Abstract
Targeted disruption of leukocyte trafficking to the gut represents a promising approach for the treatment of inflammatory bowel diseases (IBDs). CCR5, the shared receptor for MIP1α and β and RANTES, is expressed by multiple leukocytes. Here, we aimed to determine the role of CCR5 in mediating leukocyte trafficking in models of colitis and evaluate the therapeutic potential of maraviroc, an orally active CCR5 antagonist used in the treatment of CCR5-tropic HIV. Acute and chronic colitis were induced by administration of DSS or TNBS to wild-type and CCR5−/− mice or adoptive transfer of splenic naïve CD4+ T-cells from wild type or CCR5−/− mice into RAG-1−/−. CCR5 gene ablation reduced the mucosal recruitment and activation of CCR5-bearing CD4+ and CD11b+ leukocytes, resulting in profound attenuation of signs and symptoms of inflammation in the TNBS and transfer models of colitis. In the DSS/TNBS colitis and in the transfer model, maraviroc attenuated development of intestinal inflammation by selectively reducing the recruitment of CCR5 bearing leukocytes. In summary, CCR5 regulates recruitment of blood leukocytes into the colon indicating that targeting CCR5 may offer therapeutic options in IBDs.
Similar content being viewed by others
Introduction
Crohn’s disease and ulcerative colitis are two chronic inflammatory bowel diseases (IBDs) arising from inappropriate intestinal immune responsesto antigens derived from commensal microorganisms in genetically susceptible individuals. IBDs have traditionally been treated with non-specific immunosuppressants including corticosteroids and thiopurines, anti-inflammatory agents such as 5-aminosalicylic acid and more recently with tumor necrosis factor (TNF)α antagonists1. However, despite these treatments have been found effective in mild disease, a large proportion of patients with severe disease fail to achieve remission due to lack of drug response, loss of response, drug intolerance, or severe side effects that require cessation of therapy. There is therefore an urgent unmet clinical need for novel treatments that can suppress the intestinal inflammatory response in a more specific way, thereby overcoming the limitations of the currently available immunosuppressant therapies1.
A hallmark of chronic inflammatory disorders is the rapid recruitment and inappropriate retention of leukocytes at the site (s) of inflammation. Therapeutic targeting of the molecules that govern leukocyte recruitment and retention in the intestine is therefore a promising strategy for the treatment of IBDs1. Thus, the integrin α4β7 antagonist vedulizumab which attenuates inflammation by reducing leukocytes trafficking has gained approval for the treatment of IBD1. In addition to integrins, the recruitment of blood leukocytes to sites of inflammation is orchestrated by the interaction of chemokine receptors with specific ligands that are expressed in an anatomically restricted fashion2,3,4. More than 50 chemokines have been identified to date which are divided into four main families based on structural and functional characteristics2,3,4. The C-C chemokine receptor 5 (CCR5) is the shared receptor for the chemotactic mediators CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES). CCR5 is expressed by multiple cell lineages including T-cells, monocytes, macrophages and dendritic cells. Because expression of CCR5 ligands is increased in models of colitis, CCR5 antagonism might hold promise as a therapeutic strategy in IBDs5,6.
In addition to regulating leukocyte homing to the inflamed mucosa, CCR5 functions as a major co-receptor for HIV entry into target cells and selective CCR5 antagonists have been developed that inhibit the replication of CCR5-tropic viral strains7,8. Maraviroc, is a small molecule that induces a non-competitive, slowly reversible, inhibition of CCR5 and displays therapeutic efficacy against CCR5-tropic HIV infection9,10,11. In addition, by blocking the signaling of all three CCR5 ligands, maraviroc effectively inhibits the migration and effector functions of CCR5-bearing leukocytes exerting anti-inflammatory and immune-modulatory effects12,13,14,15.
In the current report, we demonstrate that recruitment of CCR5-expressing leukocytes in the colon is essential for the onset and maintenance of inflammation in mouse models of colitis and that, maraviroc, a small molecule inhibitors of CCR5, protects against colitis development targeting CCR5-bearing leukocytes. These data suggest that CCR5 inhibitors could be used to modulate leukocyte trafficking in human IBDs.
Materials and Methods
Animals
CCR5−/− (B6.129P2-Ccr5tm1Kuz/J), CCR5+/+, CX3CR1+GFP on C57BL6 background and Rag-1−/− mice were from the SPF animal facility at A*STAR, Singapore. The study was carried out in strict accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Biological Resource Centre (BRC) of Biopolis in Singapore or University of Perugia, Italy. The BRC IACUC protocol was approved by the National Advisory Committee for Laboratory Animal Research in Singapore (Permit Number: 110626) and by Perugia University’s ethical committee and Italian Ministry of Health permit no. 42/2014/B.
