Abstract
Klebsiella pneumoniae can cause community-acquired pyogenic liver abscess (PLA). Capsular polysaccharide (CPS) is important for its virulence. Among 79 capsular (K) types discovered thus far, K57 is often associated with PLA. Here, we report the identification of a K57 variant. Cps gene locus sequencing revealed differences between the K57 reference strain 4425/51 (Ref-K57) and a variant, the PLA isolate A1142. While Ref-K57 cps contained orf13 encoding a putative acetyltransferase, the insertion of a putative transposase-encoding gene at this position was detected in A1142. This variation was detected in other K57 clinical strains. Biochemical analyses indicated that A1142 was deficient in CPS acetylation. Genetic replacement and complementation verified that orf13 was responsible for CPS acetylation. Acetylation increased CPS immunoreactivity to antiserum and enhanced K. pneumoniae induction of pro-inflammatory cytokines through JNK and MAPK signaling. While acetylation diminished the serum resistance of bacteria, it promoted adhesion to intestinal epithelial cells possibly via increasing production of type I fimbriae. In conclusion, acetylation-mediated capsular variation in K57 was observed. Capsular acetylation contributed to the variety and antigenic diversity of CPS, influenced its biological activities and was involved in K. pneumoniae-host interactions. These findings have implications for vaccine design and pathogenicity of K. pneumoniae.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae is an important human pathogen in hospital-acquired and community-acquired infections1,2,3,4. This organism causes nosocomial infections, such as septicemia, pneumonia, urinary tract infections (UTIs), surgical site infections and catheter-related infections. K. pneumoniae also causes community-acquired infections, such as pyogenic liver abscess (PLA) complicated by meningitis and endophthalmitis, UTIs and pneumonia. Over the last 20 years, community-acquired K. pneumoniae PLA has become an emerging infectious disease worldwide, especially in East Asian countries5,6,7,8. This new type of invasive disease is often complicated by metastatic infections, such as meningitis and endophthalmitis. Furthermore, diabetes mellitus, a predisposing factor, has been detected in about 50% of patients with PLA4,9,10.
One of important virulence factors of K. pneumoniae is the capsule, an extracellular polysaccharide structure that protects bacteria from lethal serum factors and phagocytosis. At least 79 capsular types have been defined in Klebsiella, each representing a distinct chemical structure of the capsular polysaccharide (CPS; the K antigen). The capsular types have been related to K. pneumoniae infection and disease severity11,12. K. pneumoniae strains with the K1 and K2 capsular types are identified as the predominant virulent types and also are strongly associated with strains causing PLA8,13,14,15. In addition to K1 and K2, other K types are also implicated in PLA. Our previous studies of 42 K. pneumoniae strains causing PLA identified those with K1 (n = 35), K2 (n = 2), K57 (n = 2), K5 (n = 1) and K54 (n = 1) capsules, as well as a new type (n = 1)14. Similarly, the prevalence of 50 liver abscess isolates in Southern Taiwan revealed capsular types K1, K2, K5, K20, K54 and K57, in addition to an unidentified type16. The chromosomal capsular polysaccharide synthesis (cps) gene cluster encodes the majority of the proteins involved in the translocation and assembly of surface polysaccharides, composed of repeated sugar subunits17, for the K. pneumoniae capsule. Genotyping of cps can be used to distinguish capsular types18,19. Information about disease-related capsular types of bacterial pathogens can contribute to diagnosis and to the development of capsule-based vaccines.
To understand pathogen-host interactions and host responses, characterization of the structures and biological activities of various capsular architectures is important. Polysaccharide modifications have been described to cause capsular variation in what were originally defined as singular capsular types in some pathogens, such as Streptococcus pneumoniae20 and Escherichia coli K1 strains21. Capsular modifications also may be associated with the virulence of some bacterial strains21,22. Although modifications of CPS by O-acetyl and O-pyruvyl groups have been reported in a K. pneumoniae K1 PLA strain23, analysis of potential capsular variation and related modifications in Klebsiella is incomplete. In addition, the roles of capsular modifications in K. pneumoniae remain to be elucidated. In addition, direct links between the structural, biochemical and genetic data for some capsular types are still lacking.
K. pneumoniae K57 is one of the PLA-associated capsular types. In this study, we discovered the presence of a capsular variant in the K57 capsular type, which was based on genetic data of the cps region and biochemical analysis of CPS modification. Our group previously published the complete sequence of the K57 cps cluster of the PLA isolate, A114214. Sequencing of the cps cluster of another strain, the K57 reference strain (Ref-K57), revealed differences between the two strains at the site of a cps gene (orf13) encoding a putative acetyltransferase. Variations of this gene were also detected in other K57 clinical strains. By gene replacement and complementation analyses, we verified that orf13 is responsible for CPS acetylation, which altered K. pneumoniae K57 antigenicity, innate host response, serum resistance and cell adhesion.
Results
Identification of differences in cps gene loci in K. pneumoniae K57 strains
Our previous study focused on the cps regions of K. pneumoniae K57 strain, which is related to PLA14. Thus, we sequenced and analyzed the cps gene cluster of the K57 reference strain (Ref-K57) from the Statens Serum Institute. We compared the sequence of the Ref-K57 cps with that published for the PLA isolate, A1142, another K57 strain (Table 1). We noted an obvious difference in the region between orf12 and wbaZ (Fig. 1A). Specifically, the Ref-K57 sequence included a 981-bp orf13 (DNA residues 15948–16928) in this position; the predicted orf13 gene product exhibited 38% amino acid identity (75/196) with the acyltransferase superfamily of proteins (WP_014751172). In contrast, the corresponding gene in A1142 apparently was disrupted by the insertion of a gene encoding a putative transposase; the nominal orf13 of A1142, thus, was split into two fragments (residues 15933–16223 and 17367–17978). This difference revealed that Ref-K57 and A1142 harbored distinct orf13 in the cps gene loci.
Variation of orf13 in K57 capsular polysaccharide synthesis (cps) regions.
