Abstract
Intrahepatic cholestasis of pregnancy (ICP), a pregnancy-related liver disease, leads to complications for both mother and fetus. Circulating microRNAs (miRNAs) have emerged as candidate biomarkers for many diseases. So far, the circulating miRNAs profiling of ICP has not been investigated. To assess the urinary miRNAs as non-invasive biomarkers for ICP, a differential miRNA profiling was initially analyzed by individual quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assay in urinary samples from a screening set including 10 ICP and 10 healthy pregnancies. The selected candidate miRNAs were then validated by a validation set with 40 ICP and 50 healthy pregnancies using individual qRT-PCR assay. Compared with the expression in urine of healthy pregnant women, the expression levels of hsa-miR-151-3p and hsa-miR-300 were significantly down-regulated, whereas hsa-miR-671-3p and hsa-miR-369-5p were significantly up-regulated in urine from ICP patients (p < 0.05 and false discovery rate < 0.05). A binary logistic regression model was constructed using the four miRNAs. The area under the receiver operating characteristic curve was 0.913 (95% confidence interval = 0.847 to 0.980; sensitivity = 82.9%, specificity = 87.0%). Therefore, urinary microRNA profiling detection in ICP is feasible and maternal urinary miRNAs have the potential to be non-invasive biomarkers for the diagnosis of ICP.
Similar content being viewed by others
Introduction
Intrahepatic cholestasis of pregnancy (ICP), a pregnancy-related syndrome in which the incidence varies geographically from 0.1% to 15.6%1,2, is characterized by mild to severe pruritus and disturbed liver function tests. The disease symptoms and liver dysfunction appear mainly in the late second or third trimester of pregnancy and recover within a few days (typically 1–3) after delivery. However, ICP leads to complications for both mother and fetus. ICP is associated with intractable pruritus and high predisposition to postpartum bleeding, being the leading causes of maternal morbidity. On the other hand, ICP is associated with an increased risk of spontaneous preterm labor, fetal distress and sudden intrauterine death. Currently, the exact cause of ICP is unknown. Genetic, endocrinologic, nutritional, and environmental factors are considered to be related to the pathogenesis of the disease1,3,4,5. The diagnosis of ICP should exclude other clinical entities which are included in the differential diagnosis of cholestasis and hepatic disease. Viral hepatitis, autoimmune liver disease, gall bladder stones, tumors of the hepatobiliary tract, and other causes with elevated hepatic enzymes specified to pregnancy (e.g., namely preeclampsia and acute fatty liver) should be considered in the differential diagnosis4,5,6. ICP is related to abnormalities in the metabolism and disposition of bile acid compositions. The elevated serum total bile acids (TBA) level is considered as the most useful laboratory indicator for the diagnosis of ICP7. However, it was impossible to distinguish the ICP patients with low pruritus from normal pregnant women by serum TBA8. Further more, normal serum TBA concentrations have been observed in some cases with ICP9.
MicroRNAs (miRNAs) are a class of small noncoding RNAs, approximately 19–25 nucleotides, which negatively regulate gene expression and have a critical role in many biological and pathological processes10. miRNAs, as important post-transcription regulators, inhibit protein translation or stimulate transcript degradation though sequence-specific base pairing on the 3’untranslated regions of the target mRNAs. So miRNAs influence crucial cell processes such as differentiation, proliferation/growth, apoptosis suggesting their relevant role in human disease11,12. Besides tissue-specific expression of miRNAs, circulating mRNAs have been detected in most extracellular fluids, particularly in plasma/serum13,14. Moreover, these circulating miRNAs are highly stable and easily detected and usually reflect a tissue specific injury or expression15.