DSS And TNBS Colitis Models
DSS colitis was induced by oral administration of 5% DSS in drinking water to weight matched, 4–6 weeks old male CD1 mice for 5 or 8 (not shown) days. Mice were randomized to receive DSS alone or DSS in combination with maraviroc at dose of 5, 25 or 50 mg/kg/day or prednisolone 5 mg/kg/day16, starting on day 1 after DSS. The dose of prednisolone used in this study has been demonstrated effective in reducing inflammation in rodent models of colitis and is roughly similar to the dose used in clinical setting (1 mg/kg)16. Animals were monitored daily for weight loss and fecal score (0, normal; 1, soft but still formed; 2, very soft; 3, diarrhea; and 4, liquid stool). The criteria/scoring system for assessing macroscopic damage was as follows; 0, no ulcer, no inflammation; 1, no ulcer, local hyperemia; 2, colon wall thickening/edema; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation. Additional points were scored for the presence of indurations, intestinal adhesions and each additional site of inflammation/ulceration >1 cm2 in surface area.
For TNBS colitis, weight matched, 4–6 weeks old male wild-type and CCR5−/− mice were pretreatedwith 100 μl TNBS pre-sensitization solution (1% TNBS dissolved in 4:1acetone:olive oil solution) which was applied to shaved abdominal skin on day −717. Control mice were subjected to the same protocol but using pre-sensitization solution that lacked TNBS. After 7 d, the mice were anesthetized by i.p. injection with ketamine/xylazine solution (100 mg/ml ketamine and 20 mg/ml xylazine in saline administered at 100 μl/10 g of body weight) and then treated with TNBS (0.5 mg per mouse) which was dissolved in 50% ethanol and administered intra-rectally using a 3.5 French catheter equipped with a 1 ml syringe. To ensure uniform distribution of TNBS throughout the colon and cecum, mice were held in a vertical position for 30 s after instillation. Mice were randomized into either the control group (TNBS only, n = 6) or treatment group (TNBS plus 50 mg/kg/d maraviroc per os, n = 5–6) from day 1 after TNBS administration and then monitored daily as described above.
T-cell transfer colitis model
Splenocytes were collected from 6–10 week old CCR5−/− mice or wild-type controlmice (n = 8 per group) and naive CD4+ CD45RBhigh T-cells were isolated by cell sorting. A total of 3 × 105 CD45RBhigh cells were then injected intravenously into Rag1−/− mice that were subsequently weighed and assessed for fecal score every 20 days to evaluate IBD development18. To investigate whether maraviroc rescues from intestinal inflammation induced by transfer colitis, Rag1−/− mice were injected with CD4+ CD45RBhigh T-cells and 34 days later randomized into either a control group (no further treatment, n = 6) or treatment with maraviroc, 50 mg/kg/d maraviroc per os (n = 4) for 3 weeks, 5 d/week.
Flow cytometry and cell sorting
Cell suspensions were prepared from spleen, colonic lamina propria (LP) and mesenteric lymph nodes (MLN) and used for flow cytometry or cell sorting the next day. The following antibodies were obtained from Biolegend and used at 1:100 dilution unless specified otherwise; CD195 (CCR5)-APC or PE (clone HM-CCR5), CD44-PE/Cy7 (clone IM7), CD62L-FITC (clone MEL-14, BD Pharmingen), CD45-PE, FITC and PerCP-Cy5.5 (clone 30-F11 used at 1:200 dilution), CD4-APC/Cy7 or FITC or APC or AlexaFluor405 (clone RM4-5), CD45RB-APC/Cy7 (clone C36-16A), MHCII-APC/Cy7 (cloneM5/II4.152) GR1-Pacific blue (clone RB6-8C5, eBioscience) CD11b-PE/Cy7 and AlexaFlour700 (clone M1/70, eBioscience) Ly-6C-FITC (clone HK1.4), Ly-6G-PE (clone 1A8, BD Pharmingen) and DAPI. In the transfer colitis model, assessment of intracellular cytokine production by CD4+ T-cells was achieved by stimulating the sorted cells with PMA (0.1 μg/ml) and ionomycin (1 μg/ml) for 6.5 h ex vivo in the presence of Brefeldin A (10 μg/ml) for the final 5 h. The cells were then harvested and surface-labelled with CD4-APC/Cy7 before permeabilization using BD Cytofix/Cytoperm™ kits (BD Bioscience) and intracellular staining for 30 min at 4 °C using a 50 μl mixture of IL17-PE (clone TCII-18H101 used at 1:50 dilution), IFNγ-AlexaFluor®647 (clone XMG1.2 used at 1:50 dilution), TGF-β1-Pacific Blue (clone TW-16B4) and IL10-PE/Cy7 (clone JES5-16E3 used at 1:50 dilution).
