Abstract
Accurate translation of the genetic information from DNA to protein is maintained by multiple quality control steps from bacteria to mammals. Genetic and environmental alterations have been shown to compromise translational quality control and reduce fidelity during protein synthesis. The physiological impact of increased translational errors is not fully understood. While generally considered harmful, translational errors have recently been shown to benefit cells under certain stress conditions. In this work, we describe a novel regulatory pathway in which reduced translational fidelity downregulates expression of flagellar genes and suppresses bacterial motility. Electron microscopy imaging shows that the error-prone Escherichia coli strain lacks mature flagella. Further genetic analyses reveal that translational errors upregulate expression of a small RNA DsrA through enhancing its transcription and deleting DsrA from the error-prone strain restores motility. DsrA regulates expression of H-NS and RpoS, both of which regulate flagellar genes. We demonstrate that an increased level of DsrA in the error-prone strain suppresses motility through the H-NS pathway. Our work suggests that bacteria are capable of switching on and off the flagellar system by altering translational fidelity, which may serve as a previously unknown mechanism to improve fitness in response to environmental cues.
Similar content being viewed by others
Introduction
The genetic information is passed from DNA to RNA to protein with high fidelity. On average, amino acid misincorporation rate is approximately 10−3–10−4 1,2. Such fidelity is maintained at every step during gene expression via careful selection of cognate substrates and proofreading of incorrect products3,4,5. For example, translation of mRNA into protein requires accurate ligation of amino acids to the right transfer RNAs (tRNAs) by aminoacyl-tRNA synthetases6,7, delivery of proper aminoacyl-tRNAs to the ribosome by elongation factors8 and precise matching of codon and anticodon on the ribosome9. Despite such extensive quality control mechanisms, increased translational errors (mistranslation) are known to be caused by genetic mutations10,11,12, nutrient starvation13,14, aminoglycoside antibiotics15,16, oxidative stress17,18,19, ethanol stress20 and temperature shift21,22. Severe mistranslation causes global protein misfolding and aggregation23,24, which leads to cell death, mitochondrial defects and neurodegeneration25. A recent study also suggests that maintaining translational fidelity is critical for bacterial stringent response26. On the other hand, some levels of mistranslation are tolerated and even beneficial under defined stress conditions27,28. For example, we have recently shown that increased translational errors in Escherichia coli improve survival under oxidative stress conditions through activation of the general stress response, which is controlled by sigma factor RpoS29.
Flagella are complex molecular machines critical for cell motility and chemotaxis in bacteria30,31. A flagellum is composed of over 20 different structural proteins assembled to form the motor, the hook and the flagellar filament32,33. Expression of flagellar genes is highly regulated and hierarchical34,35. The master operon flhDC is regulated by multiple environmental cues and in turn controls transcription of flagellar structural genes. Compared to transcriptional regulation, translational regulation of flagellar synthesis is less understood. Recent work shows that Bacillus subtilis requires modification of elongation factor P to efficiently translate certain flagellar proteins36. How flagellar synthesis is affected by translational fidelity is completely unknown. In the present work, we demonstrate that mistranslation inhibits flagellar synthesis and motility in E. coli. Such inhibition is independent of RpoS, but instead requires inactivation of a histone-like nucleoid structural protein H-NS, leading to reduced expression of flhDC. We further show that a small RNA DsrA plays a critical role in mistranslation-mediated suppression of bacterial motility.