(A) Comparison of the cps between the K57 reference strain (Ref-K57) and A1142. Open reading frames (ORFs) are shown as arrows. Ref-K57 (upper) contains a 981-bp orf13 (green, residue 15948–16928) encoding a putative acyltransferase family protein. In the PLA isolate A1142 (lower), this gene is interrupted by a gene encoding a putative transposase (red, residue 16519–17193). The blue arrows indicate the positions of the orf13 PCR primers (K57-orf13-F and K57-orf13-R) used for panel B analysis (see below). (B) PCR analysis of orf13 in a total of 23 K. pneumoniae K57 strains. No. 1: strain Ref-K57; No. 2: strain A1142; No. 3–23: other collected K57 clinical strains. The arrows indicate two forms of orf13. M: DNA marker.
We next examined other K. pneumoniae K57 clinical isolates for the presence of similar orf13 variations. PCR analysis of a total of 23 distinct K57 strains revealed that orf13 was present in either of two conformations in these strains (Fig. 1B). Specifically, 15 strains (65.2%) harbored orf13 sequences of a length close to that of Ref-K57 (981 bp). The other eight strains (34.8%) carried orf13 sequences of a length similar to that of A1142 (2,046 bp). These results indicated that cps variation in gene orf13 was present in a range of different K57 clinical strains.
Ref-K57 orf13 is responsible for CPS acetylation and A1142 generates a K57 variant deficient in CPS acetylation
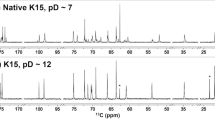
The potential acetylation of K57 CPS isolated from Ref-K57 and A1142 was initially determined and compared. 1H NMR analysis showed that CPS extracted from Ref-K57 harbored an acetyl group in the region near 2.0 1H ppm (Fig. 2A, upper panel); this modification was not detected in CPS isolated from A1142 (Fig. 2A, lower panel). Using the Hestrin method to determine the degree of CPS acetylation24,25, Ref-K57 CPS acetylation levels were higher than those observed for A1142 CPS (Fig. 2B). The main sugar compositions of the CPS polymers from Ref-K57 and A1142 were also compared; no obvious differences were found. According to the published structure of K57 CPS26, the ratio of mannose (Man) and galactose (Gal) was predicted to be 2:1. The Man:Gal ratio in both Ref-K57 CPS and A1142 CPS were nearly 2:1. Thus, the CPS polymers of Ref-K57 and A1142 differed in their acetyl modification rather than in their major sugar composition.
Comparison of CPS acetylation in K57 strains.
(A) 1H NMR spectra of extracted CPS in Ref-K57 (upper) and A1142 (lower). To avoid the effects of sugars from lipopolysaccharide (LPS), O-antigen-lacking ΔwecA mutants were analyzed (see methods). The arrows indicate acetylation in Ref-K57. (B) Quantification of CPS acetylation. Acetylation levels of extracted CPS from Ref-K57, Ref-K57::orf13::A1142orf13-tnp, A1142 and A1142::Ref-K57orf13 were determined by measuring the absorbance at 504 nm and normalized to 50 μg of CPS for each sample. Data are mean ± SEM from three independent experiments. *P < 0.05, Student’s t test. (C) The upper diagram shows the genetic replacement of orf13 in the Ref-K57 cps locus (strain Ref-K57Δorf13::A1142orf13-tnp). Ref-K57 orf13 was replaced by the corresponding gene from A1142, in which orf13 was interrupted by a gene encoding a putative transposase (A1142orf13-tnp). The lower diagram shows genetic complementation of orf13 in the A1142 cps locus (strain A1142::Ref-K57orf13). For this construct, the orf13 of Ref-K57 was inserted into the intergenic region between wbaZ and gnd of the A1142 cps locus.
To verify the function of the orf13 gene, we constructed gene replacement and complementation K57 strains (Fig. 2C). Specifically, we replaced the orf13 coding region (residues 15948–16928) of Ref-K57 with the transposase-interrupted orf13 from A1142 (residues 15933–17975), yielding an isogenic mutant strain that we designated Ref-K57Δorf13::A1142orf13-tnp (the “replacement” strain). We generated chromosomal complementation of orf13 by insertion of the intact Ref-K57 orf13 gene into the intergenic region between wbaZ and gnd in the A1142 cps cluster, yielding a strain that we designated A1142::Ref-K57orf13 (the “complementation” strain). This gene replacement or complementation did not cause significant changes in CPS production compared to that in the respective parent strain, as determined by quantification of uronic acid in K57 CPS (Fig. 3A). Ref-K57 generated less CPS than A1142. The replacement strain Ref-K57Δorf13::A1142orf13-tnp produced an amount of CPS close to that of Ref-K57 and similarly, no significant differences of CPS production were found between A1142 and A1142::Ref-K57orf13. In addition, India ink capsule staining showed no obvious differences in capsule thickness between the gene replacement or complementation strains and the respective parent strains (Fig. 3B). By microscopic observation, Ref-K57Δorf13::A1142orf13-tnp looked similar to Ref-K57 and both the A1142 parent and A1142::Ref-K57orf13 strains had thick capsules surrounding the bacterial cells.
CPS production and capsule staining of K. pneumoniae K57 strains.
(A) Quantification of K57 CPS production. The levels of extracted CPS from the equivalent amounts of overnight cultures of Ref-K57, Ref-K57Δorf13::A1142orf13-tnp, A1142 and A1142::Ref-K57orf13 strains were detected by measuring the absorbance at 520 nm. Data are presented as mean ± SEM from three independent experiments. Ref-K57 vs. Ref-K57Δorf13::A1142orf13-tnp, P = 0.8; A1142 vs. A1142::Ref-K57orf13, P = 0.45; Ref-K57 vs. A1142, P = 0.0079 (Student’s t test). (B) Capsule staining of K. pneumoniae. A drop of log-phase K. pneumoniae (as indicated) was stained with India ink and observed using a light microscope. Scale bar is 100 nm.