Inflammatory Histological Score
Sections of medial colon and small intestine were fixed in buffered formalin, routine 5 μm sections were prepared (~150 μm between each section, 4–8 per fragment) and then stained with hematoxylin and eosin. Stained sections were examined and scored in a blinded fashion. The histologic scoring system was as follows: A) degree of inflammation was graded semi-quantitatively from 0 to 4 (0, no signs of inflammation; 1, very low level; 2, low level of leukocyte infiltration; 3, high level of leukocyte infiltration, high vascular density and thickening of the colon wall; and 4, transmural infiltration, loss of goblet cells, high vascular density and thickening of the colon wall). B) Extent of inflammation from 0 to 4 (0 = none; 1 = mucosal; 2 = submucosal; 3 = mucosal + submucosal; 4 = full thickness). The total inflammatory score was obtained by multiplying the ‘degree’ by ‘extent’ scores (minimum 0, maximum score 16).
ELISA assay
Lamina propria mononuclear cells (LPMC) were obtained from mice with TNBS-induced colitis (6 days duration) and seeded into 96 well plates (200,000 cells/well) for stimulation with LPS (5 μg/ml) in RPMI complete medium for 36 h at 37 °C16,17. Alternatively, CD4+ cells were sorted from the MLN after 3–4 d duration of TNBS colitis and then seeded into 96 well plates (100,000 cells/well) for stimulation with plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (2 μg/ml) in RPMI complete medium for 72 h at 37 °C. After incubation, the culture supernatant was analyzed for cytokine content by ELISA. Measurement of cytokine/chemokine release in the DSS model was achieved using a Bio-Plex® Multiplex Immunoassay kit (BioRad, Italy).
Statistical analysis
Data are expressed as mean ± standard error. Two-tailed, unpaired Student’s t tests were used to compare 2 groups of data, as indicated in the respective figures. P < 0.05 was considered significant. When more than two groups were considered, one way ANOVA followed by Bonferroni was used. GraphPad Prism software version 5.0 was used to prepare the graphics and perform all statistical analyses (GraphPad Software, San Diego, CA).
Results
Maraviroc Induces A Dose-Dependent Decrease In Mucosal Inflammation In DSS Colitis
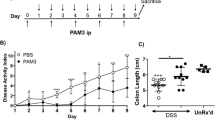
In order to assess the efficacy of CCR5 blockade as a therapeutic approach in colonic inflammation, mice administered with DSS were treated with maraviroc (5, 25 or 50 mg/kg/day) or prednisolone (5 mg/kg/day) for the following 5 days. At a dose of 50 mg/kg/day, maraviroc attenuated the development of signs and symptoms of colitis with an efficacy that was comparable to 5 mg/kg/day prednisolone (Fig. 1). All three doses of maraviroc effectively blocked the recruitment of leukocytes into the colon, as assessed by measurement of MPO activity, although some of the therapeutic benefit of maraviroc treatment appeared to be lost at the lower doses (Fig. 1A–D). Maravirocalso protected against development of macroscopic and microscopic damage and colonic shortening and was as effective as prednisolone at preventing DSS-induced changes in colonic levels of IL-2, IL-4, IL-5, IL-6, IL-17 and GM-CSF thought the some of these effects were likely of minor mechanistic relevance (Fig. 1E,F). These protective effect persisted for up to 8 days (data not shown).
Maraviroc induces a dose-dependent decrease in mucosal inflammation in DSS Colitis.
Mice were administered DSS (5%) in drinking water for 5 days and then treated with placebo or maraviroc (5, 25 or 50 mg/kg/d) or prednisolone (5 mg/Kg/d) for 4 days starting on day 2. (A) The disease activity index (DAI) was calculated daily for each individual mouse based on weight loss, rectal bleeding and stool consistency. (B,C) Macroscopic injury (lesion area in mm2) and colon length. (D) MPO activity (mU/mg protein). (E) Histopathology analysis of colon in mice administered DSS alone or in combination with maraviroc or prednisolone. Data Are mean ± SE of 5 mice per group. (F) Assessment of tissue biomarkers by Bio-Plex® Multiplex immunoassay. Data were normalized to total tissue proteins. In each panel at least 5 animals per group are included. Markers for the Th1 phenotype are labeled in blue, for Th2 phenotype in violet and for regulatory phenotype/chemokine in black. *P < 0.05 versus control; **P < 0.05 versus DSS.