Results
Mistranslation suppresses motility and flagellar assembly
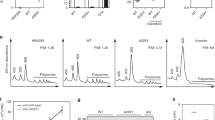
To investigate the physiological impact of mistranslation, we previously engineered an E. coli error-prone strain by introducing a point mutation (I199N) into the chromosomal rpsD gene, which encodes a protein component of the ribosomal small subunit29. The resulting rpsD* strain (Table S1) displays 5-fold increased readthrough of the UAG stop codon compared to the parent strain MG1655, but does not show a decreased protein synthesis rate29. Mutations in the rpsD gene decrease accuracy during codon-anticodon pairing to cause global mistranslation of all mRNAs and may decrease fidelity of initiation, elongation and termination during protein synthesis10. RNA sequencing of rpsD* cells grown at 37 °C29 reveals that flagellar assembly is the most significantly downregulated pathway compared to wild-type (WT) MG1655 (P = 1.9 × 10−25). Because even WT MG1655 shows low expression of flagellar genes and slow motility at 37 °C, we tested the motility of WT and rpsD* strains at room temperature (25 °C). Our results showed that the rpsD* strain was defective in motility on soft agar plates (Fig. 1). The motility defect was rescued by either reverting the chromosomal rpsD* mutation or introducing a second mutation (K42N) in the rpsL gene to reduce translational errors (Fig. 1). The K42N mutation is located near the ribosomal A site and restricts pairing between codon and anticodon and has been shown to increase decoding fidelity37. In addition to mistranslation caused by the rpsD* mutation, codon-specific mistranslation caused by addition of canavanine (an arginine analogue recognized by arginyl-tRNA synthetase and mistranslates arginine codons) also decreases motility (Fig. S1). Next, we used negative-staining electron microscopy to visualize the flagella of WT and rpsD* strains. Whereas WT cells contained multiple flagella per cell, most rpsD* displayed no mature flagella at all (Fig. 2). These results suggest that the motility defect caused by mistranslation is due to impaired flagellar assembly.
Motility defect of error-prone rpsD* strain.
Motility of WT, rpsD*, rpsD* revertant and rpsD*/L* strains were tested on soft-agar plates. In panel B, relative motility was calculated as the percentage of the spot diameter relative to the WT strain. The quantitative results are the average of at least three repeats with error bars indicating standard deviations.
Mistranslation decreases expression of flagellar genes
We next tested the expression levels of flagellar genes in the WT and rpsD* strains at 25 °C using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The rpsD* mutation significantly decreased the mRNA levels of all tested flagellar genes, including flgB (encoding a flagellar basal-body rod protein), flgK (encoding a hook-filament junction protein), fliA (encoding Sigma 28 involved in synthesis of later-stage flagellar genes), fliF (encoding an MS-ring structural protein) and flhDC (encoding the master regulator of flagellar genes FlhD and FlhC) (Figs 3 and 4). Among these genes, transcription of flgB and fliA is dependent on the FlhDC complex and flgK and fliF are controlled by both FlhDC and FliA34,35.
Mistranslation downregulates flhDC expression.
(A) qRT-PCR of flhD and flhC mRNA. (B) Western blot of FLAG-FlhD protein. Quantitation of FlhD protein level is normalized with loading control RpoB. (C) Time course of FLAG-FlhD degradation. The quantitative results are the average of at least three repeats with error bars indicating standard deviations.
To determine how translational errors affect the protein level of FlhD, we inserted a Flag tag at the 3′-end of the chromosomal flhD gene at the native locus. Western blot using an anti-Flag antibody revealed that the FlhD protein level decreased 60% in the rpsD* strain compared to the WT (Fig. 4B). We further showed that such decrease was not due to accelerated degradation (Fig. 4C), suggesting that mistranslation downregulates FlhD at the transcriptional and/or translational level.
Small RNA DsrA inhibits motility in error-prone strain
We have previously shown that translational errors activate the general stress response, which is controlled by RpoS29. The increase of RpoS level under error-prone conditions at 37 °C depends on a small RNA DsrA29. It has been suggested that RpoS negatively regulates expression of FliA and cell motility in E. coli38. We thus tested whether mistranslation suppresses motility through upregulation of RpoS. Deleting rpoS in the rpsD* strain was not able to restore motility (Fig. 5), suggesting that RpoS does not play a major role in flagellar synthesis under error-prone conditions. However, deleting dsrA fully rescued the motility defect of the rpsD* strain (Fig. 5). Consistently, overexpressing DsrA from a plasmid in the WT strain suppressed motility (Fig. 5). To test the role of DsrA in regulating expression of flagellar genes, we constructed a lacZ reporter under the control of flgB promoter. In line with the qRT-PCR results (Fig. 3), the activity of flgB promoter (controlled by FlhDC) decreased 60% in the rpsD* strain compared to the WT (Fig. 6). Deleting DsrA enhanced transcription of flgB to almost the same level as the WT. Addition of canavanine also decreased the activity of flgB promoter (Fig. S1B).
