum antigens (PfCSP, PfDBLα and PfVAR2CSA). The homologous antigen pairs PfCSP/PvCSP (correlation of 0.67) and PfAMA1/PvAMA1 (correlation of 0.44) displayed the strongest levels of across-species association.
Antibody levels were determined in all available postpartum women (n = 201) and control women (n = 201) (A) Seroprevalence against P. falciparum and P. vivax amongst postpartum (black circles) and control women (grey triangles). Bars indicate 95% confidence intervals. (B) Box and whiskers plots of IgG levels (log2(MFI) for PfVAR2CSA, log2(OD) for all other antibodies) against P. falciparum and P. vivax antigens amongst postpartum (black) and control (grey) women. Horizontal lines in box indicates median, box indicates the interquartile range, whiskers indicate the highest and lowest values within 1.5*interquartile range of the first and third quartiles, dots represent outliers. A single asterix denotes p < 0.05, a double asterix denotes p < 0.01 from Wilcoxon rank-sum and chi square tests.
Antibody dynamics in postpartum and control women
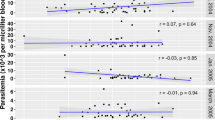
Availability of multiple serological measurements for each woman allowed us to assess postpartum antibody dynamics and relate these findings to control women. Due to the large number of merozoite responses determined and the considerable collinearity between merozoite responses, an average P. falciparum merozoite response and P. vivax merozoite response were generated for ease of interpretation. In the absence of an infection, P. falciparum and P. vivax antibody levels appeared relatively stable in postpartum and control women during the 12-week follow-up period (Supplementary Figures 1 and 2). Residual variances were significantly lower in those who did not experience species-specific infection than in those who did experience species-specific infection (likelihood-ratio test, all p < 0.001, except PfVAR2CSA p = 0.05). Many postpartum and control women who were PfVAR2CSA seropositive at baseline maintained their responses over the course of the study (Fig. 2). PfVAR2CSA seropositive women with a documented infection during pregnancy had a significant negative slope of antibodies over time (−0.06 (95% Confidence Interval: −0.11, −0.02; p = 0.01) while in the other PfVAR2CSA seropositive women there was no significant decline in antibodies (p = 0.50).
Levels of VAR2CSA antibodies (log2(MFI)) over time in (A) postpartum women with a P. falciparum infection detected post-delivery, (B) control women with a P. falciparum infection detected, (C) postpartum women without any P. falciparum infection detected post-delivery and (D) control women without any P. falciparum infection. Each individual woman’s VAR2CSA antibodies over time are represented by a series of connected dots.
In order to assess differences in antibody responses at baseline and over time in postpartum and control women, multivariable linear mixed-effects models were constructed for each antibody response (Table 2; Supplementary Tables 2 and 3). Age and a history of working outdoors, both indicators of past exposure, were associated with increased antibody levels to P. falciparum and P. vivax targets. Adjusted mean levels of antibodies to PfVAR2CSA expressing parasites were 0.29 (95% CI 0.15–0.42) higher in women with at least three prior pregnancies compared to those with two or fewer prior pregnancies (Table 2, p < 0.001). At baseline, postpartum women had lower mean levels of P. falciparum and P. vivax antibodies targeting merozoites, sporozoites and infected erythrocytes compared to control women (adjusted mean difference 0.16 to 0.32 (Table 2)).
Mean antibody levels did not change significantly over time in control women (Table 2, all p > 0.14). In contrast, the mean level of antibodies in postpartum women increased during the follow-up period; with a 0.01–0.03 mean increase per week follow-up (Table 2). As such, over the course of the 12 weeks of follow-up the initial difference in merozoite, sporozoite and infected erythrocyte antibody levels between postpartum and control women reduced over time as mean antibody levels of postpartum and control women converged (Fig. 3). Adjusting for haematocrit in the models did not alter these observations. Similarly, in a subset analysis of postpartum women accounting for species-specific infections during pregnancy (which were associated with increased baseline antibody levels), mean postpartum antibody levels increased with time (p = 0.10 for Pf merozoite immunity, p < 0.05 for all others) (Supplementary Table 4).