CCR5 Gene Ablation Or Maraviroc Therapy Reduces Inflammation In TNBS Colitis
We next investigated the involvement of CCR5 in the pathology of a T-cell-driven colon inflammation using a TNBS-induced model of colitis in both wild-type mice and CCR5−/− animals that received either placebo or maraviroc treatment (50 mg/kg for 5 days, starting one day after colitis induction) (Fig. 2A–C). In wild-type animals, TNBS-induced colitis was associated with a robust influx of CD45+ CD11b+ leukocytes into the LP (Fig. 2D,E). Analysis of the expression of MHC-II and myeloid differentiation antigen Gr-1 in these cells allowed the identification of three major subsets (Fig. 2F): population 1 comprised MHC-II+ Gr-1− CD11bint cells (P1; putative macrophages), whereas population 2 was made-up of MHC-II−Gr-1−CD11blow cells (P2; putative monocyticcells) and population 3 cells were MHC-II−Gr-1+ CD11bhigh (P3; putative granulocytes). Further analysis of Ly6C, Ly6G, CD11c and CX3CR1 expression among the LP infiltrating leukocytes indicated that population P2 included a substantial fraction of Ly6C+ i.e. blood derived monocytes and that exposure to TNBS increased substantially the proportion of these cells (Table 1 and Supplementary Figure 1)19,20. In wild-type animals, leukocyte infiltration of the colon was associated with development of phenotypic features of acute colitis (Fig. 2A–C). In contrast, CCR5−/− mice exhibited milder weight-loss, reduced severity of diarrhea and only limited thickening of the colon wall compared with CCR5+/+ animals (Fig. 2A–C; P < 0.05 versus wild type). Importantly, maraviroc rescued wild-type animals from the development of wasting disease, diarrhea and macroscopic inflammation (Fig. 2A–C; P < 0.05 versus DSS wild type). Consistent with the concept that CCR5 blockade achieves these beneficial effects by impairing the mucosal recruitment of multiple leukocyte lineages, we also observed that disruption of CCR5 signaling either by gene ablation or small molecule antagonism significantly attenuated the influx of myeloid cells into the LP (Fig. 2D–F).
CCR5-knockout mice are protected against TNBS-induced acute colitis.
(A,B) Severity of TNBS-induced colitis (weight loss and stool consistency) was reduced in CCR5−/− mice. (C) Macroscopic inflammation. n = 6 per group (*P < 0.05, **P < 0.01, ***P < 0.005). (D) Absolute number of CD45+ and CD11b+ cells detected in the colonic LP of individual naïve and coltic mice. (E) Representative flow cytometry analysis of CCR5+/+ mice treated with TNBS showing staining of total colonic leucocytes for CD11b+ cells (upper panel, after exclusion of MHC-IIhigh CD11chigh putative dendritic cells) and their relative expressions of MHC-II and GR1 (lower panel). (F) Absolute number of cells in the colonic LP corresponding to Population 1 (P1) (MHC-II+/Gr-1−/CD11bint), Population 2 (P2) (MHC-II−/Gr-1−/CD11blow) and Population 3 (P3) (MHC-II−/Gr-1+/CD11bhigh) in untreated and TNBS coliticmice that were treated or not by oral administration of maraviroc. Data shown mean ± SE n = 3 per group (*P < 0.05, **P < 0.01, ***P < 0.005; two-tailed, unpaired Student’s t test).
CCR5 Expression In Both The Innate And Adaptive Immune Compartments Contributes To Murine Intestinal Inflammation
Further analysis of the CD11b+ myeloid cells detected in wild-type colitic mice revealed an increase in CCR5 expression among these cells after exposure to TNBS (Fig. 3A). This increase in CCR5 expression in the myeloid cell compartment was accompanied by a shift away from the predominant macrophage population detected in the steady-state (CCR5+ CD11bint MHC-II+ Gr-1− cells), towards an inflammatory infiltrate made up primarily of monocyte-like cells (CCR5+ CD11blow MHC-II−Gr-1−) (Fig. 3B; *p < 0.05, ***p < 0.005). CCR5 antagonism using maraviroc therapy abrogated the accumulation of CCR5+ CD11b+ leukocytes in the inflamed colonic mucosa (Fig. 3A and Supplementary Figure 2, *p < 0.05) and was associated with a robust reduction of pro-inflammatory mediators including TNFα, IL-6 and IL-1β (Fig. 3C).
CCR5 expression in myeloid cells in steady state condition and during colitis.
(A) CD11b+ cells in the colonic LP exhibit up-regulation of CCR5 during colitis which can be blocked by administration of maraviroc therapy (n = 3 per group; *P < 0.05). (B) The CD11b+ CCR5+ cell colonic infiltrate was analyzed for differential expression of MHCII and GR1; the upper panel shows a representative dot plot and the lower panel shows the relative proportions of P1, P2 and P3 cells detected in untreated and colitic mice that were treated or not with maraviroc. (C) Cytokine production by colonic LP leukocytes upon ex vivo stimulation with LPS for 36 h. Values indicate mean ± SE; n = 3/group (*P < 0.05, **P < 0.01, ***P < 0.005).
Importantly, CCR5 also contributed to the mucosal recruitment of pro-inflammatory T cells. After 6 days of TNBS administration, we detected a substantial number of CD4+ cells in the LP, but only in mice that were sufficient in CCR5 (Fig. 4A). CD44 expression has previously been described as a marker of memory T-cells with tissue homing potential21.