Promoter activity of flgB.
The promoter of E. coli flgB was fused with lacZ gene on a low copy number plasmid and transformed into various E. coli strains. The β-galactosidase activity was determined and shown as Miller Units. The rpsD* mutation decreased flgB promoter activity and deleting DsrA fully restored transcription of flgB promoter. The results are the average of at least three repeats with error bars indicating standard deviations.
DsrA is induced at low temperatures (e.g., at 25 °C) through enhanced transcription and improved stabilization39. Using qRT-PCR, we found that the RNA level of DsrA was increased 3-fold by the rpsD* mutation at 25 °C (Fig. 7A). To further investigate how mistranslation enhances DsrA level, we tested transcription of dsrA using a yellow fluorescent protein reporter under the control of dsrA promoter. Transcription of dsrA promoter increased 2.5-fold in the rpsD* strain compared to the WT (Fig. 7B). Next, we determined the stability of DsrA by inhibiting transcription with rifampicin (Rif) and following the RNA level over time. The rpsD* mutation did not enhance the stability of DsrA (Fig. 7C), suggesting that the increase in DsrA RNA occurred at the transcriptional level. Collectively, our data suggest that mistranslation elevates the DsrA RNA level, which in turn downregulates expression of flagellar genes and suppresses motility.
Mistranslation increases DsrA expression.
(A) Steady state level of DsrA RNA determined by qRT-PCR. (B) Promoter activity of DsrA determined using a YFP reporter. (C) Degradation of DsrA in E. coli strains. The results are the average of at least three repeats with error bars indicating standard deviations.
DsrA-mediated motility suppression depends on H-NS
In addition to RpoS, another major target regulated by DsrA is H-NS40. We showed that deleting dsrA in the rpsD* strain restored motility (Figs 5 and 8A). In the absence of hns, deleting dsrA no longer increased motility of rpsD* cells (Fig. 8A). In contrast, deleting rpoS did not completely prevent the rescuing effect of dsrA deletion (Fig. 8A). To dissect the roles of the RpoS and H-NS pathways in regulation of motility by DsrA, we further took advantage of previously reported DsrA mutants that specifically impair regulation of rpoS (dsrA *R) or hns (dsrA *H)41. In the complementation assay, overexpressing WT DsrA or DsrA *R, both of which are able to inhibit H-NS activity, substantially reduced motility of the WT ΔdsrA strain (Fig. 8B). In contrast, overexpressing DsrA *H, which does not directly affect the H-NS pathway, showed only a minor decrease in motility (Fig. 8B).
DsrA suppresses bacterial motility through H-NS.
(A) Deleting H-NS abolishes the effect of DsrA deletion that restores motility in rpsD* cells. (B) Overexpressing H-NS specific DsrA (dsrA *R) suppressed motility in WT cells. The results are the average of at least three repeats with error bars indicating standard deviations.
DsrA regulates H-NS at the translational level42. In line with this, we found that the mRNA level of hns was unchanged by the rpsD* mutation (Fig. S2A). However, the activity of an H-NS repressed promoter (hdeA) increased significantly in the rpsD* strain (Fig. S2B), suggesting that the overall H-NS activity is lowered by the rpsD* mutation. In addition, the mRNA levels of flhDC were downregulated in the rpsD* strain (Fig. 4A), which is consistent with previous reports that H-NS stimulates transcription of flhDC43. Our results therefore suggest that DsrA regulates flagellar synthesis and motility mainly through the H-NS pathway.
Discussion
Bacteria utilize flagella for movement in the environment. Flagella are also used as bacterial mechanosensors to initiate biofilm formation44 and are important for virulence in many bacterial pathogens32. On the other hand, biosynthesis and functioning of flagella consume substantial cellular resources45 and flagella also activate the host immune response that inhibits and kills invading bacteria46,47,48. Flexible modulation of flagellar synthesis is thus important for bacterial adaptation to frequently changing natural environments. In this study, we demonstrate that reducing translational fidelity leads to reduced flagellar synthesis and loss of motility in E. coli. We have previously shown that reduced translational fidelity activates the general stress response, promoting bacterial survival under stress conditions29. Suppressing flagellar synthesis would allow cellular resources to be conserved for essential activities to maintain cell viability, e.g., synthesis of stress response effector proteins.