Mean predicted values (line) and 95% confidence intervals (shading) of antibody reactivity to (A) Pf merozoite, (B) PfVAR2CSA, (C) PfCSP, (D) Pv merozoite (log2(MFI) for PfVAR2CSA and log2(OD) for all others) are plotted for postpartum (red) and non-pregnant control (blue) women. There was strong evidence for an interaction between postpartum and time for PfCSP antibodies (likelihood-ratio test p value for interaction between postpartum and time) (PfCSP p < 0.001), moderate evidence for Pf merozoite immunity (p = 0.09), weak evidence for Pv merozoite immunity (p = 0.25) and PfVAR2CSA (p = 0.19).
The effect of species-specific infection on P. falciparum and P. vivax antibody levels in postpartum and control women
To investigate the effect of species-specific infection on antibody levels, and whether postpartum status modified this effect, the previous models were expanded to incorporate species-specific infection, detected by light microscopy, during follow-up as a time-varying variable (Table 3 and Supplementary Table 5). There was strong evidence of boosting with species-specific infection in antibody responses to Pf merozoites, Pv merozoites, PfCSP, PvCSP, and PfDBLα (mean increase 0.85 (95% CI: 0.57, 1.13), 0.35 (0.19, 0.50), 0.36 (0.18, 0.54), 0.18 (0.04, 0.31), 0.18 (0.01, 0.35) respectively; all p < 0.04). In contrast, antibodies targeting PfVAR2CSA-expressing parasites did not show evidence of boosting in response to P. falciparum infection in postpartum and control women (Table 3, p = 0.89). The magnitude of boosting of antibody responses to P. falciparum and P. vivax antigens in response to a species-specific infection was similar in postpartum and control women (Table 3, p > 0.08 for interaction terms). Antibodies to P. falciparum merozoite antigens showed the greatest change with infection, with mean levels 0.85 (95% CI: 0.57, 1.13) higher after a P. falciparum infection than no infection in all women (p < 0.001).
To investigate the impact of heterologous species-infection on antibody levels, models were examined incorporating heterologous-species infection during follow-up as a time-varying variable. Infections with one species did not boost antibody responses against blood-stage antigens (Table 3, p > 0.40). However, antibody responses to the circumsporozoite proteins, PfCSP and PvCSP, demonstrated apparent boosting in response to heterologous infection (Table 3, p < 0.02).
Discussion
This is the first study comparing humoral immunity to malaria over time between postpartum women and control women. Antibody responses were relatively stable in the absence of infection but more variable in those who experienced microscopically detectable infections during follow-up. Postpartum women had reduced antibody levels to P. falciparum and P. vivax antigens after delivery when compared with control women, however, postpartum levels recovered to control levels after 12 weeks. The majority of antibodies were boosted in response to species-specific infection, but there was no evidence of any boosting of antibodies against VAR2CSA-expressing parasites in the postpartum period. There were no detectable differences in the magnitude of boosting in response to infection in postpartum and control women.
The observation that antibody levels to Plasmodium spp. antigens increased in postpartum women over 12 weeks of follow-up suggests that humoral immunity is transitioning back to normal levels after pregnancy. This concurs with two studies in Africa that observed increased antibody levels at a single time-point (1 month and 6 weeks) postpartum compared to pregnancy21,32. Levels of circulating antibodies against merozoites and infected erythrocytes have declined during pregnancy in other studies in Africa and the Pacific25,33,34; the increase observed postpartum is likely reflective of a return to pre-pregnancy levels. Antibodies are preferentially transported to the fetus during pregnancy35, so post-pregnancy antibody levels may then increase to pre-pregnancy homeostatic levels. The haemodilution that occurs during pregnancy is followed by a period of haemoconcentration after delivery36; this change would be expected to result in increased antibody concentrations. Haematocrit levels in this cohort of postpartum women increased over 12 weeks postpartum9, however postpartum antibody levels increased even when adjusting for increasing haemconcentration suggesting that other factors play a role in changing antibody levels, such as the cessation of maternofetal antibody transfer.
Antibody responses specific to P. falciparum and P. vivax blood-stage antigens increased with homologous species-specific infections in concordance with numerous studies including a study in pregnant women from the same population20. We found no strong evidence of species-transcending boosting of immunity against blood-stage antigens. Antibodies against PfCSP and PvCSP appeared to boost in response to both species of infection, suggesting that some of the humoral response directed against CSP demonstrates species-transcending recognition, a finding that concurs with evidence from animal models37. We found no evidence of differences in the boosting of blood-stage or sporozoite antibodies in response to infection in postpartum women compared with control women indicating that the postpartum humoral immune system responds to Plasmodium spp. infection similarly to control women.