So far, circulating miRNAs are used as biomarkers for various forms of acute and chronic liver disorders, such as drug-induced liver injury16, chronic viral hepatitis17,18, hepatocellular carcinoma19 and nonalcohol-related fatty liver disease20. The search for miRNAs that might serve as biomarkers for pregnancy complications was initially started by Chim and colleagues in 200821. Luo et al.22 demonstrated that miRNAs are exported to the circulation via exosome and suggested that circulating trophoblast derived miRNAs reflected the physiological status of the pregnancy and could be used diagnostically. Subsequently, placental-specific miRNAs were identified in plasma and serum in several other studies23,24. Urine, as a readily available compartment and a non-invasive source for circulating miRNAs, has been used to diagnose in many diseases17,25. So far, the circulating miRNAs profile of ICP, as a special type of cholestasis in pregnancy, has not been investigated. In the present study, we systematically screened urine miRNAs expression profiling of normal pregnant women and pregnant women with ICP by individual quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assay. We also performed validations by using individual qRT-PCR assay, in order to identify the potential biomarker of miRNAs for diagnosis of ICP.
Results
Patient description
The characteristics of participants are summarized in Table 1. The cases and controls were well matched for age and gestational week. However, the serum TBA, alanine transaminase (ALT), aspartate transferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) of patients with ICP were significantly different from those of the normal controls.
Differential urinary miRNA profiling in ICP and healthy pregnancies
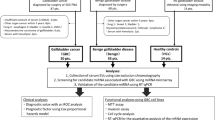
To estimate whether there was a difference in the generalizable urinary miRNA signatures between ICP and healthy pregnancies, we employed individual qRT-PCR assays to test 348 mature human miRNAs of 10 ICP and 10 healthy pregnancies (Supplementary dataset). Comparing the ICP and the control group, 24 miRNAs presented significant differential expression levels. Among them, 15 miRNAs were up-regulated (p < 0.05) and 9 were down-regulated (p < 0.05) in the ICP group. The candidate miRNAs selected for validation included 24 differential expression miRNAs and other 8 miRNAs that did not have statistically significant differential expression but |log2FC| were more than 1.5 (Table 2).
Validation of miRNA differential expression profiling for diagnosis of ICP
The expression of 32 candidate miRNAs selected from the previous step was confirmed by individual qRT-PCR in an independent cohort of 90 (50 control vs. 40 ICP) urine samples. The fold change of miRNA expression in ICP urine sample relative to the average expression in normal was calculated by the equation 2−∆∆Ct and parametric moderated t-test was used to compare miRNA expression between patients with ICP and controls. Nine of the 32 miRNAs presented significantly different expression levels between the ICP and control group and six miRNAs of nine passed the FDR correction (FDR < 0.05) using adjusted FDR bonferroni-hochberg method. The six differential expression miRNAs between the ICP and control group are shown in Fig. 1 and the fold change (FC) of these miRNA expression is shown in Fig. 2. Compared with the expression in urine of healthy pregnant women, the expression levels of hsa-miR-151-3p, has-miR-489, and hsa-miR-300 were significantly down-regulated, whereas hsa-miR-16, hsa-miR-671-3p, and hsa-miR-369-5p were significantly up-regulated in urine of ICP patients.
The expression of candidate miRNAs was confirmed by individual qRT-PCR in an independent cohort of 90 (50 control vs 40 ICP) urine samples. The expression levels of hsa-miR-369-5p, hsa-miR-16, hsa-miR-671-3p, hsa-miR-151-3p, has-miR-489 and hsa-miR-300 were significantly different between ICP and control groups (p < 0.05and FDR < 0.05). The values are normalized by geometric mean normalization of cel-miR-1832 and cel-miR-5594-5p, shown as relative expression at y-axis. The upper and lower limits of the boxes and the lines inside the boxes indicate the 75th and 25th percentiles and the median, respectively. Upper and lower horizontal bars denote the 90th and 10th percentiles.
A binary logistic regression model to estimate the risk of being diagnosed with ICP was applied to the validation set (50 controls vs. 40 ICP urine samples). Four of the six miRNAs turned out to be significant predictors (threshold: enter variable, if p < 0.05; remove variable, if p > 0.1). The predicted probability of being diagnosed with ICP was determined by the four miRNAs (hsa-miR-151-3p, hsa-miR-671-3p, hsa-miR-369-5p and hsa-miR-300) panel logit model and used to construct the receiver operating characteristic (ROC) curve. The miRNA panel area under the curve (AUC) was 0.913 (95%CI = 0.847 to 0.980; sensitivity = 82.9%, specificity = 87.0%, Fig. 3).