We show that mistranslation suppresses flagellar synthesis and motility through enhanced transcription of DsrA. DsrA is a small RNA found in multiple Gram negative bacteria, including Escherichia, Salmonella and Shigella. DsrA RNA level is significantly increased at low temperatures due to both increased transcription and decreased degradation39 and temperature regulation of dsrA transcription depends on complex promoter architecture49. Our results show that transcription driven by dsrA promoter is enhanced in the error-prone strain (Fig. 7). To date, the only known transcriptional regulator of DsrA is LeuO, which represses DsrA transcription50. In our previous RNA sequence results29, LeuO mRNA level is increased in the rpsD* strain compared to the WT. Exactly how DsrA transcription is regulated by mistranslation remains to be clarified in the future. It is likely that another unknown transcriptional regulator of DsrA is affected by global protein mistranslation, e.g., through stabilization of a transcriptional activator due to titration of available proteases by an increased level of mistranslated proteins. It is also possible that mistranslation causes LeuO to misfold and lose its activity.
Small RNAs have been shown to regulate motility via diverse mechanisms51. Our data suggest that the effect of DsrA on bacterial motility requires H-NS instead of RpoS. DsrA blocks synthesis of H-NS protein by base pairing with the translational start site of its mRNA42. Increased expression of DsrA in the error-prone strain is thus expected to lower H-NS activity. We have used an H-NS repressed promoter hdeA as a reporter to test the activity of H-NS and show that the H-NS activity is suppressed in the rpsD* strain compared to the WT (Fig. S2). H-NS regulates a large number of genes, including activation of flhDC transcription40,43. A recent study also suggests that H-NS influences bacterial motility via FlhDC-independent pathways52. We show that overexpression of FlhC is sufficient to restore motility of the error-prone strain (Fig. S3), suggesting that mistranslation suppresses motility mainly through downregulation of flhDC in a process that requires DsrA and H-NS. Collectively, our results have revealed a previously unknown linkage between translational fidelity and flagellar synthesis, which may play an important role in bacterial adaptation to ever changing environmental conditions.
Materials and Methods
Strains, plasmids, growth conditions and reagents
Strains and plasmids used in this study are listed in Table S1 and the oligos are listed in Table S2. E. coli was grown in Lennox broth (LB) at 37 °C with agitation unless otherwise indicated. Antibiotics were used at the following concentrations: ampicillin (Amp), 100 μg/ml; chloramphenicol (Chl), 25 μg/ml. Antibiotics and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and RNase-free DNase I was from Thermo Scientific (Rockford, IL).
Genome engineering of bacterial strains
All strains used in this study are derivatives of E. coli K-12 strain MG1655 (WT), which was obtained from The E. coli Genetic Stock Center at Yale University. All in-frame gene deletion mutants were constructed as described using chloramphenicol as the resistance marker53. All mutants were verified by PCR and the antibiotic resistance marker was subsequently removed from the deletion strains using plasmid pCP20, which was cured at 42 °C afterwards. The marker-free deletion mutants were verified by both loss of resistance and PCR.
The fusion of 3 × FLAG tag at the 3′ end of flhD was conducted as follows. A cassette containing the toxin encoding gene ccdB under control of araBAD promoter and a kanamycin resistance gene (Ranquet et al., submitted, deposit patent number: FR11/60169, 08/11/2011, UJF/BGene) was amplified from template genomic DNA of CR201 strain (obtained from N. De Lay) using the primers FlhD-KN1 and FlhD-CCDB1 and introduced into chromosome by λ red recombinase-mediated gene replacement. The kan-ccdB cassette fused with flhD in the chromosome was then replaced with the gBlock fragment of 3 × FLAG tag (FlhD-FLAG, synthesized from Integrated DNA Technology). The successful recombinants (YF56 and YF57) were obtained by selection for growth in the presence of arabinose (1%) and verified by PCR.