Less than 10% of women were seropositive at baseline for antibodies against VAR2CSA expressing parasites, reflecting the low endemicity of P. falciparum in this population; and the frequent monitoring and early treatment of malaria during pregnancy by the SMRU. Consistent with the literature19, antibodies against VAR2CSA were higher in women who had experienced more pregnancies. Whether VAR2CSA-specific antibody responses are maintained at adequate levels outside of pregnancy in the relative absence of VAR2CSA expressing parasites has been the matter of some uncertainty. It has also been proposed that antibodies to VAR2CSA may rapidly decay postpartum38. However, we found that antibodies to VAR2CSA only decayed rapidly amongst those recently exposed in pregnancy, while in other individuals VAR2CSA antibodies were relatively stable. This agrees with observed biphasic decay in antibody responses post antigen exposure39 and recent research investigating VAR2CSA-specific B cell memory which found that memory can be maintained for many years in the absence of antigen exposure40. Importantly, our study found no evidence of any boosting of antibodies against VAR2CSA-expressing parasites with infections in postpartum or control women, which would seem to support the paradigm that exposure to VAR2CSA is minimal outside of pregnancy. Our data supports the notion that antibody responses against VAR2CSA acquired in earlier pregnancies can be maintained through to future pregnancies.
Strengths of this study included the recruitment of a control group, which enabled direct comparisons between postpartum antibody responses with other women; and the weekly sampling for Plasmodium infection by light microscopy. However, it is probable that some women had submicroscopic infections41; which may have accounted for the few fluctuations observed in uninfected women. Submicroscopic carriage of parasites may also assist in the maintenance of antibody levels and differences in prevalence of submicroscopic infections between postpartum and control women may exist, though this could not be assessed in the present study. The frequent monitoring and prompt treatment of women in this study is a higher standard of treatment than many women would receive elsewhere; it is possible that untreated infections would result in a greater magnitude of antibody boosting. Additionally, this study took place in an area of low P. vivax and P. falciparum transmission, so findings may not be generalisable to areas of higher endemicity. Antibodies in this study were measured via ELISA and flow cytometry, and as yet there are no agreed upon standard or defined threshold for protection, so the clinical relevance of the reduced levels of antibodies postpartum is yet to be determined. A transient reduction in malaria-specific antibody levels would be expected to increase susceptibility to clinical malaria in some women, but as the correlates of antibody-mediated protection remain poorly defined42, further research is needed to assess the clinical implications of the reduction observed.
In conclusion, we observed that postpartum women had lower levels of antibodies to a variety of malaria antigens after delivery but were in the process of recovering to control levels. Despite slightly lower antibody levels, postpartum women showed no signs of impaired boosting in response to infection. Evidence from population studies in areas of higher endemicity suggests a threshold of humoral immunity is required for protective immunity42, a reduction in circulating levels of antibodies in postpartum women may render previously protected women susceptible in other study settings. Further studies are required to examine the immune response postpartum in different transmission settings and to determine the impact of transitioning immune responses and risk of malaria postpartum.
Additional Information
How to cite this article: McLean, A. R. D. et al. Antibody responses to Plasmodium falciparum and Plasmodium vivax blood-stage and sporozoite antigens in the postpartum period. Sci. Rep. 6, 32159; doi: 10.1038/srep32159 (2016).
References
Dellicour, S., Guerra, C. A., Kuile, F. O. t., Snow, R. W. & Tatem, A. J. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. Plos Med. 7, e1000221 (2010).
Desai, M. et al. Review: Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases 7, 93–104, doi: 10.1016/s1473-3099(07)70021-x (2007).
Jamieson, D. J., Theiler, R. N. & Rasmussen, S. A. Emerging infections and pregnancy. Emerg. Infect. Dis. 12, 1638–1643, doi: 10.3201/eid1211.060152 (2006).
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395, 851–852, doi: 10.1038/27570 (1998).
Salanti, A. et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200, 1197–1204 (2004).
Hviid, L. The immuno-epidemiology of pregnancy-associated Plasmodium falciparum malaria: a variant surface antigen-specific perspective. Parasite Immunol. 26, 477–486, doi: 10.1111/j.0141-9838.2004.00733.x (2004).