Discussion
Circulating miRNAs were reported to serve as an improved biomarker for several diseases19,26,27,28 because of their robustness against severe conditions that would normally degrade most RNAs, such as enzymatic degradation, freezing, and thawing, or extreme pH conditions14,29. In addition to robustness, easy access is another important criterion for biomarkers. miRNAs are detectable in almost all body fluids and excretions, including urine, feces, saliva, tear, ascitic, pleural, and amniotic fluid13,15,30, which could provide a new set of diagnostic tools for a variety of diseases.
ICP is associated with an increased risk of spontaneous preterm labor, fetal distress and sudden intrauterine death. Therefore, an accurate and early diagnosis of ICP is essential31. Usually, diagnosis of ICP is based on pruritus with abnormal liver function32. However, pruritus in pregnancy is a common symptom but it could be the only evidence in ICP. Moreover, it is often difficult to make an accurate diagnosis by performing solely routine laboratory tests because liver function is also altered in some other conditions of pregnant women. Serum TBA more than 10 μmol/L is considered as one of the most important standards in diagnosing ICP7. Five pregnant women in our study who were finally diagnosed as ICP after delivery had serum TBA less than 10 μmol/L. The five patients were misdiagnosed using serum TBA as the biomarker. Fortunately, only one patient was misdiagnosed using the miRNA panel. The circulating miRNAs profile has not been investigated so far. However, the circulating miRNAs profiling of pregnancy-specific disease and in primary biliary cirrhosis (PBC), a cholestatic disease had been reported. A prospective longitudinal cohort study was designed by Hromadnikova et al. to predict subsequent onset of gestational hypertension. The result showed that the up-regulation of miR-516-5p, miR-517*, miR-520 h and miR-518b was associated with a risk of later development of gestational hypertension33. Ura B et al. found serum miR-1233 might represent a potential marker of early sPE34. However, a research by Luque et al. showed maternal serum miRNA assessment at first-trimester of pregnancy does not appear to have any predictive value for early preeclampsia23. A multistage retrospective nested case-control study designed by ChunZhao et al. showed serum miRNAs (miR-29a, miR-222 and miR-132) are differentially expressed between GDM women and controls and could be candidate biomarkers for predicting GDM. Due to the significant limitations of currently available noninvasive tools to diagnose and monitor liver damage in various forms of liver diseases, and growing evidence for a prominent role of specific miRNA in liver pathobiology, research about circulating miRNA profiling in liver disease are rapidly growing35. For cholestatic disease, available data on circulating miRNAs and PBC appear in the literature in recent study. Recently, a human study in PBC patients showed that the expression of miR-505-3p and 197-3p in serum was reduced in PBC patients compared with healthy controls and patients with viral hepatitis36. Tan et al. found that miR-122-5p, miR-144-3p, and miR-26b-5p in serum were with high diagnostic accuracy for PBC37.