Analysis of CPS acetylation levels (Fig. 2B) revealed that compared to Ref-K57, CPS acetylation of Ref-K57Δorf13::A1142orf13-tnp (the replacement strain) was decreased. On the other hand, A1142::Ref-K57orf13 (the complementation strain) exhibited increased CPS acetylation compared to the A1142 parent. These results indicated that orf13 in K57 was responsible for CPS acetylation and was inactivated by disruption of the gene in A1142.
Acetylation enhances the immunoreactivity of K57 CPS
The surface carbohydrate comprising the K. pneumoniae capsule typically exhibits strong antigenicity. We, therefore, investigated whether acetylation affected the immunoreactivity of K57 CPS. Immunoblot analysis of equivalent amounts of CPS from each strain was undertaken using commercial anti-K57 serum, which was generated using the Ref-K57 strain by the Statens Serum Institute. As shown in Fig. 4A, both Ref-K57 and A1142 CPS reacted to the anti-Ref-K57 antiserum; however, the Ref-K57 CPS had stronger reactivity. Compared to Ref-K57 CPS, Ref-K57Δorf13::A1142orf13-tnp CPS exhibited decreased immunoreactivity, suggesting that acetylation was important for Ref-K57 CPS recognition by anti-Ref-K57 antiserum. Interestingly, A1142::Ref-K57orf13 CPS exhibited stronger reactivity than parental CPS, indicating that acetylation complementation enhanced the reactivity to anti-Ref-K57 antiserum. To quantify the immunoreactivity, signals derived from the same immunoblot were analyzed by densitometry (Fig. 4B). In Ref-K57Δorf13::A1142orf13-tnp, gene replacement reduced the immunoreactivity of Ref-K57 CPS (set as 100%) to ~19%. Compared to Ref-K57 CPS, A1142 CPS exhibited lower immunoreactivity (~29%), which was increased to ~69% in A1142orf13-tnp.
Effects of acetylation on the immunoreactivity of K57 CPS.
(A) Representative immunoblot of K. pneumoniae CPS against anti-K57 antiserum. Equivalent amounts of extracted CPS (2.5 μg per sample) from K57 strains, including Ref-K57, the replacement strain (Ref-K57Δorf13::A1142orf13-tnp), A1142 and the acetylation complementation strain of A1142 (A1142::Ref-K57orf13), was analyzed against commercial rabbit anti-Ref-K57 antiserum from the Statens Serum Institute. (B) Quantification of the immunoreactivity of different K57 CPS (as indicated). Bands on the same immunoblot were densitometrically analyzed using Image J software. Relative activity was presented by comparing to Ref-K57 (set as 100%). Data are mean ± SEM from three independent experiments. *P < 0.05, Student’s t test.
CPS acetylation enhances induction of pro-inflammatory cytokines by K57 K. pneumoniae
Microbial infection often triggers the production of host pro-inflammatory cytokines. The potential impact of K57 K. pneumoniae on host innate responses was assessed. The pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are secreted primarily by activated macrophages, mediating multiple biological effects, including activation of immune responses. A1142 stimulated TNF-α and IL-6 secretion by mouse macrophage Raw 264.7 cells as detected at ~1 and ~6 h after infection (Fig. 5A,B). Ref-K57 did not efficiently induce TNF-α and IL-6 secretion, which may be due to the lower CPS levels than that of A1142. No obvious differences were found between the Ref-K57Δorf13::A1142orf13-tnp and Ref-K57 strains (Fig. 5A,B). Notably, the complementation strain, A1142::Ref-K57orf13, induced TNF-α and IL-6 secretion more efficiently than the A1142 parent strain. Compared to the A1142 parental strain, A1142::Ref-K57orf13 induced secretion of higher levels of TNF-α at 4 and 6 h post-infection (Fig. 5A). For IL-6 secretion, earlier induction by A1142::Ref-K57orf13, from <4 h post-infection, was observed (Fig. 5B). In addition, the levels of IL-6 induced by A1142::Ref-K57orf13 at 6 h and 24 h post-infection were higher than those induced at respective time points by the A1142 parental strain. These results suggested that CPS acetylation enhanced K57 K. pneumoniae induction of pro-inflammatory cytokines.
Effects of acetylation on cytokine induction by K57 K. pneumoniae.
Induction of pro-inflammatory cytokines: (A) TNF-α (B) IL-6. Mouse macrophage RAW 264.7 cells were incubated with A1142, A1142::Ref-K57orf13, Ref-K57, or Ref-K57Δorf13::A1142orf13-tnp. The levels of secreted cytokines in the medium were measured at the indicated time points. Data are presented as mean ± SEM from three independent experiments. *P < 0.05, Student’s t test. (C) Western blotting of cell signaling induced by A1142 or A1142::Ref-K57orf13. Total protein lysates of Raw246.7 cells were subjected to detection for phosphorylation status of NF-κB, JNK, p38 MAPK and ERK1/2.
To investigate underlying mechanisms, we determined the effect of CPS acetylation on the activation of NF-κB, JNK, p38 MAPK and ERK1/2 in Raw 264.7 cell lysates by Western blotting (Fig. 5C). Consistent with the cytokine secretion data, the acetylation complementation strain induced the phosphorylation of NF-κB, JNK and p38 MAPK more efficiently than the A1142 parental strain. Therefore, acetylation of CPS possibly increased K. pneumoniae-induced cytokine production due to enhanced activation of JNK and MAPK signaling pathways.
CPS acetylation decreases serum resistance of K. pneumoniae but promotes cell adhesion
In mice inoculated with K. pneumoniae A1142 or the complementation strain, A1142::Ref-K57orf13, in vivo competition assays revealed no significant differences in the virulence between the strains (Fig. 6A; results of CFU viable counts shown in Figure S1). This assay reflected in vivo virulence of test strain in mice. Each test strain (LacZ-positive; blue colonies on the plates containing IPTG and X-Gal) was inoculated with the isogenic lacZ promoter deletion strain (white colonies) at a 1:1 ratio and the numbers of bacterial colonies recovered from mice were compared. The colony numbers of the A1142 parent and A1142::Ref-K57orf13 were both similar to that of A1142ΔplacZ (Figure S1) and no significant differences between A1142 and A1142::Ref-K57orf13 in the competitive index (CI) were found (Fig. 6A). Thus, acetylation of CPS may not significantly change the in vivo virulence of A1142.