Ramharter, M. et al. Clinical and parasitological characteristics of puerperal malaria. J. Infect. Dis. 191, 1005–1009, doi: 10.1086/427781 (2005).
Diagne, N. et al. Increased susceptibility to malaria during the early postpartum period. N. Engl. J. Med. 343, 598–603, doi: 10.1056/nejm200008313430901 (2000).
Boel, M. E. et al. Malaria in the post-partum period; a prospective cohort study. Plos One 8, e57890, doi: 10.1371/journal.pone.0057890 (2013).
Doolan, D. L., Dobano, C. & Baird, J. K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36, Table of Contents, doi: 10.1128/cmr.00025-08 (2009).
Osier, F. H. A. et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 76, 2240–2248 (2008).
Richards, J. S. et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 51, e50–60, doi: 10.1086/656413 (2010).
Rono, J. et al. Breadth of anti-merozoite antibody responses is associated with the genetic diversity of asymptomatic Plasmodium falciparum infections and protection against clinical malaria. Clin. Infect. Dis. 57, 1409–1416, doi: 10.1093/cid/cit556 (2013).
Richards, J. S. et al. Identification and Prioritization of Merozoite Antigens as Targets of Protective Human Immunity to Plasmodium falciparum Malaria for Vaccine and Biomarker Development. J. Immunol. 191, 795–809, doi: 10.4049/jimmunol.1300778 (2013).
Elliott, S. R. et al. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep 6, 100, doi: 10.12703/p6-100 (2014).
McLean, A. R., Ataide, R., Simpson, J. A., Beeson, J. G. & Fowkes, F. J. Malaria and immunity during pregnancy and postpartum: a tale of two species. Parasitology, 1–17, doi: 10.1017/s0031182015000074 (2015).
Fried, M. & Duffy, P. E. Adherence of Plasmodium falciparum to Chondroitin Sulfate A in the Human Placenta. Science 272, 1502–1504 (1996).
Salanti, A. et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49, 179–191 (2003).
Ataide, R., Mayor, A. & Rogerson, S. J. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol. 30, 85–94, doi: 10.1016/j.pt.2013.12.007 (2013).
Fowkes, F. J. et al. New insights into acquisition, boosting and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206, 1612–1621, doi: 10.1093/infdis/jis566 (2012).
Mayor, A. et al. Immunoglobulins against the surface of Plasmodium falciparum-infected erythrocytes increase one month after delivery. Malar. J. 11, 130, doi: 10.1186/1475-2875-11-130 (2012).
Fievet, N. et al. Immune response to Plasmodium falciparum antigens in Cameroonian primigravidae: evolution after delivery and during second pregnancy. Clin. Exp. Immunol. 107, 462–467 (1997).
Ampomah, P., Stevenson, L., Ofori, M. F., Barfod, L. & Hviid, L. Kinetics of B Cell Responses to Plasmodium falciparum Erythrocyte Membrane Protein 1 in Ghanaian Women Naturally Exposed to Malaria Parasites. J. Immunol. 192, 5236–5244, doi: 10.4049/jimmunol.1400325 (2014).
Cox, S. E. et al. Rapid acquisition of isolate-specific antibodies to chondroitin sulfate A-adherent plasmodium falciparum isolates in Ghanaian primigravidae. Infect. Immun. 73, 2841–2847 (2005).
Aitken, E. H. et al. Antibodies to Chondroitin Sulfate A-Binding Infected Erythrocytes: Dynamics and Protection during Pregnancy in Women Receiving Intermittent Preventive Treatment. J. Infect. Dis. 201, 1316–1325, doi: 10.1086/651578 (2010).
Staalsoe, T. et al. Acquisition and Decay of Antibodies to Pregnancy-Associated Variant Antigens on the Surface of Plasmodium falciparum-lnfected Erythrocytes That Protect against Placental Parasitemia. The Journal of Infectious Diseases 184, 618–626 (2001).
Rogerson, S. J., Chaiyaroj, S. C., Ng, K., Reeder, J. C. & Brown, G. V. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. The Journal Of Experimental Medicine 182, 15–20 (1995).
Cutts, J. C. et al. Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis. BMC Med. 12, 150, doi: 10.1186/preaccept-1041462276129106 (2014).