As the same as tissue-specific miRNA profiling determination, qRT-PCR and its variations are the most frequently used techniques for circulating miRNA profiling test in body fluids. However, enrichment before the analysis is a crucial step for the circulating miRNAs expression profiling. A common problem that may limit the quantity of miRNAs accessible for expression analysis is their stability and resistance to storage handling. Fortunately, circulating miRNAs are resistant to severe conditions. Accordingly, our study explored urinary miRNA profiling in ICP patients by individual qRT-PCR both for screening and validation stage. Compared with the expression in urine of healthy pregnant women, the expression levels of hsa-miR-151-3p and hsa-miR-300 were significantly down-regulated, whereas hsa-miR-671-3p and hsa-miR-369-5p were significantly up-regulated in urine of ICP patients. MiR-151, as an oncogenic miRNA hosted by the FAK gene, is associated with various forms of cancer with a tendency to be up-regulated38. Even though miR-151–3p expression was found to be decreased in the development of cardiac hypertrophy39, peripheral blood mononuclear cells of schizophrenia patients40. and polycystic ovary syndrome patient follicular fluid41. miR-671-3p has been certified to be associated with MMP-9 gene polymorphism in different carcinoma42. miR-300 was found to be down-regulated in cancer cells that have undergone epithelial–mesenchymal transition comparing with typical epithelial phenotype carcinoma cells43. Meanwhile, miRNA-369-5p was up-regulated in mesenchymal cells compared with epithelial cells in endometrial carcinosarcomas series44. Yabushita et al.45 identified miRNA-369-5p was significantly increased in the serum of pancreatic ductal adenocarcinoma rats compared to that in the control rats. An inverse correlation between expression of miR-369-5p and its target gene was observed by Z. Xu et al.46 miR-369-5p was down-regulated in DSA+ LT while its gene targets in TGF-β signal pathways were up-regulated. TGF-β may be involved in the pathogenesis of ICP through inhibiting the production of TNF-α and IFN-γ46.
In conclusion, with this pilot trial we demonstrate the feasibility to detect an ICP dependent miRNA profiling in urine. The test enables us to specifically discriminate patients with ICP from healthy pregnant women. We could identify four significantly altered and specifically regulated miRNAs (hsa-miR-151-3p, hsa-miR-671-3p, hsa-miR-369-5p and hsa-miR-300) in ICP patients from those in healthy controls. Our present results show typical expression patterns of miRNA in the urine of ICP patients. This sustains the potential role of urinary miRNAs as non-invasive innovative biomarkers in diagnosis of ICP. Since this study examines only a limited number of samples, extended future studies are needed to confirm these observations.
Methods
Research subjects and design
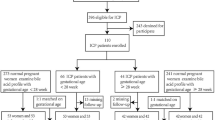
We designed case-control study, including urine from 50 cases of ICP and 60 cases of healthy pregnant women to find the maternal circulating miRNAs for diagnosis of ICP. This study was performed at the First Affiliated Hospital of Chongqing Medical University, Chongqing, and designed conformed to the ethics guidelines given in the Declaration of Helsinki. Written informed consent was obtained from all subjects and all experimental protocols were approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University. Maternal urine samples were obtained from pregnancies who received prenatal care at the third-trimester (≥28 gestational weeks) from October 2013 to August 2015. The enrollment criteria for ICP were as follows: pruritus and jaundice in the third trimester of pregnancy without signs of chronic liver diseases, skin diseases, or symptomatic cholelithiasis; elevated levels of aminotransferases and total serum bile acid; and normalization of cholestasis after delivery. For the control group, only those healthy pregnant women matched with cases on age and pregnant weeks were enrolled during the same period, excluding women with a history of gallstones or cholecystopathy, pruritus, drugs consumption, hepatitis, or any other diseases damaging hepatobiliary function. Women with ICP underwent fetal monitoring from the time of diagnosis until delivery, and received drug therapy such as ursodeoxycholic acid (UDCA) for symptom relief. Samples from the women with ICP were collected at the first visit to confirm diagnosis before drug treatment. Serum liver function tests, including serum liver function tests, including TBA, TBIL, DBIL, ALT, AST, ALP, and GGT were performed on an automated Olympus Chemistry Analyzer (AU5400, Olympus, Japan).