Effects of CPS acetylation on serum resistance and cell adhesion.
(A) In vivo competition of the A1142 parent strain and the acetylation complementation strain (A1142::Ref-K57orf13) was tested in mice. Each test strain was compared with the A1142ΔplacZ mutant strain and the ratio of LacZ-positive to LacZ-negative colonies in the liver or spleen of each mouse was determined. A1142ΔplacZ was fully virulent as the parental A1142. The competitive index (CI) was defined as (outputΔplacZ/outputtest strain)/(inputΔplacZ/inputtest strain). Each symbol represents the CI for each inoculum, with the medians shown by bars. A1142 and A1142::Ref-K57orf13, P = 0.31 for liver and P = 0.81 for spleen, Wilcoxon signed rank test. (B) Serum resistance assays. K. pneumoniae strains were incubated with human serum (75%) at 37 °C for 1 h. The numbers of recovered CFU were determined and the survival ratio was expressed as recovered CFU/inoculum CFU. The CPS-lacking mutant, A1142Δwzy, which is sensitive to serum killing, was analyzed for comparison. Data are presented as mean ± SEM from three independent experiments. *P < 0.05, Student’s t test. (C) Adhesion of K. pneumoniae to Caco-2 cells. The adhesion rate was expressed as the proportion of the inoculum that adhered. Data are presented as mean ± SEM from three independent experiments. *P < 0.05, Student’s t test. (D) Relative expression of the fimbriae genes, fimA, fimC and mrkD, was determined by RT-qPCR. The acetylation complementation strain, A1142::Ref-K57orf13 (black bars, ◼), was compared to the A1142 parent strain (white bars, ◻, set as 1). Data are presented as mean ± SEM from three independent experiments. *P < 0.05, Student’s t test.
Given that the capsule protects K. pneumoniae from killing by the host sera in the immune system, we evaluated whether CPS acetylation affects the serum resistance of K. pneumoniae. In vitro serum resistance assays showed that although the A1142 strain grew in human serum (Fig. 6B), the A1142::Ref K57orf13 strain became sensitive to serum-killing, indicating that CPS acetylation decreased the serum resistance of K. pneumoniae and diminished the protective properties of the K57 capsule. Acetyl modification of CPS may reduce the ability of K. pneumoniae to escape from the host immune system.
The capsule of K. pneumoniae has also been implicated in adhesion of bacteria to host cells27. We analyzed the effect of capsular acetylation on adhesion in a cell culture assay (Fig. 6C; results of CFU viable counts shown in Figure S2). Notably, A1142::Ref-K57orf13 exhibited a higher rate of adhesion to human Caco-2 (intestinal epithelium-derived) cells than observed with the A1142 parental strain. Thus, CPS acetylation enhanced attachment of K. pneumoniae to host intestinal cells and may play a role in promoting its colonization and interactions with host cells.
Since India ink staining showed that the capsule thickness of A1142::Ref-K57orf13 was not significantly changed, we investigated whether fimbriae, the surface factors important for K. pneumoniae adhesion, were altered. Expression of the type I fimbriae genes, fimA and fimC, and the type III fimbriae gene, mrkD, was determined by quantitative real-time reverse-transcription PCR (RT-qPCR) (Fig. 6D). Compared to the A1142 parental strain, the expression of fimA and fimC in A1142::Ref-K57orf13 increased. No significant differences in mrkD were detected. Thus, the enhanced cell adhesion of the acetylation complementation strain could be due to higher levels of type I fimbriae.
Discussion
Conventional definitions of the K serotypes of encapsulated bacteria assume that each type has a specific capsular polysaccharide structure and is a genetically distinct entity. The presence of variants in a K serotype has been described in some bacteria28,29,30. Here, we characterized a variant of the K57 capsular serotype, a type that is associated with K. pneumoniae-induced PLA. Our work revealed that this capsular variation reflected changes in CPS acetylation. Although acetylation has been widely studied in the capsule of several pathogenic bacteria, such as the E. coli K1 strain, S. pneumoniae and N. meningitidis20,21,22,31,32, this modification has been poorly characterized in Klebsiella capsular types. Our studies identified a gene whose product is responsible for the acetylation of K57 CPS and demonstrated its impact. These results have implications for K. pneumoniae vaccine design and pathogenicity.
Based on PCR genotyping of the cps cluster and immunoserotyping, the PLA isolate, A1142, was defined as a K57 capsular type14. However, it harbored differences in the cps gene (orf13) encoding a putative acetyltransferase. In A1142, orf13 is interrupted by insertion of a putative transposase-encoding gene and presumably inactivated. NMR, biochemical and genetic analyses indicated that the intact orf13 of Ref-K57 mediates CPS acetylation and A1142 is a K57 variant that is deficient in CPS acetylation. Capsular variants involving acetylation have been described in other bacterial species. For instance, in E. coli with K1 capsular polysaccharides, variation in polysaccharide O-acetylation is mediated by a phase-variable gene encoding an O-acetyltransferase32,33. The determinant gene, neuO, is associated with the prophage CUS-3 and not part of the cps cluster. In S. pneumoniae, the polysaccharide structures of serotypes 11A and 11E appear to be identical except for the presence of O-acetylation21. This modification depends on the presence of the O-acetyltransferase-encoding gene, wcjE, in the 11A cps locus. Strains of serotype 11E contain various disruptive mutations in wcjE that result in inactivation of the gene. Another example is the reversible serotype switching between S. pneumoniae 15B and 15C, a variation that has been attributed to reversible slipped-strand mutations in the wciZ gene of the cps cluster. Because this gene encodes an O-acetyltransferase, strand slippage during replication results in phase-variable O-acetylation of capsule polysaccharides34,35. Our studies of K. pneumoniae demonstrated that varied acetylation of CPS in K57 strains was mediated by disruptive insertion of a putative transposase-encoding gene in the cps region at the site of the gene encoding acetyltransferase, indicating another mechanism of serotype variation in bacteria.