Electron microscopy
Overnight culture of bacteria were diluted 1:100 into fresh LB and grown to OD600 ~ 0.8 at 25 °C with agitation. Cells were collected and washed in 0.1 M NaCl and resuspended in phosphate-buffered saline. To examine cells by electron microscopy, 7 μl of culture was placed onto carbon-coated nickel grids (Electron Microscopy Sciences) for 1 minute, washed three times with sterile water and then negatively stained with 0.2% uranyl acetate for 30 seconds. The samples were visualized using a JEOL JEM-1400 electron microscope. Cells were randomly selected to count the number of flagella.
Swimming motility assay
Overnight culture of bacteria were diluted 1:100 into fresh LB and grown to OD600 ~ 0.8 at 25 °C with agitation. All cultures were normalized to the same OD600 before being spotted on freshly made tryptone broth (10 g/L of tryptone and 5 g/L of NaCl) plates containing 0.25% agar. For strains harboring plasmids, appropriate antibiotics were added into the tryptone broth motility plates. Plates were incubated at 25 °C overnight before taking pictures and measuring diameters of spots. The quantitative results represent the percentage of the diameter compared to that of the WT strain on the same plate.
Quantitative reverse transcription-PCR
Mid-log phase cells grown in LB medium at 25 °C was normalized to the same OD600 and harvested. Total RNA was extracted using hot phenol and residual chromosomal DNA was removed as previously described54, except that glycogen was used to precipitate RNA samples. To test RNA degradation, freshly made rifampicin (250 μg/ml final concentration) was added into normalized bacterial cultures to fully stop transcription at time zero.
Reverse transcription and quantitative PCR were performed using the iScript cDNA Synthesis Kit and the SsoAdvanced Universal SYBR Green Supermix Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. 16S rRNA was used as an internal reference for normalization. The ΔΔCt method was used to obtain the fold change of target genes in the mutant strains compared to those in the WT strains.
Determination of FlhD protein expression
To determine expression of the FlhD, a 3 × FLAG tag was fused at the C terminal of flhD right before the stop codon in both WT and rpsD* strains. Mid-log phase cells grown in LB medium at 25 °C was normalized to the same OD600 and harvested. Same volume of bacterial cultures was used to prepare total protein using the standard trichloroacetic acid/acetone protein precipitation protocol. For sample preparation to test FlhD degradation, freshly made chloramphenicol (100 μg/ml final concentration) was added into normalized bacterial cultures to fully stop translation at time zero and same volume of cultures was collected for protein preparation at specific time point. Western blot was performed according to standard procedures55 using a primary anti-FLAG antibody.
Bacterial fluorescence protein and lacZ reporter
To measure the fluorescence intensity of reporter strains, overnight culture of bacteria was diluted to 0.01 OD600 in LB. Cells were further grown in 96-well plates incubated at 25 °C in the plate reader (BioTek) with shaking. Both OD600 and fluorescence were measured at 15 minute intervals for a total of 20 hours. Strains carrying pZS*11 were used as positive controls to eliminate the differences of protein synthesis rate between different strains. For lacZ reporter measurement, β-galactosidase assay was conducted as described24.
Additional Information
How to cite this article: Fan, Y. et al. Reduced Protein Synthesis Fidelity Inhibits Flagellar Biosynthesis and Motility. Sci. Rep. 6, 30960; doi: 10.1038/srep30960 (2016).
References
Kurland, C. G. Translational accuracy and the fitness of bacteria. Annual Review of Genetics 26, 29–50 (1992).
Kramer, E. B., Vallabhaneni, H., Mayer, L. M. & Farabaugh, P. J. A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA 16, 1797–1808 (2010).
Francklyn, C. S. DNA polymerases and aminoacyl-tRNA synthetases: shared mechanisms for ensuring the fidelity of gene expression. Biochemistry 47, 11695–11703 (2008).
Traverse, C. C. & Ochman, H. Conserved rates and patterns of transcription errors across bacterial growth states and lifestyles. Proceedings of the National Academy of Sciences of the United States of America 113, 3311–3316 (2016).
Gordon, A. J., Satory, D., Halliday, J. A. & Herman, C. Lost in transcription: transient errors in information transfer. Current Opinion in Microbiology 24, 80–87 (2015).
Mascarenhas, A. P., An, S., Rosen, A. E., Martinis, S. A. & Musier-Forsyth, K. Fidelity Mechanisms of the Aminoacyl-tRNA Synthetases. In: Protein Engineering (ed^(eds RajBhandary UL, Köhrer C) Springer-Verlag (2008).