CCR5 expression by colonic CD4+ T-cell subsets is up-regulated in colitis and decreased by maraviroc therapy.
(A) Absolute number of CD4+ T-cells detected in the colonic LP of mice after 6 days of TNBS colitis with or without maraviroc therapy for the final 5 days. (B) CCR5 expression level within the CD44low and CD44high populations of colonic CD4+ T-cells. (C) Representative contour plots showing CD44 and CCR5 expression levels among CD4+ T-cells in the colonic LP in both control and colitic mice that were treated or not with maraviroc or vehicle. Values are mean ± SE; n = 3 (*P < 0.05).
CCR5 is considered to be a phenotypic marker for effector/memory T-cells22,23. Consistent with this view, CD4+ T-cells with a CD44high ‘memory’ phenotype exhibited stable high expression of CCR5 in the mouse LP (Fig. 4B). Furthermore, TNBS colitis associated with increased levels of CCR5 expression within the T-cell pool, but this was largely restricted to the CD44low immature subset, suggesting maturation of these cells towards an effector/memory phenotype during inflammation (Fig. 4B). CCR5 upregulation by colitis in CD4+ CD44low cells was abrogated by co treatment with maraviroc (Fig. 4B,C).
Mucosal Inflammatory Responses Of CCR5+ Leukocytes Exhibit An Effector/Memory Th17 Profile
Within the CD44high subset of LP CD4+ T-cells, we further identified that CCR5+ cells were substantially larger in size than CCR5− cells in both the steady-state and during inflammation, suggesting selective cell cycle entry and clonal expansion of the CCR5+ population in this model24. Similarly, while the CD44low subset of CD4+ LP exhibited fairly uniform size in the steady-state, there was a robust increase in the size of the CCR5+ cells following exposure to TNBS (Fig. 5A). These data are consistent with the known ability of chemokines to influence T-cell differentiation25.
Differential morphology and CCR5 expression by memory CD4+ T-cells during TNBS colitis.
(A) CD4+ T-cell morphology of the CCR5+ subset (red contour) and CCR5- subset (blue contour) within the CD44high and CD44low populations of colonic CD4+ T-cells obtained from mice that were either untreated or subjected to TNBS colitis for 7 days. (n = 3–4/group; *P < 0.05, **P < 0.05). (B) Contour plot showing the percentage of CCR5+ cells with the CD4+ T-cell pool of colonic LP and mesenteric lymph nodes after 3–4 days of TNBS colitis. (C) CD4+ T-cell morphology of the CCR5+ subset (red contour) and CCR5- population (blue contour) obtained from mesenteric lymph nodes of TNBS colitic mice. Proliferation (D) and cytokine production (E) by CCR5+ and CCR5- populations of sorted CFSE-labeled CD4+ T-cells after in vitro stimulation with CD3/CD28 for 72 hours. Values are mean ± SE; n = 4 (***P < 0.005).
Previous reports suggest that CCR5 is selectively expressed on IFN-γ producing Th1 cells and allowing targeted recruitment of these cells to inflammatory sites23,24. Therefore we next sought to determine whether CCR5-expressing leukocytes in the TNBS model produce cytokines consistent with a Th1 phenotype. For this purpose, we FACS-sorted CCR5- and CCR5+ populations of CD4+ T-cells from the LP and MLN of colitic mice and then stimulated these cells with anti-CD3/CD28 mAb. Of relevance, we found that almost all colonic CCR5+ CD4+ T-cells died during the 3-day stimulation period (data not shown), likely due to activation-induced cell death, whereas there was no difference in the rate of cell death and proliferation detected among MLN-derived cells (data not shown and Fig. 5D, respectively). Assessment of cytokine levels in culture supernatants of MLN-derived cells demonstrated that CCR5+ CD4+ cells displayed a profile consistent with Th17 polarization during TNBS colitis (Fig. 5E).
Leukocyte Expression Of CCR5 Is Required For Both Acute And Chronic Murine Colitis
The role of CCR5 in the context of chronic inflammation was investigated using the adoptive transfer model of T-cell-mediated colitis in animals grafted with either CCR5+/+ or CCR5−/− T-cells. Adoptive transfer of naïve CD4+ CD45RBhigh T-cells from wild-type miceinto RAG1−/− recipients conferred gut pathology characterized bya severe weight loss, diarrhea, macroscopic inflammation and colonic thickening (Fig. 6A–D). In contrast, mice that were transferred with naïve CD4+ CD45RBhigh T-cells that lacked CCR5 exhibited milder disease and fewer total colonic leukocytes as well as reduced infiltration of CD4+ effector/memory T-cells into the gut mucosa (Fig. 6E). Indeed, unlike CD4+ T-cells from wild-type donors, CCR5−/− CD4+ T-cells appeared unable to efficiently mature into effector cells and exhibited significantly reduced production of IFNγ and IL-17 when analyzed ex vivo (Fig. 6F). A similar immune profile was also observed in the MLN of mice transferred with CCR5−/− CD4+ T-cells (Supplementary Figures 3 and 4). Together, these data indicated that CCR5 ablation in CD4+ T-cells abolishes a Th1/Th17-type inflammatory pathology of the gut which resembles that of human IBD26.