Sample collection and miRNA extraction
The urine samples were obtained and processed within 2 h. Processing involved centrifugation at 3,000 g for 10 min at 4 °C, followed by centrifugation at 12,000 g for 10 min at 4 °C. The supernatant was removed and stored at −80 °C until analysis. The miRNA from urine was isolated using the QuantoBio’s microRNA Purification Kit (QuantoBio Biotek Corporation, Beijing, China) according to the manufacturer’s protocol. In brief, 300 μL of lysis buffer was added to 200 μL of urine sample. The sample was mixed in a tube followed by adding 550 μL of acidified phenol:chloroform. After mixing vigorously for 30 s, the sample was then centrifuged at 15,000 g for 10 min at 4 °C. The 200 μL supernatant was carefully transferred to a new collection tube, and 500 μL volume of ethanol containing binding buffer from the kit was added and mixed. The sample was then applied directly to a silica membrane containing column and the miRNA was retained and cleaned by using wash buffer provided in the kit. The immobilized cleaned miRNA was then eluted from the membrane into a collection tube with RNase-free water. The quantity and purity of the RNA was evaluated by A260/A280 ratio using a Nanodrop 2000c spectrometer (Thermo Fisher Scientific, USA). The prepared miRNA samples were stored at −80 °C.
Reverse transcription
The miRNA of isolation was reversely transcribed into cDNA using the Quanto-miR cDNA Synthesis Kit (QuantoBio Biotek Corporation, Beijing, China) according to the manufacturer’s protocol. In brief, E. coli polyA polymerase was used to add adenines at the 3′ end of RNA molecules lacking a polyA tail. After oligodT annealing, a universal tag was attached to the 3′ end of cDNAs during cDNA synthesis. With the addition of this universal tag, individual miRNAs were detected with miRNAs-pecific forward primers and a reverse universal primer mix. These cDNA samples were stored at −80 °C.
Quantitative realtime-PCR
A SYBR Green-based real-time PCR method was used to quantify the relative expression of miRNAs. In the miRNA expression profiling array, a total of 348 human mature miRNAs were evaluated in urine of ICP and healthy pregnant women. qRT-PCR was carried out on ViiA7 (Life technology, USA) in a total reaction volume of 10 μL (including 8 μL SYBR Green I mix, 1 μL primer, and 1 μL reverse transcription product) according to the manufacturer’s protocol (QuantoBio Biotek Corporation, Beijing, China). The Echo 550 liquid handler (Labcyte, USA) was used for liquid handling. A robot (HighRes, USA) integrated PCR and liquid handler was used in the qPCR analysis. The reactions were initiated in a 384-well optical plate at 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s.
Data analysis
The expression of miRNA profile and candidate miRNA were normalized by global expression level normalization and two geometric mean normalization of two external standards (synthetic C.elegans miRNA cel-miR-1832 and cel-miR-5594-5p) added in the steps of RNA isolation and reverse transcription, respectively. Differential miRNA expression was determined by parametric moderated t-test and corrected by bonferroni-hochberg false discovery rate (FDR), both with significance level set at 0.05. The relative expression levels of target miRNAs were calculated based on the threshold cycle (CT) value. The fold change (FC) of miRNA expression in ICP urine sample relative to the average expression in normal was the equation 2−ΔΔCT, in which ΔΔCT = (CTmiRNA − CTnormalization) ICP − (CTmiRNA -CTnormalization) control.
Statistical Analysis
The characteristics of participants were compared between controls and ICP women using independent samples-t tests. Binary logistic regression was utilized to screen out the combination biomarker for diagnosis of ICP. All the statistical analyses were performed with SPSS Statistics. v17.0.0 (SPSS, Inc., Chicago, USA). P value less than 0.05 was considered statistically significant, and all tests were two tailed.
Additional Information
How to cite this article: Ma, L. et al. Feasibility of urinary microRNA profiling detection in intrahepatic cholestasis of pregnancy and its potential as a non-invasive biomarker. Sci. Rep. 6, 31535; doi: 10.1038/srep31535 (2016).
References
Ozkan, S., Ceylan, Y., Ozkan, O. V. & Yildirim, S. Review of a challenging clinical issue: Intrahepatic cholestasis of pregnancy. World journal of gastroenterology 21, 7134–7141 (2015).
Lee, N.-M. Liver disease in pregnancy. World journal of gastroenterology 15, 897 (2009).