Ling, J., Reynolds, N. & Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annual Review of Microbiology 63, 61–78 (2009).
LaRiviere, F. J., Wolfson, A. D. & Uhlenbeck, O. C. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science 294, 165–168 (2001).
Zaher, H. S. & Green, R. Fidelity at the molecular level: lessons from protein synthesis. Cell 136, 746–762 (2009).
Ogle, J. M. & Ramakrishnan, V. Structural insights into translational fidelity. Annual Review of Biochemistry 74, 129–177 (2005).
Li, L. et al. Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proceedings of the National Academy of Sciences of the United States of America 108, 9378–9383 (2011).
Liu, Z. et al. Homologous trans-editing factors with broad tRNA specificity prevent mistranslation caused by serine/threonine misactivation. Proceedings of the National Academy of Sciences of the United States of America 112, 6027–6032 (2015).
Ballesteros, M., Fredriksson, A., Henriksson, J. & Nystrom, T. Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO Journal 20, 5280–5289 (2001).
Zaborske, J. M., DuMont, V. L., Wallace, E. W., Pan, T., Aquadro, C. F. & Drummond, D. A. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biology 12, e1002015 (2014).
Davies, J., Gilbert, W. & Gorini, L. Streptomycin, suppression and the code. Proceedings of the National Academy of Sciences of the United States of America 51, 883–890 (1964).
Gromadski, K. B. & Rodnina, M. V. Streptomycin interferes with conformational coupling between codon recognition and GTPase activation on the ribosome. Nature Structural Molecular Biology 11, 316–322 (2004).
Netzer, N. et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462, 522–526 (2009).
Ling, J. & Söll, D. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proceedings of the National Academy of Sciences of the United States of America 107, 4028–4033 (2010).
Bullwinkle, T. et al. Oxidation of cellular amino acid pools leads to cytotoxic mistranslation of the genetic code. eLife e02501 (2014).
Haft, R. J. et al. Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria. Proceedings of the National Academy of Sciences of the United States of America 111, E2576–E2585 (2014).
Meyerovich, M., Mamou, G. & Ben-Yehuda, S. Visualizing high error levels during gene expression in living bacterial cells. Proceedings of the National Academy of Sciences of the United States of America 107, 11543–11548 (2010).
Schwartz, M. H. & Pan, T. Temperature dependent mistranslation in a hyperthermophile adapts proteins to lower temperatures. Nucleic Acids Research 44, 294–303 (2016).
Lee, J. W. et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 (2006).
Ling, J., Cho, C., Guo, L. T., Aerni, H. R., Rinehart, J. & Söll, D. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Molecular Cell 48, 713–722 (2012).
Reynolds, N. M., Lazazzera, B. A. & Ibba, M. Cellular mechanisms that control mistranslation. Nature Review of Microbiology 8, 849–856 (2010).
Bullwinkle, T. J. & Ibba, M. Translation quality control is critical for bacterial responses to amino acid stress. Proceedings of the National Academy of Sciences of the United States of America 113, 2252–2257 (2016).
Pan, T. Adaptive translation as a mechanism of stress response and adaptation. Annual Review of Genetics 47, 121–137 (2013).
Pouplana, L. R., Santos, M. A., Zhu, J. H., Farabaugh, P. J. & Javid, B. Protein mistranslation: friend or foe? Trends in Biochemical Sciences 39, 355–362 (2014).
Fan, Y., Wu, J., Ung, M. H., De Lay, N., Cheng, C. & Ling, J. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Research 43, 1740–1748 (2015).
Kearns, D. B. A field guide to bacterial swarming motility. Nature reviews Microbiology 8, 634–644 (2010).
Yuan, J., Branch, R. W., Hosu, B. G. & Berg, H. C. Adaptation at the output of the chemotaxis signalling pathway. Nature 484, 233–236 (2012).
Zhao, X., Norris, S. J. & Liu, J. Molecular architecture of the bacterial flagellar motor in cells. Biochemistry 53, 4323–4333 (2014).
Chevance, F. F. & Hughes, K. T. Coordinating assembly of a bacterial macromolecular machine. Nature reviews Microbiology 6, 455–465 (2008).