CCR5+ CD4+ T-cells are critically required for the onset and maintenance of chronic colitis.
(A–D) Colitis severity (weight loss, stool consistency, macroscopic and microscopic inflammation and colon length) and representative photo of mouse colons after adoptive transfer of CD4+ T-cells from the spleens of CCR5+/+ and CCR5KO mice into Rag1−/− recipient animals (3 × 105 CD45RBhigh cells/mouse). Values indicate mean ± SE; n = 7 (*P < 0.05). (E) Phenotypic analysis of colonic leukocytes showing the total number of CD45+ cells, total CD4+, CD4+ naïve cells (CD62Lpos/CD44low) and CD4+ activated/memory cells (CD62Lneg/CD44high), with representative dot plots showing expression of CD62L and CD44 within the CD4+ T-cell compartment. (F) Cytokine production by CD4+ T-cells obtained from LP of colitic mice. Values are mean ± SE; n = 6/group (*P < 0.05, **P < 0.01).
CCR5 blockade attenuates sign and symptoms of chronic colitis
To investigate whether the therapeutic effects observed in acute models of colitis was maintained over time, Rag1−/− rendered colitic by transfer of CD4+ T cells from wild type donors, were administered with maraviroc 50 mg/kg/d for 3 weeks starting on day 34 after colitis induction. As shown in Fig. 7 and Supplementary Figure 5, we found that maraviroc attenuates colon inflammation in this setting and reduced signs and symptoms of colitis, as well as the inflow of CD45+ cells into the LP. Analysis expression of markers of activation demonstrated that long term administration of maraviroc reduced the number of IFNγ+ cells, but had no effect on IL-10 and TGFβ stained cells. Altogether these data confirm that the maraviroc attenuates inflammation in chronic models of colitis.
CCR5 Inhibition reduces chronic colitis severity.
(A,B) Colitis severity (weight loss from the day of maraviroc treatment, stool consistency, macroscopic inflammation and colon length) and representative photo of mouse colons after adoptive transfer of CD4+ T-cells from the spleens of CCR5+/+ mice into Rag1−/− recipient animals (3 × 105 CD45RBhigh cells/mouse) alone or after 3 weeks of treatment with maraviroc (50 mg mg/kg/d, 5 days for week). Values indicate mean ± standard error of n = 4 and 6 per group (*P < 0.05). (C) Phenotypic analysis of colonic leukocytes showing the total number of CD45+ cells, total CD4+, CD4+ naïve cells (CD62Lpos/CD44low) and CD4+ activated/memory cells (CD44high). (D) Cytokine production by CD4+ T-cells obtained from LP of colitic mice alone or treated with maraviroc. Values indicate mean ± standard error of n = 4/group (*P < 0.05).
Discussion
In the present study we provide evidence that CCR5, a chemokine receptor expressed by different leukocytes exerts a critical role in the development of acute and chronic inflammation in murine models of colitis. Analysis of CCR5 expression on LP leukocytes in mice administered TNBS revealed that colitis development in this modelis mediated by the recruitment of three major leukocyte populations: MHC-II+ Gr-1− CD11bint cells (putative macrophages), MHC-II−Gr-1− CD11blow cells (likely monocytes/macrophages) and MHC-II− Gr-1+ CD11bhigh cells (putative granulocytes). A further characterization of these cells, revealed that the monocytes/macrophages compartment in the TNBS model is largely composed by Ly6C+ cells. Previous studies have shown that in the steady state the intestinal monocytes/macrophages population in mice is maintained by the continuous recruitment of Ly6C+ monocytes (CD14+ cells in humans) from the blood stream25. Under resting conditions, these monocytes undergo local differentiation into reparative, anti-inflammatory, macrophages (MHCII+ CD11bInt and CX3CR1+) that are highly phagocytic, hypo-responsive to pro-inflammatory stimuli producing large amounts of IL-10 in inflammatory milieus. The maturation of these Ly6Chigh blood monocytes into tissue-resident macrophages (MHCIIpos Gr1neg CD11b+) is governed by signals received from the local micro environment and involves cell transition through several intermediate phenotypes. However, the normal pattern of monocyte differentiation is disrupted during inflammation, leading to the recruitment and accumulation of Ly6C+ MHCIIpos CX3CR1int effector monocytes in the intestine, where theycan generate TNFα and other soluble mediators. In the present study, we observed that in the steady state themonocytic cell population of the colonic LP is largely made up byLy6C+ cells (up to 60%) and the predominance of Ly6C+ cells in this compartment increases further in the TNBS colitis (>90% of the monocytic cell infiltrate), strongly indicating the selective recruitment of Ly6C+ effector monocytes from the blood stream23,24. Consistent with this view, while only 10% of gut monocytic cells expressed CCR5 in the steady state, the proportion of CCR5+ cells increased to ~70% in response to TNBS, supporting the notion that colitis development in this model is supported by recruitment of circulating Ly6C+ monocytes via a mechanism that involves CCR5. Importantly, these Ly6C+ CCR5+ cells displayed a strong polarization towards an effector phenotype and produced pro-inflammatory cytokines including IL-1β, IL-6 and TNFα.