Egerman, R. S. & Riely, C. A. Predicting fetal outcome in intrahepatic cholestasis of pregnancy: is the bile acid level sufficient? Hepatology 40, 287–288 (2004).
Hepburn, I. S. & Schade, R. R. Pregnancy-associated liver disorders. Digestive diseases and sciences 53, 2334–2358 (2008).
Mays, J. K. The active management of intrahepatic cholestasis of pregnancy. Current opinion in obstetrics & gynecology 22, 100–103 (2010).
Bacq, Y. Liver diseases unique to pregnancy: a 2010 update. Clinics and research in hepatology and gastroenterology 35, 182–193 (2011).
Lammert, F., Marschall, H. U., Glantz, A. & Matern, S. Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. Journal of hepatology 33, 1012–1021 (2000).
Martinefski, M., Contin, M., Lucangioli, S., Di Carlo, M. B. & Tripodi, V. In search of an accurate evaluation of intrahepatic cholestasis of pregnancy. Scientifica 2012, 496489 (2012).
Muresan, D., Ona, D., Cruciat, G., Rotar, I. & Stamatian, F. Recurrent intrahepatic cholestasis of pregnancy. A case report. Journal of gastrointestinal and liver diseases : JGLD 17, 323–325 (2008).
Giordano, S. & Columbano, A. MicroRNAs: new tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma? Hepatology 57, 840–847 (2013).
Pal, M. K. et al. MicroRNA: a new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer biology & medicine 12, 328–341 (2015).
Jeon, T. I. & Osborne, T. F. miRNA and cholesterol homeostasis. Biochimica et biophysica acta (2016).
Chen, X. et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research 18, 997–1006 (2008).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America 105, 10513–10518 (2008).
Yang, Y. et al. Urine miRNAs: potential biomarkers for monitoring progression of early stages of diabetic nephropathy. Medical hypotheses 81, 274–278 (2013).
Wang, K. et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proceedings of the National Academy of Sciences of the United States of America 106, 4402–4407 (2009).
Dubin, P. H. et al. Micro-RNA-122 levels in acute liver failure and chronic hepatitis C. Journal of medical virology 86, 1507–1514 (2014).
Winther, T. N., Bang-Berthelsen, C. H., Heiberg, I. L., Pociot, F. & Hogh, B. Differential plasma microRNA profiles in HBeAg positive and HBeAg negative children with chronic hepatitis B. PloS one 8, e58236 (2013).
Qi, J., Wang, J., Katayama, H., Sen, S. & Liu, S. M. Circulating microRNAs (cmiRNAs) as novel potential biomarkers for hepatocellular carcinoma. Neoplasma 60, 135–142 (2013).
DiStefano, J. K. & Gerhard, G. S. Circulating microRNAs in nonalcoholic fatty liver disease. Expert review of gastroenterology & hepatology, 1–3 (2015).
Chim, S. S. et al. Detection and characterization of placental microRNAs in maternal plasma. Clinical chemistry 54, 482–490 (2008).
Luo, S. S. et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biology of reproduction 81, 717–729 (2009).
Luque, A. et al. Usefulness of circulating microRNAs for the prediction of early preeclampsia at first-trimester of pregnancy. Scientific reports 4, 4882 (2014).
Kotlabova, K., Doucha, J. & Hromadnikova, I. Placental-specific microRNA in maternal circulation–identification of appropriate pregnancy-associated microRNAs with diagnostic potential. Journal of reproductive immunology 89, 185–191 (2011).
Corcoran, C., Rani, S. & O’Driscoll, L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. The Prostate 74, 1320–1334 (2014).
Silva, J. et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. The European respiratory journal 37, 617–623 (2011).
Lawrie, C. H. et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. British journal of haematology 141, 672–675 (2008).
Cantara, S. et al. Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population. The Journal of clinical endocrinology and metabolism 99, 4190–4198 (2014).
Kroh, E. M., Parkin, R. K., Mitchell, P. S. & Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50, 298–301 (2010).
Link, A. et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 19, 1766–1774 (2010).