Chilcott, G. S. & Hughes, K. T. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiology and molecular biology reviews : MMBR 64, 694–708 (2000).
Liu, R. & Ochman, H. Stepwise formation of the bacterial flagellar system. Proceedings of the National Academy of Sciences of the United States of America 104, 7116–7121 (2007).
Rajkovic, A. et al. Translation control of swarming proficiency in Bacillus subtilis by 5-amino-pentanolylated elongation factor P. The Journal of biological chemistry (2016).
Kramer, E. B. & Farabaugh, P. J. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 13, 87–96 (2007).
Dong, T., Yu, R. & Schellhorn, H. Antagonistic regulation of motility and transcriptome expression by RpoN and RpoS in Escherichia coli. Molecular microbiology 79, 375–386 (2011).
Repoila, F. & Gottesman, S. Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. Journal of Bacteriology 183, 4012–4023 (2001).
Bertin, P. et al. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. Journal of bacteriology 176, 5537–5540 (1994).
Lease, R. A., Cusick, M. E. & Belfort, M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proceedings of the National Academy of Sciences of the United States of America 95, 12456–12461 (1998).
Lalaouna, D., Morissette, A., Carrier, M. C. & Masse, E. DsrA regulatory RNA represses both hns and rbsD mRNAs through distinct mechanisms in Escherichia coli. Molecular microbiology 98, 357–369 (2015).
Soutourina, O. et al. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. Journal of bacteriology 181, 7500–7508 (1999).
Belas, R. Biofilms, flagella and mechanosensing of surfaces by bacteria. Trends in microbiology 22, 517–527 (2014).
Guttenplan, S. B. & Kearns, D. B. Regulation of flagellar motility during biofilm formation. FEMS microbiology reviews 37, 849–871 (2013).
Ramos, H. C., Rumbo, M. & Sirard, J. C. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends in microbiology 12, 509–517 (2004).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nature immunology 7, 576–582 (2006).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature immunology 7, 569–575 (2006).
Repoila, F. & Gottesman, S. Temperature sensing by the dsrA promoter. Journal of bacteriology 185, 6609–6614 (2003).
Klauck, E., Bohringer, J. & Hengge-Aronis, R. The LysR-like regulator LeuO in Escherichia coli is involved in the translational regulation of rpoS by affecting the expression of the small regulatory DsrA-RNA. Molecular Microbiology 25, 559–569 (1997).
De Lay, N. & Gottesman, S. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Mol Microbiol 86, 524–538 (2012).
Kim, E. A. & Blair, D. F. Function of the Histone-Like Protein H-NS in Motility of Escherichia coli: Multiple Regulatory Roles Rather than Direct Action at the Flagellar Motor. Journal of bacteriology 197, 3110–3120 (2015).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America 97, 6640–6645 (2000).
Masse, E., Vanderpool, C. K. & Gottesman, S. Effect of RyhB small RNA on global iron use in Escherichia coli. Journal of bacteriology 187, 6962–6971 (2005).
Miller, J. H. Experiments in molecular genetics (1972).
Acknowledgements
We thank Dr. Susan Gottesman (National Institutes of Health) and Dr. Nicholas De Lay (The University of Texas Health Science Center at Houston) for providing us plasmids, strains and constructive comments. This work is funded by The National Institute of General Medical Sciences R01 GM115431 (to JL).
Author information
Authors and Affiliations
Contributions
Y.F. and J.L. designed experiments, analyzed data and wrote the manuscript. Y.F. and C.R.E. performed experiments.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fan, Y., Evans, C. & Ling, J. Reduced Protein Synthesis Fidelity Inhibits Flagellar Biosynthesis and Motility. Sci Rep 6, 30960 (2016). https://doi.org/10.1038/srep30960
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30960
This article is cited by
-
Transcriptome analysis of Escherichia coli K1 after therapy with hesperidin conjugated with silver nanoparticles
BMC Microbiology (2021)
-
Biosynthesis of selenium nanoparticles and effects of selenite, selenate, and selenomethionine on cell growth and morphology in Rahnella aquatilis HX2
Applied Microbiology and Biotechnology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.