In addition to regulating the trafficking of innate leukocytes, CCR5 is also critically required for the recruitment of circulating T-cells to the intestinal mucosa. Thus CCR5−/− mice were essentially unable to recruit CD4+ cells into the LP in response to TNBS27. Phenotypic and functional characterization of LP lymphocytes demonstrated that ~15% of CD4+ cells in the gut express CCR5 in the steady state and that exposure to TNBS promoted the recruitment of additional CCR5+ CD4+ T-cells with robust polarization towards a Th17 profile. These data underlined a broad role of CCR5 in regulating the mucosal recruitment and functional maturation of both innate immune cells and T-cells arriving from the blood.
Consistent with the data obtained from chemical colitis models, the adoptive transfer of naive CD4+ CD45RBhigh T-cells into RAG-1−/− mice demonstrated that CCR5-deficent T-cells were unable to replicate the chronic colitis that was induced by CCR5-sufficient T-cells. Transplantation of CCR5−/− T-cells associated with a substantial reduction in the number of CD4+ and CD45+ cells recruited in the LP of grafted animals, as well as reduced weight loss, lower diarrhea score, milder macroscopic inflammation and reduced colonic shortening. In contrast to the acute chemical colitis models, attenuation of gut inflammation by CCR5 gene ablation, however, was due largely to the impaired infiltration of CD4+ T-cells and did not depend on changes in the CD11b+ myeloid compartment. In addition to disrupting the mucosal recruitment of CD4+ T-cells, genetic ablation of CCR5 also reducedthe proportion of cells with an activated phenotype (CD44highCD62L−) and impaired the ability of these cells toproduce IFNγ and IL-17 without any discernable loss of immuno regulatory function (IL-10+ TGF-β+ CD4+ T-cell frequency).
The critical role of CCR5 in regulating leukocyte trafficking in intestinal inflammation was confirmed by the protective effects exerted by maraviroc in three model of colitis, i.e. the acute colitis induced by DSS and TNBS and in the chronic colitis induced by transfer of CD4+ T cells into Rag1−/− mice. Maraviroc is an orally active, allosteric modulator of CCR5 that binds to the extracellular portion of the receptor, thereby preventing the conformational changes required for signal transduction after engagement of physiological ligands (CCL3, 4 and 5) or viral proteins (HIV GP120)8,9. Here, we have shown that administration of maraviroc attenuates gutinflammation in rodent models of colitis by selectively depleting CD11b+ CCR5+ cells from the colonic mucosa (in the TNBS model) and modulating the production of signature cytokines such as IL -1β, IL-6 and TNFα in both DSS-induced and TNBS models of colitis. Of relevance, the therapeutic effect of maraviroc was maintained in the chronic model of colitis resulting in a robust attenuation of recruitment of CD45+/CD11b+ cells in the LP.
While the data we report strongly support a role for CCR5 in rodent colitis, CCR5 gene mutations do not overtly modulate human IBD phenotypes, as demonstrated by the fact thata common 32-bp ‘loss-of-function’ deletion (Δ32) of CCR5 fails to confer protection against IBD28,29,30,31,32,33. In contrast, the Δ32 mutation confers protection against development of primary sclerosing cholangitis in IBD34. These data suggest that alternative mechanisms of leukocyte trafficking to the gut may compensate for the absence of CCR5 signaling in IBD patients with this mutation and conversely that the benefits of maraviroc therapy may be restricted to IBD patients with a functional CCR5 gene.
In conclusion, we have provided compelling evidence that CCR5 mediates the trafficking of both innate and adaptive immune cells in rodent models of colitis. Pharmacological inhibition or genetic ablation of CCR5 rescued mice from colitis in both acute and chronic models, hence the clinically-approved small molecule antagonist of CCR5, maraviroc may represent a novel approach to reducing the mucosal trafficking of blood leukocytes in human IBDs.
Additional Information
How to cite this article: Mencarelli, A. et al. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci. Rep. 6, 30802; doi: 10.1038/srep30802 (2016).
References
Danese, S. & Panés, J. Development of drugs to target interactions between leukocytes and endothelial cells and treatment algorithms for inflammatory bowel diseases. Gastroenterology. 147, 981–989 (2014).