The capsular polysaccharides of K. pneumoniae are immunogenic. Antibodies targeting the capsule have been shown to provide hosts with increased resistance to capsulated pathogens36,37. Modifications of the CPS may influence the properties of polysaccharides. Acetylation affects the antigenicity or immunogenicity of other bacterial polysaccharides32,38,39,40, such as K1 Escherichia coli, groups W-135, Y and C meningococci and group B Streptococcus capsular polysaccharides. In agreement, our data demonstrated that the acetyl group influenced the immunoreactivity of K. pneumoniae K57 polysaccharides to anti-K57 antibodies. Immunoblotting of Ref-K57 CPS against anti-Ref-K57 antiserum showed that the immunoreactivity was significantly decreased by acetylation gene replacement (Ref-K57Δorf13::A1142orf13-tnp). Therefore, the acetyl group may constitute part of the epitope of K. pneumoniae K57 CPS recognized by this antibody. CPS from A1142 displayed lower reactivity to anti-Ref-K57 antiserum and complementation of acetylation enhanced the immunoreactivity of CPS in this strain. These results indicate that acetyl modification plays an important role in K. pneumoniae CPS antigenicity and antibody recognition, likely as the part of the polysaccharide epitope. Analysis of acetylation prevalence in K. pneumoniae clinical strains, including other PLA predominant capsular types, merits further survey. These results also suggest that polysaccharide modifications should be considered in the development of capsule-based vaccines for K. pneumoniae.
We also demonstrated the effects of CPS acetylation on innate immune responses. Notably, acetylation enhanced K57 K. pneumoniae induction of TNF-α and IL-6. This result is consistent with the previous demonstration that chemical removal of O-acetyl and O-pyruvyl groups from extracted K1 CPS attenuates the induction of pro-inflammatory cytokines23. In the same study, K1 CPS was shown to induce cytokine expression through activation of Toll-like receptor 4 (TLR4) and MAPK signaling pathways23. Our studies of K57 K. pneumoniae further revealed a role for CPS acetylation in host signal transduction. Thus, acetylation may improve CPS induction of cytokines via activating cell signaling pathways, including JNK and MAPK. We propose that acetylation possibly mediates the interactions between polysaccharides and host receptors to initiate cell signaling pathways, therefore, contributing to polysaccharide induction of cytokine expression and innate immune responses in the host.
Alternation of surface polysaccharide architecture by acetylation has been proposed to influence the virulence of several bacterial pathogens, including E. coli, N. meningitidis and Staphylococcus aureus21,22,31. Epidemiological association of O-acetylated CPS and increased virulence was also reported in bacteremia-inducing E. coli K1 strains21. One study of S. aureus serotype 5 showed that a mutant lacking O-acetylation has reduced bacterial virulence in opsonophagocytic assays and in mice31. Our study of K. pneumoniae suggested that capsular acetylation contributed to pathogenicity; however, complementation of acetylation did not significantly change the in vivo virulence of the non-acetylated PLA strain A1142 in mice. Instead, acetylation influenced K. pneumoniae resistance against human serum and adhesion to human intestinal cells although with opposing effects. While acetylation reduced serum resistance of A1142, it promoted bacterial adhesion to the host cells, which may explain the unchanged in vivo virulence observed in the mouse experiments. Additionally, many other factors could also be involved in the in vivo results, i.e. an overall consequence. For example, K. pneumoniae might regulate their CPS production or acetylation levels in the host environment, or acetylation could affect other unidentified host responses. Our data imply that acetylation in K. pneumoniae K57 has different impacts on bacterial survival and adaptation. This capsular modification may play a variety of roles in K. pneumoniae pathogenicity, requiring further studies using different clinical strains and other K-type K. pneumoniae.
We demonstrated that acetylation of CPS promoted cell adhesion of K. pneumoniae. No significant changes in CPS production and the capsule thickness were found. Thus, one possibility for the better cell adhesion could be that acetylation enhanced interactions between bacterial surfaces with the host cells despite expressing similar amounts of CPS. RT-qPCR revealed that the expression of type I fimbriae genes was higher in the acetylated strain. Therefore, one explanation for increased adhesion might be due to more production of fimbriae in the acetylated CPS strain. Why and how complementation of acetylation could affect fimbriae expression is still unclear. Potential gene regulation networks or interplay between cps and fim genes should be further characterized.
Capsular typing of K. pneumoniae is important for determining its prevalence for epidemiological research. The inability to determine the types for some clinical isolates is not unusual. For example, an Australian survey using antisera for typing reported that out of 293 K. pneumoniae isolates, 88 (30%) could not be typed and 54 (18%) had a positive reaction for more than one capsular type41. Klebsiella capsules can be typed using different methods and each has some limitations that can preclude serotype determination in clinical isolates42. The presence of CPS variants like that described in the present study may provide another explanation. Serological diagnosis is commonly used to determine Klebsiella capsular serotypes of clinical isolates often exhibit different sensitivities. Varied acetylation of CPS may render some clinical strains less immunoreactive to antiserum. Since modifications increase the complexity of the capsule, investigators may need to employ more than one approach to determine the type accurately.
In summary, DNA sequence analysis of the cps cluster permitted identification of a candidate CPS acetyltransferase-encoding gene with distinct alleles in different K57 strains. We correlated this genetic variation with acetylation-mediated changes in K. pneumoniae PLA-related capsular serotype K57. We further showed the impacts of acetylation on K. pneumoniae CPS immunoreactivity, host innate response, serum resistance and host cell adhesion. We suggest that acetylation contributes to the diversity and antigenic heterogeneity of the capsule and K. pneumoniae-host interactions. Our findings increase our knowledge about the variety of Klebsiella CPS and provide further insights into the role of capsular modification in bacterial pathogenesis.