Fowkes, F. J. I., Richards, J. S., Simpson, J. A. & Beeson, J. G. The Relationship between Anti-merozoite Antibodies and Incidence of Plasmodium falciparum Malaria: A Systematic Review and Meta-analysis. Plos Med. 7, 1–20, doi: 10.1371/journal.pmed.1000218 (2010).
Hommel, M. et al. Evaluation of the Antigenic Diversity of Placenta-Binding Plasmodium falciparum Variants and the Antibody Repertoire among Pregnant Women. Infect. Immun. 78, 1963–1978 (2010).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Cox, S. E. et al. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Trop. Med. Int. Health 10, 1286–1297, doi: 10.1111/j.1365-3156.2005.01515.x (2005).
Teo, A. et al. Malaria preventive therapy in pregnancy and its potential impact on immunity to malaria in an area of declining transmission. Malar. J. 14, 215, doi: 10.1186/s12936-015-0736-x (2015).
Campbell, C. C., Martinez, J. M. & Collins, W. E. Seroepidemiological studies of malaria in pregnant women and newborns from coastal El Salvador. Am. J. Trop. Med. Hyg. 29, 151–157 (1980).
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A. & Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646, doi: 10.1155/2012/985646 (2012).
Pritchard, J. A. Changes in the blood volume during pregnancy and delivery. Anesthesiology 26, 393–399 (1965).
Yadava, A., Nurmukhambetova, S., Pichugin, A. V. & Lumsden, J. M. Cross-species immunity following immunization with a circumsporozoite protein-based vaccine for malaria. J. Infect. Dis. 205, 1456–1463, doi: 10.1093/infdis/jis220 (2012).
Hviid, L., Barfod, L. & Fowkes, F. J. Trying to remember: immunological B cell memory to malaria. Trends Parasitol., doi: 10.1016/j.pt.2014.12.009 (2015).
White, M. T. et al. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J. Infect. Dis. 210, 1115–1122, doi: 10.1093/infdis/jiu219 (2014).
Ampomah, P., Stevenson, L., Ofori, M. F., Barfod, L. & Hviid, L. B-Cell Responses to Pregnancy-Restricted and -Unrestricted Plasmodium falciparum Erythrocyte Membrane Protein 1 Antigens in Ghanaian Women Naturally Exposed to Malaria Parasites. Infect. Immun. 82, 1860–1871, doi: 10.1128/iai.01514-13 (2014).
Imwong, M. et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar. J. 14, 381, doi: 10.1186/s12936-015-0906-x (2015).
Stanisic, D. I. et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children: influence of age, force of infection, and magnitude of response. Infect. Immun., doi: 10.1128/iai.02398-14 (2014).
Acknowledgements
We wish to thank Rosanna Powell, Gaoqian Feng, Andrew Guy, Xi Zen Yap, Vashti Irani and Kerryn Moore for technical assistance; The Australian Red Cross Blood service for providing whole blood samples; Robin Anders, Christine Langer, Annie Mo, David Narum and Joseph Smith for provision of proteins; the SMRU staff for their contributions; all the study participants for their participation. This work was supported by the National Health and Medical Research Council of Australia (project grant (#1049213) to FF; training award to FF; Infrastructure for Research Institutes Support Scheme Grant; Senior Research Fellowship to JS and JB); Australian Research Council (Future Fellowship to FF); Victorian State Government Operational Infrastructure Support (grant to the Burnet Institute); The Christophe and Rodolphe Mérieux Foundation (prize (2008) to FN); Australian Government (Australian Postgraduate Award to AM); SMRU is part of the Mahidol Oxford University Tropical Medicine Research Unit supported by the Wellcome Trust of Great Britain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.R.D.M., R.A., D.D., T.T., J.G.B. and F.J.I.F. developed and implemented methods for sample processing, antigen production and antibody determination. A.R.D.M., J.A.S. and F.J.I.F. carried out statistical analysis. R.M., M.E.B. and F.N. carried out clinical studies to obtain samples for antibody determination. All authors interpreted data, contributed to the writing of, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
McLean, A., Boel, M., McGready, R. et al. Antibody responses to Plasmodium falciparum and Plasmodium vivax blood-stage and sporozoite antigens in the postpartum period. Sci Rep 6, 32159 (2016). https://doi.org/10.1038/srep32159
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32159
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.