Mitroulis, I. et al. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 147C, 123–135 (2015).
Zimmerman, N. P., Vongsa, R. A., Wendt, M. K. & Dwinell, M. B. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis. 14, 1000–1011 (2008).
Griffith, J. W., Sokol, C. L. & Luster, A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 32, 659–702 (2014).
Oppermann, M. Chemokine receptor CCR5: insights into structure, function and regulation. Cellular Signalling 16, 1201–1210 (2004).
Andres, P. G. et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 164, 6303–6312 (2000).
Klasse, P. J. The molecular basis of HIV entry. Cell Microbiol. 14, 1183–1192 (2012).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 370, 901–910 (2014).
Lagane, B., Garcia-Perez, J. & Kellenberger, E. Modeling the allosteric modulation of CCR5 function by Maraviroc. Drug Discov Today Technol. 10, e297–e305 (2013).
Tan, Q. et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341, 1387–1390 (2013).
Garcia-Perez, J. et al. Allosteric model of maraviroc binding to CC chemokine receptor 5 (CCR5). J Biol Chem. 286, 33409–33421 (2011).
Ermocida, A. et al. Extravirologic modulation of immune response by an NRTI-sparingantiretroviral regimen including darunavir and maraviroc. New Microbiol. 37, 225–229 (2014).
Arberas, H. et al. In vitro effects of the CCR5 inhibitor maraviroc on human T cell function. Antimicrob Chemother. 68, 577–586 (2013).
Reshef, R. et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 367, 135–145 (2012).
Cipriani, S. et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation 127, 2114–2124 (2013).
Fiorucci, S. et al. NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA 99, 15770–15775 (2002).
Fiorucci, S. et al. Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis. Immunity 17, 769–780 (2002).
Ostanin, D. V. et al. T cell transfer model of chronic colitis: concepts, considerations and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 296, G135–G146 (2009).
Bain, C. C. & Mowat, A. M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 260, 102–117 (2014).
Cerovic, V. et al. Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 8, 38–48 (2015).
Tietz, W. & Hamann, A. The migratory behavior of murine CD4+ cells of memory phenotype. Eur J Immunol. 27, 2225–2232 (1997).
Bleul, C. C., Wu, L., Hoxie, J. A., Springer, T. A. & Mackay, C. R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA 94, 1925–1930 (1997).
Mackay, C. R. Cxcr3(+)ccr5(+) t cells and autoimmune diseases: guilty as charged? J clin invest 124, 3682–3684 (2014).
Bain, C. C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 15, 929–937 (2014).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Weber, B., Saurer, L., Schenk, M., Dickgreber, N. & Mueller, C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur J Immunol. 41, 773–779 (2011).
Luther, S. A. & Cyster, J. G. Chemokines as regulators of T cell differentiation. Nat Immunol. 2, 102–107 (2001).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Platt, A. M., Bain, C. C., Bordon, Y., Sester, D. P. & Mowat, A. M. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 184, 6843–6854 (2010).
Rugtveit, J. et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology 112, 1493–1505 (1997).
Rector, A. et al. Analysis of the CC chemokine receptor 5 (CCR5) delta-32 polymorphism in inflammatory bowel disease. Hum Genet. 108, 190–193 (2001).
Henckaerts, L. et al. CC-type chemokine receptor 5-Delta32 mutation protects against primary sclerosing cholangitis. Inflamm Bowel Dis. 12, 272–277 (2006).
Acknowledgements
The authors would like to thank I. Low, T. Seng, S. Mustafah and N. Shadan from the SIgN flow-cytometry facility for help with cell sorting and analysis. This study was supported in part by an unrestricted grant from ViiV Healthcare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study. Andrea Mencarelli was supported by the BMRC, A*STAR, Singapore. Author should like to thanks L. Robinson and N. McCarthy, Insight Editing London for English revision.
Author information
Authors and Affiliations
Contributions
Study concept and design: S.F., A.M., E.D., L.S., F.B. and D.F. Acquisition of data: A.M. and S.C. Analysis and interpretation of data: A.M., S.F., E.D. and S.C. Drafting of the manuscript: A.M., S.F., E.D. and L.S. Study supervision: S.F. and F.B.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mencarelli, A., Cipriani, S., Francisci, D. et al. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci Rep 6, 30802 (2016). https://doi.org/10.1038/srep30802
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30802
This article is cited by
-
CCR5 maintains macrophages in the bone marrow and drives hematopoietic failure in a mouse model of severe aplastic anemia
Leukemia (2021)
-
Targeted 1H NMR metabolomics and immunological phenotyping of human fresh blood and serum samples discriminate between healthy individuals and inflammatory bowel disease patients treated with anti-TNF
Journal of Molecular Medicine (2021)
-
The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer
Mucosal Immunology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.