Methods
Bacterial strains, cps-PCR and cps sequencing
The Ref-K57 strain, 4425/51, was purchased from the Statens Serum Institute (Copenhagen, Denmark). Twenty-two K. pneumoniae K57 clinical isolates, including PLA and bacteremia strains, were collected from the National Taiwan University Hospital, En Chu Kong Hospital, Chang Gung Memorial Hospital and Taipei Veterans General Hospital in Taiwan14,19,43,44 and were gifts from Hong Kong and Finland14,44. The K57 capsular type of the clinical strains was determined using wzy-targeting, PCR-based cps genotyping14,43. PCR analysis of orf13, the cps gene encoding a putative acetyltransferase, was performed using the following primers: K57-orf13-F (atgggtaaaaatatcaaagagcg) and K57-orf13-R (ttagaataggaaccataatcttttcc).
For cps sequencing, the cps region of the Ref-K57 strain (between the conserved genes, galF and gnd) was amplified as previously described14. The products were sequenced by primer walking, providing DNA sequences for the cps regions. Genes were annotated by NCBI-BLAST.
Analysis of CPS acetylation
The degree of acetylation was quantified according to a modified Hestrin method24,25. Extracellular polysaccharides were extracted with hot phenol as previously reported19 and further purified by ethanol re-precipitation. Each sample was resuspended in water (0.5 mL) and combined with 1 mL of alkaline hydroxylamine (1M hydroxylamine in 1.75M NaOH) and the mixture was incubated for 30 min before addition of 0.5 mL each of 4M HCl and 0.37M FeCl3 in 0.1M HCl. Absorbance was measured at 504 nm (A504). For comparison, data are presented as A504 normalized to 50 μg of CPS for each sample.
To analyze potential acetylation of K. pneumoniae CPS using 1H NMR23, the O-antigen-lacking mutants, Ref-K57ΔwecA and A1142ΔwecA, were constructed using an unmarked deletion method with the temperature-sensitive plasmid pKO3-Km45 to avoid the effects of sugars from lipopolysaccharide (LPS). K. pneumoniae CPS was isolated according to the previously described methods23 with modifications as needed. Briefly, bacteria suspended in distilled water were heated for 10 min at 100 °C to release capsular materials; CPS was precipitated with 80% (v/v) acetone at 4 °C. Dried precipitate was resuspended in 20mM Tris-HCl, pH 7.5 and treated with ribonuclease, deoxyribonuclease I and proteinase K. The sample was then dialyzed extensively against water using an 80-kDa cutoff membrane and lyophilized. The CPS was further purified on a TSK HW-65F column, followed by dialysis and re-lyophilization. Sugar composition was determined by methanolysis and trimethylsilylation, followed by GC-MS analysis46.
Quantification of CPS
K57 CPS, which contains uronic acid, was quantified according to previously described methods47,48. Briefly, extracted samples from the equivalent amounts of bacterial overnight cultures were resuspended in 0.1 mL of water and combined with 1.2 mL of 12.5 mM tetraborate in concentrated H2SO4. After vigorous vortexing, the mixture was boiled for 5 min. After cooling, 20 μL of 0.15% 3-hydroxydiphenol (Sigma-Aldrich, St. Louis, MO) was added. Then, the absorbance at 520 nm was measured.
Immunoblotting of CPS
For immunoblot analysis of CPS, rabbit anti-K57 antiserum was purchased from the Statens Serum Institute. Extracted polysaccharide samples were vacuum-spotted onto a nitrocellulose membrane using a slot blot device. The membrane was overlapped with a piece of filter and both were rinsed with Western transfer buffer containing 47.8 mM Tris, 38.6 mM glycine, 20% MeOH and 0.037% sodium dodecyl sulfate. The membrane was dried and non-specific sites were blocked by soaking the membrane in 1× phosphate-buffered saline with 0.5% Tween 20 (PBST) plus 5% milk for 1 h at room temperature. The membrane then was incubated with anti-K57 antiserum (1:1000 dilution) dissolved in PBST plus milk at 4 °C overnight, washed four times with PBST for 10 min each, incubated with the secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG-HRP, 1:10 000) for 1 h at room temperature and washed three times with PBST for 10 min each. The ECL reagent was added for 3 min and the membrane was exposed to X-ray film in the dark. To quantify the immunoreactivity, the resulting bands were analyzed by densitometry using Image J software (NIH, USA).
Gene replacement and complementation in K. pneumoniae
The isogenic replacement mutant of Ref-K57 was constructed using the temperature-sensitive plasmid, pKO3-Km43,45. In this replacement mutant, the coding region of the putative acetyltransferase (orf13) in the cps locus of Ref-K57 was replaced by the corresponding gene (disrupted by an ORF encoding a putative transposase) from A1142 (residues 15933–17975). For chromosomal complementation of orf13 in the cps cluster of A1142, a single copy of the Ref-K57 orf13 was cloned into the intergenic region between wbaZ and gnd using the pKO3-Km vector according to a previously reported method49. The gene replacement or complementation was confirmed by PCR analysis and DNA sequencing.
Analysis of pro-inflammatory cytokine secretion and cell signaling
RAW 264.7 murine macrophage-like cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1× penicillin/streptomycin (PAA) and maintained at 37 °C in a humidified incubator at 95% air-5% CO2. Culture medium and supplements were obtained from GIBCO/BRL (Gaithersburg, MD, USA). For infection, cells (1 × 106 cells per well) in FBS-free DMEM medium in 24-well plates were infected with mid-log-phase (A600 = 0.4 to 0.6) K. pneumoniae at a multiplicity of infection of 10 (MOI 10; bacteria/cells). After 1 h of infection, the cells were treated with 100 μg/mL of gentamicin and supernatants were collected at various time points as indicated. The levels of mouse TNF-α and IL-6 in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
For Western blot detection of cell signaling, RAW 264.7 cells were harvested after K. pneumoniae induction (as described above). Total cellular protein extracts were obtained by lysing cells in ice cold RIPA buffer, followed by centrifugation and protein quantification using a Coomassie blue protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of the protein extracts were subjected to SDS-PAGE electrophoresis and subsequent electrotransfer onto a PVDF membrane. After blocking with 5% skimmed milk in PBST for 1 h at room temperature, the blots were incubated with primary antibodies overnight at 4 °C. Antibodies against phospho-ERK1/2 (Thr202/185 and Tyr204/187), phospho-p38 MAPK (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-NF-κB p65 (Ser536) and those against respective total proteins were obtained from Cell Signaling (Beverly, MA, USA). Antibodies against β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). After washes with PBST, the blots were incubated with HRP-conjugated secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA, USA) and the signals were detected using a digital imaging system (UVP, Upland, CA, USA).