Meng, L. J. et al. Effects of ursodeoxycholic acid on conjugated bile acids and progesterone metabolites in serum and urine of patients with intrahepatic cholestasis of pregnancy. Journal of hepatology 27, 1029–1040 (1997).
Reyes, H. & Simon, F. R. Intrahepatic cholestasis of pregnancy: an estrogen-related disease. Seminars in liver disease 13, 289–301 (1993).
Hromadnikova, I., Kotlabova, K., Hympanova, L., Doucha, J. & Krofta, L. First trimester screening of circulating C19MC microRNAs can predict subsequent onset of gestational hypertension. PloS one 9, e113735 (2014).
Ura, B. et al. Potential role of circulating microRNAs as early markers of preeclampsia. Taiwanese journal of obstetrics & gynecology 53, 232–234 (2014).
Szabo, G. & Bala, S. MicroRNAs in liver disease. Nature reviews. Gastroenterology & hepatology 10, 542–552 (2013).
Ninomiya, M. et al. Distinct microRNAs expression profile in primary biliary cirrhosis and evaluation of miR 505-3p and miR197-3p as novel biomarkers. PloS one 8, e66086 (2013).
Tan, Y. W. et al. Serum MicroRNAs as Potential Biomarkers of Primary Biliary Cirrhosis. PloS one 9 (2014).
Sung, C. O. et al. Integrative analysis of copy number alteration and gene expression profiling in ovarian clear cell adenocarcinoma. Cancer genetics 206, 145–153 (2013).
Wei, H. et al. microRNA-151-3p regulates slow muscle gene expression by targeting ATP2a2 in skeletal muscle cells. J Cell Physiol 230, 1003–1012 (2015).
Gardiner, E. et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry 17, 827–840 (2012).
Sorensen, A. E., Wissing, M. L., Englund, A. L. & Dalgaard, L. T. MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. The Journal of clinical endocrinology and metabolism, jc20153588 (2016).
Duellman, T., Warren, C. & Yang, J. Single nucleotide polymorphism-specific regulation of matrix metalloproteinase-9 by multiple miRNAs targeting the coding exon. Nucleic Acids Res 42, 5518–5531 (2014).
Yu, J., Xie, F., Bao, X., Chen, W. & Xu, Q. miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer 13, 121 (2014).
Castilla, M. A. et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol 223, 72–80 (2011).
Yabushita, S. et al. Circulating microRNAs in serum of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Pancreas 41, 1013–1018 (2012).
Xu, Z. et al. Dysregulated MicroRNA Expression and Chronic Lung Allograft Rejection in Recipients With Antibodies to Donor HLA. Am J Transplant 15, 1933–1947 (2015).
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No: 81401228, 81471473), and Research Fund for the Doctoral Program of Higher Education of China (20115503110013).
Author information
Authors and Affiliations
Contributions
M.D., L.M. and X.-Q.Z. conceived and designed the experiments. L.M. and D.-X.Z. performed the experiments and analyzed the data. Y.C., T.Y., L.L.D. and Y.S. performed clinical work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ma, L., Zhang, XQ., Zhou, DX. et al. Feasibility of urinary microRNA profiling detection in intrahepatic cholestasis of pregnancy and its potential as a non-invasive biomarker. Sci Rep 6, 31535 (2016). https://doi.org/10.1038/srep31535
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31535
This article is cited by
-
DIA-based proteomics analysis of serum-derived exosomal proteins as potential candidate biomarkers for intrahepatic cholestasis in pregnancy
Archives of Gynecology and Obstetrics (2022)
-
MicroRNA: a novel implication for damage and protection against ionizing radiation
Environmental Science and Pollution Research (2021)
-
Identification of Reference Genes for Analysis of microRNA Expression Patterns in Equine Chorioallantoic Membrane and Serum
Molecular Biotechnology (2018)
-
Urinary extracellular vesicles. A promising shortcut to novel biomarker discoveries
Cell and Tissue Research (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.