In vivo competition assays
In vivo competition of K. pneumoniae in mice was analyzed as outlined in previous work49. Briefly, the parental A1142 or A1142::Ref-K57orf13 strains were mixed with the isogenic lacZ promoter deletion mutant (A1142ΔplacZ) at a 1:1 ratio and a per-mouse dose of 5 × 106 colony-forming units (CFUs) in 100 μL of saline solution was inoculated intraperitoneally into 5-week-old BALB/c mice. Each mouse was killed 24 h post-inoculation and the liver and spleen were removed and homogenized in 1× PBS. The number of LacZ-positive and LacZ-negative colonies on LB plates containing 1 mM IPTG/mL and 50 μg/mL of X-Gal were counted. The competitive index (CI) was defined as (outputΔplacZ/outputtest strain)/(inputΔplacZ/inputtest strain). All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council, USA and all animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the National Taiwan University College of Medicine (NTUCM).
Serum resistance assays
The serum resistance of the K. pneumoniae strains was analyzed as previously described44. Briefly, an inoculum of 2.5 × 104 CFU bacteria in 25 μL was mixed with 75 μL human serum from healthy volunteers. The mixture was incubated at 37 °C for 1 h. After serial dilution and plating, the numbers of CFU were determined. The survival ratio was calculated; values of ≧1 were defined as serum resistance. The experiments were repeated independently three times.
Cell adhesion assays
Adhesion assays using human enterocyte-like Caco-2 cells were performed according to previously described methods50. Caco-2 cells were maintained in DMEM medium supplemented with 10% heat-inactivated FBS and 1% nonessential amino acids. For adhesion assays, the cells seeded in 24-well plates (~5 × 105 cells per well) and were prewashed with Hanks’ Balanced Salt Solution (HBSS). Mid-log-phase (A600 = 0.4 to 0.6) K. pneumoniae in FBS-free DMEM medium were added to each well at a MOI of 50. After a 20-min incubation in a humidified 5% CO2 atmosphere at 37 °C, the wells were washed with HBSS three times and bacteria were released by the addition of 0.2% Triton X-100 (Sigma-Aldrich). The experiments were conducted in duplicate and repeated independently three times.
Quantitative real-time reverse-transcription PCR (RT-qPCR)
K. pneumoniae gene expression was determined by RT-qPCR49. Total RNA from K. pneumoniae strains was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) with DNase I to remove contaminating genomic DNA. A 400-ng sample of purified total RNA was reverse-transcribed and amplified by PCR with SYBR green dye (Invitrogen) in an ABI 7900 thermocycler (Applied Biosystems, Foster City, CA, USA). For each gene, the calculated threshold cycle (Ct) was normalized to the Ct of the 23S ribosomal RNA gene from the same complementary DNA sample. The relative RNA expression was calculated based on the ΔΔCt value. Primers used are listed in Table S1.
Statistical analysis
Comparisons of mean values were assessed by a two-tailed Student’s t test using Prism 5 (Graphpad) software. P values of <0.05 were considered significant.
Additional Information
How to cite this article: Hsu, C.-R. et al. Identification of a capsular variant and characterization of capsular acetylation in Klebsiella pneumoniae PLA-associated type K57. Sci. Rep. 6, 31946; doi: 10.1038/srep31946 (2016).
References
Ko, W. C. et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8, 160–166 (2002).
Lin, Y. T., Jeng, Y. Y., Chen, T. L. & Fung, C. P. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect Dis 10, 307 (2010).
Podschun, R. & Ullmann, U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods and pathogenicity factors. Clin Microbiol Rev 11, 589–603 (1998).
Tsai, F. C., Huang, Y. T., Chang, L. Y. & Wang, J. T. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 14, 1592–1600 (2008).
Chung, D. R. et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 54, 578–583 (2007).
Lederman, E. R. & Crum, N. F. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 100, 322–331 (2005).
Yang, C. C., Yen, C. H., Ho, M. W. & Wang, J. H. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 37, 176–184 (2004).
Fung, C. P. et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50, 420–424 (2002).
Chuang, H. C. et al. Clinical and bacteriological characteristics of pyogenic liver abscess in non-diabetic patients. J Microbiol Immunol Infect 42, 385–392 (2009).
Thomsen, R. W., Jepsen, P. & Sorensen, H. T. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis 44, 1194–1201 (2007).
Cortes, G. et al. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70, 2583–2590 (2002).
Mizuta, K. et al. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun 40, 56–61 (1983).
Fang, C. T. et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45, 284–293 (2007).
Pan, Y. J. et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol 46, 2231–2240 (2008).
Struve, C., Bojer, M., Nielsen, E. M., Hansen, D. S. & Krogfelt, K. A. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol 54, 1111–1113 (2005).
Yu, W. L. et al. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis 62, 1–6 (2008).
Erbing, C., Kenne, L., Lindberg, B. & Lonngren, J. Structural studies of the capsular polysaccharide from Klebsiella Type 1. Carbohydr Res 50, 115–120 (1976).
Ayling-Smith, B. & Pitt, T. L. State of the art in typing: Klebsiella spp. J Hosp Infect 16, 287–295 (1990).
Chuang, Y. P., Fang, C. T., Lai, S. Y., Chang, S. C. & Wang, J. T. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193, 645–654 (2006).
Calix, J. J. & Nahm, M. H. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis 202, 29–38 (2010).
Frasa, H. et al. Escherichia coli in bacteremia: O-acetylated K1 strains appear to be more virulent than non-O-acetylated K1 strains. J Clin Microbiol 31, 3174–3178 (1993).
Lee, H. J. et al. Structural and kinetic characterizations of the polysialic acid O-acetyltransferase OatWY from Neisseria meningitidis. J Biol Chem 284, 24501–24511 (2009).
Yang, F. L. et al. Structure and immunological characterization of the capsular polysaccharide of a pyrogenic liver abscess caused by Klebsiella pneumoniae: activation of macrophages through Toll-like receptor 4. J Biol Chem 286, 21041–21051 (2011).
Hestrin, S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine and its analytical application. J Biol Chem 180, 249–261 (1949).
Naran, R., Chen, G. & Carpita, N. C. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol 148, 132–141 (2008).
Kamerling, J. P., Lindberg, B., Lonngren, J. & Nimmich, W. Structural studies of the Klebsiella type 57 capsular polysaccharide. Acta Chem Scand B 29, 593–598 (1975).
Sahly, H. et al. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect Immun 68, 6744–6749 (2000).
Orskov, F. et al. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med 149, 669–685 (1979).
Lewis, A. L., Nizet, V. & Varki, A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci USA 101, 11123–11128 (2004).
Zartler, E. R. et al. Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J Biol Chem 284, 7318–7329 (2009).
Bhasin, N. et al. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol Microbiol 27, 9–21 (1998).
Deszo, E. L., Steenbergen, S. M., Freedberg, D. I. & Vimr, E. R. Escherichia coli K1 polysialic acid O-acetyltransferase gene, neuO and the mechanism of capsule form variation involving a mobile contingency locus. Proc Natl Acad Sci USA 102, 5564–5569 (2005).
Stummeyer, K. et al. Evolution of bacteriophages infecting encapsulated bacteria: lessons from Escherichia coli K1-specific phages. Mol Microbiol 60, 1123–1135 (2006).
Jones, C. & Lemercinier, X. Full NMR assignment and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 15B. Carbohydr Res 340, 403–409 (2005).
van Selm, S., van Cann, L. M., Kolkman, M. A., van der Zeijst, B. A. & van Putten, J. P. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect Immun 71, 6192–6198 (2003).
Meng, C. et al. Development of 5-valent conjugate pneumococcal protein A-Capsular polysaccharide pneumococcal vaccine against invasive pneumococcal disease. Microb Pathog 47, 151–156 (2009).
Robbins, J. B. et al. Prevention of invasive bacterial diseases by immunization with polysaccharide-protein conjugates. Curr Top Microbiol Immunol 146, 169–180 (1989).
Fattom, A. I., Sarwar, J., Basham, L., Ennifar, S. & Naso, R. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect Immun 66, 4588–4592 (1998).
Fusco, P. C., Farley, E. K., Huang, C. H., Moore, S. & Michon, F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin Vaccine Immunol 14, 577–584 (2007).
Arakere, G. & Frasch, C. E. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun 59, 4349–4356 (1991).
Jenney, A. W. et al. Seroepidemiology of Klebsiella pneumoniae in an Australian Tertiary Hospital and its implications for vaccine development. J Clin Microbiol 44, 102–107 (2006).
Hsu, C. R., Lin, T. L., Pan, Y. J., Hsieh, P. F. & Wang, J. T. Isolation of a bacteriophage specific for a new capsular type of Klebsiella pneumoniae and characterization of its polysaccharide depolymerase. PloS One 8, e70092 (2013).
Hsu, C. R., Lin, T. L., Chen, Y. C., Chou, H. C. & Wang, J. T. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology(SGM) 157, 3446–3457 (2011).
Fang, C. T., Chuang, Y. P., Shun, C. T., Chang, S. C. & Wang, J. T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199, 697–705 (2004).
Hsieh, P. F., Lin, T. L., Lee, C. Z., Tsai, S. F. & Wang, J. T. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197, 1717–1727 (2008).
Yang, F. L. et al. Structural determination of the polar glycoglycerolipids from thermophilic bacteria Meiothermus taiwanensis. Eur J Biochem/FEBS 271, 4545–4551 (2004).
Blumenkrantz, N. & Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal Biochem 54, 484–489 (1973).
Domenico, P., Schwartz, S. & Cunha, B. A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun 57, 3778–3782 (1989).
Hsieh, P. F., Lin, H. H., Lin, T. L. & Wang, J. T. CadC regulates cad and tdc operons in response to gastrointestinal stresses and enhances intestinal colonization of Klebsiella pneumoniae. J Infect Dis 202, 52–64 (2010).
Hsu, C. R. et al. Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase- and phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect Immun 83, 769–779 (2015).
Acknowledgements
We thank Dr. Ying-Hsien Kao and Mr. Po-Han Chen at the Department of Medical Research, E-Da Hospital, for their kind help with Western blot analysis. We thank Miss Jui-Tzu Wang for her help with RT-qPCR. This study was supported by grants from the Ministry of Science and Technology, National Taiwan University, National Taiwan University Hospital, E-Da Hospital (EDAHT104036, EDAHT104043, EDAHT104048), Liver Disease Prevention and Treatment Research Foundation, E-Da Hospital-National Taiwan University Hospital Joint Research Program (104-EDN04) and Far Eastern Memorial Hospital-National Taiwan University Hospital Joint Research Program.
Author information
Authors and Affiliations
Contributions
C.-R.H., C.-H.L. and J.-T.W. conceived and designed the experiments. C.-R.H., T.-L.L., H.-R.Y., F.-L.Y., P.F.H. and S.-H.W. performed the experiments. C.-R.H. and C.-H.L. analyzed and interpreted the data. C.-R.H. and J.-T.W. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hsu, CR., Liao, CH., Lin, TL. et al. Identification of a capsular variant and characterization of capsular acetylation in Klebsiella pneumoniae PLA-associated type K57. Sci Rep 6, 31946 (2016). https://doi.org/10.1038/srep31946
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31946
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.